Abstract
The H7N9 influenza virus caused significant mortality and morbidity in infected humans during an outbreak in China in 2013 stimulating vaccine development efforts. As previous H7-based vaccines have been poorly immunogenic in humans we sought to determine the immunogenic and protective properties of an inactivated whole virus vaccine derived from a 2013 H7N9 virus in ferrets. As whole virus vaccine preparations have been shown to be more immunogenic in humans, but less likely to be used, than split or surface antigen formulations, we vaccinated ferrets with a single dose of 15, 30, or 50 μg of the vaccine and subsequently challenged with wild-type A/Anhui/1/2013 (H7N9) either by direct instillation or by contact with infected animals. Although ferrets vaccinated with higher doses of vaccine had higher serum hemagglutinin inhibition (HI) titers, the titers were still low. During subsequent instillation challenge, however, ferrets vaccinated with 50 μg of vaccine showed no illness and shed significantly less virus than mock vaccinated controls. All vaccinated ferrets had lower virus loads in their lungs as compared to controls. In a separate study where unvaccinated-infected ferrets were placed in the same cage with vaccinated-uninfected ferrets, vaccination did not prevent infection in the contact ferrets, although they showed a trend of lower viral load. Overall, we conclude that inactivated whole-virus H7N9 vaccine was able to reduce the severity of infection and viral load, despite the lack of hemagglutinin-inhibiting antibodies.
Keywords: H7N9, vaccine, ferrets
Introduction
A novel subtype of avian influenza virus to cause human infections, H7N9, emerged in China in 2013 and has since infected more than 200 humans [1], with unusually high mortality [2]. Unlike highly pathogenic avian strains of human concern, H7N9 is a low-pathogenic avian virus, causing subclinical symptoms in avian species. This presents a considerable obstacle in identification and control of the outbreak sources. Although most H7N9 isolates are susceptible to neuraminidase inhibitors, resistant phenotypes have been identified in patients who received treatment [3, 4]. Surveillance studies suggest that the H7 subtype viruses are prevalent in live-bird markets, even in regions outside the reported outbreak areas [5]. Their prevalence and the difficulty of detecting H7N9 circulation in poultry mean that they will likely continue to be a zoonotic threat for the foreseeable future. For these reasons, national and international agencies have begun development of intervention strategies. Vaccination remains an effective strategy to prepare for a pandemic since it provides protection against infection and induces herd immunity to limit virus spread. The World Health Organization (WHO) considers vaccination “a key component in the response and preparedness efforts against a pandemic potential, including avian influenza A (H5N1), A (H9N2), and A (H7N9)” [6]. The reemergence of H7N9 early this year emphasizes the importance in developing an effective and immunogenic vaccine.
For the present situation, an H7N9 vaccine has two major hurdles: vaccines against avian influenza strains typically are poorly immunogenic [7, 8], and the elderly, who have been disproportionately affected by H7N9, generally respond poorly to influenza vaccines [9]. This presents a compound challenge to developing an effective H7N9 vaccine. Most seasonal inactivated influenza vaccines are comprised of split-virion or surface antigen products due to their lower reactogenicity as compared to inactivated whole-virus vaccines. However, the latter is more immunogenic when compared head-to-head in an unprimed population, eliciting a stronger antibody response with just a single dose [10, 11]. Split-virion vaccines for avian influenza viruses, however are known to be poorly immunogenic in humans (summarized in Table 1 in [12]). Clinical trials with split-virion H7N7 vaccines have reported very low seroconversion rates in vaccinees despite receiving two doses at 90 μg HA each [13] and this vaccine was unable to protect mice from the lethal effects of homologous virus infection (unpublished data). Due to these data, the pending human H7N9 vaccine clinical trials (www.clinicaltrial.gov; study identifier: NCT01995695, NCT01928472,NCT01942265, NCT01938742), and the predictions that similar immunogenicity issues may be apparent with H7N9 based vaccines [14], we sought to determine if an inactivated whole-virus based H7N9 vaccine is able to induce protective antibody levels. As manufacturing burden can dictate the timely supply of vaccine and whole virus preparations are likely to be more immunogenic than the more commonly used split or surface antigen preparations, we chose to evaluate if a single dose of this vaccine is sufficiently protective. The reference vaccine strain was developed by the US Centers for Disease Control and Prevention, Atlanta, and consists of the major antigenic proteins, hemagglutinin (HA) and neuraminidase (NA), from one of the human isolates of H7N9 virus, A/Shanghai/2/2013, that was recommended by the WHO as a candidate vaccine strain, combined with the six internal genes from a high-growth donor strain [15]. Here, we describe the efficacy of an inactivated, whole-virus vaccine based on reverse genetics recombinant H7N9 virus to protect ferrets from infection in a challenge and natural exposure study.
Table 1.
Hemagglutinin inhibition (HI) titer in ferrets that received PBS or 15, 30, or 50 μg of inactivated H7N9 vaccine. Data are presented as geometric mean titer with upper and lower 95% confidence interval in parentheses.
| Serum antibody titer, geometric mean titer (95% CI) | |||
|---|---|---|---|
| Vaccine group | Pre-vaccination | Day 21 post-vaccination | No. of ferrets with titers >10 |
| PBS | <10 | 5# (5) | 0/9 |
| 15 μg | < 10 | 7.3 (4.6-11.7) | 3/9 |
| 30 μg | < 10 | 11.7 (8.2-16.6)* | 8/9 |
| 50 μg | < 10 | 14.7 (10.0-21.6)* | 8/9 |
Statistically significant, p<0.05 by Mann-Whitney test compared with PBS control.
HI titers of <10 were arbitrarily set at 5.
Materials and Methods
Viruses and cells
The challenge virus A/Anhui/1/2013 (H7N9) is antigenically similar to the vaccine strain [16] and was received as a kind gift from Dr. Yue Long Shu of the Chinese Centre for Disease Control. Virus stock was prepared by propagation in 10-day-old embryonated chicken eggs at 35°C for 36 hours, aliquoted, and stored at –70°C. Marin-Darby canine kidney (MDCK) cells used for virus titration were propagated in minimal essential medium (MEM) supplemented with 10% fetal calf serum, vitamins, L-glutamine, and antibiotics in a humidified 5% CO2 environment.
Generation of the candidate vaccine
The recombinant vaccine seed strain A/Shanghai/2/2013-PR8-IDCDC-RG32A was developed by and received from the Centers for Disease Control and Prevention. The vaccine strain was a reverse-genetics–based strain bearing the HA and NA from the human isolate of A/Shanghai/2/2013 (H7N9) and six of the internal genes from the A/Puerto Rico/8/1938 strain [17]. Virus stock was prepared at our laboratory by propagation in eggs at 35°C for 48 hours. Virus-containing allantoic fluid was harvested and inactivated with β-propiolactone at a ratio of 1:2000 (vol/vol) for 72 hours. Inactivated virus stock was then concentrated using Amicon ultrafiltration and ultracentrifugation through a 25% and 70% sucrose cushion, pelleted at 76,000 × g at 4°C for an hour, and purified as previously described [18]. The total protein content was determined using the Bradford Assay (Biorad) according to the manufacturer's specifications. To estimate the HA content in the vaccine, vaccine stock and a dilution series of bovine serum albumin (BSA) standards were run on a SDS-PAGE gel and Coomasie-stained. ImageJ software was used to perform densitometry analysis on the protein bands. The HA content was estimated based on the non-linear regression curve generated with the BSA standards (see Supplemental Figure 1). HA content (total of HA0 and HA1) was determined to be between 28 to 31% of total protein. Immunization dose was formulated based on HA content being 25% of total protein content.
Immunization and challenge
Specific-pathogen–free ferrets, 4 to 6 months old, were purchased from Triple F Farms (Bradford County, Pennsylvania), divided into four groups of nine ferrets each and bled for baseline sera. Ferrets in each group were then vaccinated with 15, 30, or 50 μg of vaccine, diluted in phosphate-buffered saline (PBS) in 0.5 ml volume, by intramuscular injection. A control group received 0.5 ml of PBS only. Three weeks later, sera were collected and tested for antibody titers as described below. Ferrets were subsequently divided into two experiments: 6 vaccinated ferrets were challenged with wild-type H7N9 virus via intranasal instillation, while the remaining 3 were used as vaccinated contacts in a transmission experiment. For the PBS-control group, 3 ferrets were challenged with wild-type virus, 3 served as mock vaccinated contacts, and the remaining 3 served as donor ferrets in the transmission experiment.
For the challenge experiment, ferrets were anesthetized with isoflurane and inoculated intranasally with 1 ml of 106 EID50 of wild-type H7N9, A/Anhui/1/2013 virus. Clinical signs of infection, weight and body temperature were monitored daily for 7 days post-challenge.
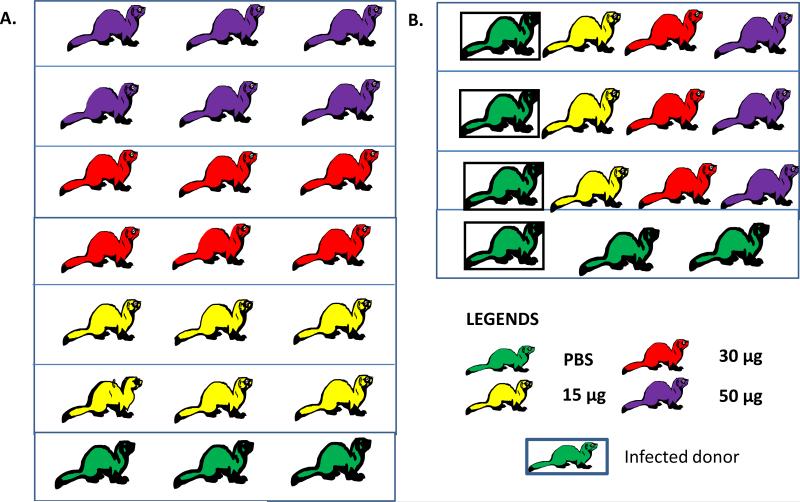
For the transmission experiment, 3 mock vaccinated ferrets were anesthetized with isoflurane and similarly inoculated with wild-type H7N9 virus to serve as donors. One day post-inoculation, vaccinated and mock vaccinated ferrets were placed into the same cage as the donor ferret to serve as direct contacts. The transmission experiment setup consists of 1 infected ferret and 1 ferret from each vaccination group, for a total of 4 ferrets per cage (Figure 1). Clinical signs of infection, weight and body temperature were monitored daily for 7 days post-challenge.
Figure 1.
Experimental setup for the (A) challenge study and (B) transmission study.
Nasal wash and tissue samples
On days 3, 5, and 7 after virus inoculation, ferrets were anesthetized by ketamine (25 mg/kg), and nasal washes were collected in 1 ml of PBS. On day 5, 3 ferrets from each vaccination group in the challenge experiment were euthanized with a mix of ketamine and xylazine according to institutional protocol. Lung tissues were collected from each of the 5 lung lobes and pooled as a single sample, homogenized and titrated on MDCK cells. After three days, a hemagglutinin assay was performed and the virus titer was determined using the Reed and Muench method [19]. The limit of virus detection was < 1 log10 TCID50/ml.
Serologic testing
Serum samples were treated with receptor-destroying enzyme (Denka Seiken, Japan), heat-inactivated at 56°C for 30 minutes, and tested in an HA inhibition (HI) assay with 0.5% chicken red blood cells [20].
Statistical tests
Data collected were analyzed and graphed using Graphpad's Prism version 5.03. HI titers were expressed as geometric mean titers with a 95% confidence interval, while viral titers were expressed as mean titers ± standard deviation in log10 TCID50/ml (or g). Statistical analysis was performed using analysis of variance (ANOVA) with Bonferroni's post-test, with p<0.05 deemed as statistically significant unless otherwise stated.
Results
Immunogenicity of A/Shanghai/2/2013-PR8-IDCDC-RG32A
We tested the serum HI titer of ferrets vaccinated with the different doses of HA at 3 weeks post-vaccination. After single doses of 15, 30, and 50 μg of vaccine, the geometric mean HI titers were 7.3, 11.7, and 14.7, respectively (Table 1), while the mock vaccinated ferrets did not show any increase in HI titers. The number of ferrets that showed detectable titers also increased with the higher vaccine doses. Although ferrets showed higher HI titers with higher doses of vaccine, none of them had titers ≥40, typically considered a minimal protective level for seasonal influenza vaccines in humans [21, 22]. Thus, although inducing relatively low titers, a single dose of this vaccine was able to induce hemagglutination inhibiting antibodies in a dose-dependent manner.
Protection against challenge with wild-type virus
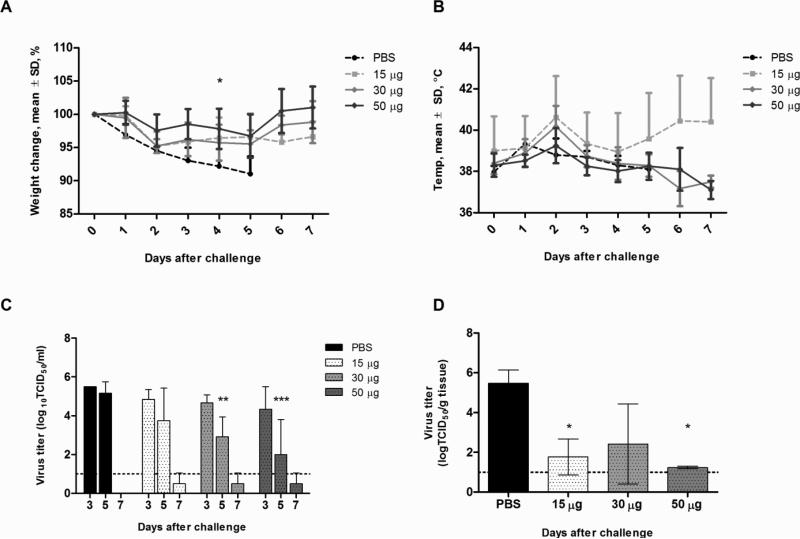
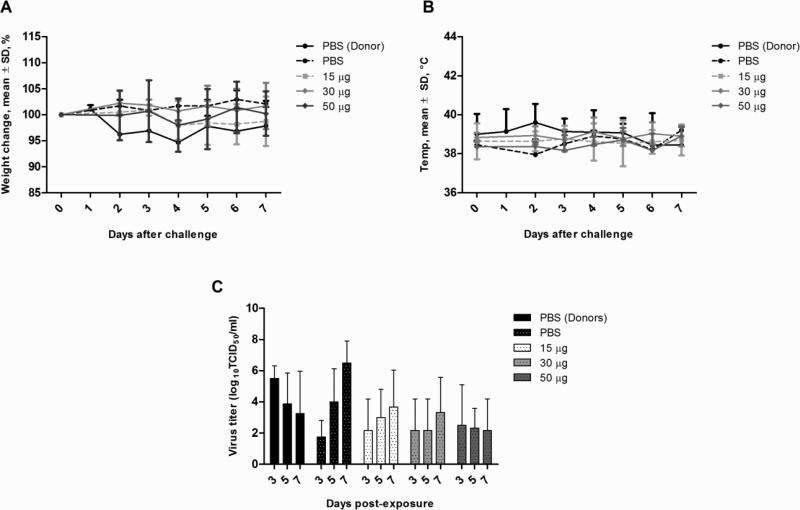
Three weeks after vaccination, the first cohort of ferrets were challenged with 106 EID50 of wild-type H7N9 virus, A/Anhui/1/2013, via intranasal instillation. Mock vaccinated ferrets showed lethargy, lack of appetite, steady weight loss (an average of up to 9% of initial body weight), and elevated temperatures as early as day 1 after challenge (Figure 2A and 2B). Ferrets that received 15 or 30 μg of vaccine also showed moderate weight loss (less than 5% of initial body weight) and elevated temperatures by day 2 after challenge but showed signs of recovery by day 3. By day 5, mock vaccinated ferrets had lost significantly more weight than the vaccinated ferrets (p<0.05). Most ferrets’ temperature had returned to baseline by day 5 with the exception of 1 ferret in the 15 μg group, resulting in an upward curve in the temperature graph (Figure 2B). Ferrets that received 50 μg of vaccine showed the least weight loss (3.2% of initial body weight) and temperature elevation. This weight loss difference from day 3 onward was statistically significant compared with the control group (p<0.01).
Figure 2.
Clinical signs and viral load in vaccinated ferrets challenged with wild-type H7N9 virus. Groups of 3 ferrets were vaccinated and challenged with 106 EID50 of virus 3 weeks later. Ferrets were monitored for weight (A) and temperature changes (B) daily up to 7 days post-infection. Data are presented as mean ± standard deviation of percentage of weight change over baseline (day 0, pre-infection). Viral shedding (C) was determined from nasal washes collected on days 3, 5, and 7 post-infection. Lung titers (D) were determined from three ferrets euthanized on day 5 post-infection. Viral titer data are presented as mean log10 TCID50/ml in nasal washes and mean log10 TCID50/g of tissue for lung titers. Statistical significance was determined using the ANOVA test with p<0.05 deemed significant compared with the PBS control group.
Vaccination also reduced the amount and duration of virus shed in the nasal cavities of infected ferrets. Although there were no differences during early infection, ferrets that were vaccinated with 30 and 50 μg of vaccine shed 2.9 ± 1.0 and 2.0 ± 1.8 log10 TCID50/ml respectively by day 5, which were significantly lower than controls (5.1 ± 0.5 log10 TCID50/ml) (p<0.05). Although no samples were available from a parallel control group, the unvaccinated ferrets in the transmission group, infected with the same dose of virus shed virus up to day 7 (Figure 3C), with a mean titer of 3.3 ± 1.4 log10 TCID50/ml. In contrast, none of the vaccinated ferrets had detectable virus titers by day 7 post-challenge.
Figure 3.
Clinical signs and viral load in vaccinated contact and donor ferrets in the transmission experiment. Groups of 3 ferrets were vaccinated and 3 weeks later placed into the same cage with a donor ferret infected with 106 EID50 of virus. Ferrets were monitored for weight (A) and temperature changes (B) daily up to 7 days post-infection. Data are presented as mean ± standard deviation of percentage of weight change over baseline (day 0, pre-infection). Viral shedding (C) was determined from nasal washes collected on days 3, 5, and 7 post-infection. Viral titer data are presented as mean log10 TCID50/ml in nasal washes. Statistical testing was done using the ANOVA test with p<0.05 deemed significant compared with the PBS control-contact group.
In the lungs, all 3 mock vaccinated ferrets showed detectable virus titers, resulting in a mean titer of 5.5 ± 0.6 log10 TCID50/g. In contrast, only 2 vaccinated ferrets, 1 each in the 15 and 30 μg vaccine groups, had detectable virus titers. The ferret in the 15 μg group had 2.8 log10 TCID50/g, while the ferret in the 30 μg group had 4.7 log10 TCID50/g. Overall, all vaccinated ferrets had significantly lower lung titers (p<0.05) with the exception of the one ferret in the 30 μg group. In addition, based on macroscopic lung lesions observed during tissue collection, the extent of lung damage corresponded to the level of viral titers in the lungs (Supplemental Figure 2). Mock vaccinated ferrets had large hemorrhagic lesions in multiple lung lobes, while the two vaccinated ferrets with detectable titers also had similar lesions in multiple lung lobes. Ferrets in the 50 μg group also showed foci of localized lesions, but the damage appeared to be less severe than in the control group.
All challenged ferrets seroconverted by day 7 post-infection, except for those in the PBS group (Table 2).
Table 2.
Serum hemagglutinin inhibition (HI) titers of ferrets at 7 days post-infection or contact exposure in the challenge or transmission experiment.
| Experiment | No. of ferrets seroconverted (HI titer of each ferret) |
|||
|---|---|---|---|---|
| PBS | 15 μg | 30 μg | 50 μg | |
| Challenge | 0/4* | 3/3 (320,320,640) | 2/2# (640,1280) | 2/2# (640,640) |
| Transmission (contacts) | 0/2 (5,5) | 1/3 (5,5,320) | 0/3 (10,10,20) | 1/3 (20,20,40) |
Infected donors in transmission experiment.
Sera from only 2 ferrets were obtained.
In summary, high doses of the inactivated whole-virus vaccine reduced amount and duration of virus shed and resulted in milder infection despite the lack of a robust HI antibody titer.
Vaccination did not protect from transmission
To determine whether vaccination is able to prevent infection by natural exposure, a transmission experiment was conducted, in which unvaccinated infected ferrets (donors) were housed in the same cage with uninfected, vaccinated contact ferrets. Thus, each cage held a donor ferret and three contact ferrets, each vaccinated with 15, 30, or 50 μg of vaccine. Weight, temperature, and symptoms were monitored, and nasal swabs were after collected 1 day post-contact. Donor ferrets lost weight, showed elevated temperatures that peaked at day 2 after infection, and shed virus up to 7 days post-infection. All contact ferrets, except for 1 each in the 15 and 30 μg vaccine groups, shed detectable virus during the study period. Mock vaccinated contact ferrets had increasing viral loads, with peak virus titer of 6.5 ± 1.4 log10 TCID50/ml on day 7. The vaccinated contact ferrets generally showed lower viral titers throughout the sampling periods, with peak virus titers for the 15, 30 and 50 μg at 3.7± 2.3, 3.3 ± 2.2 and 2.5 ± 2.6 log10 TCID50/ml respectively. However, the differences in virus titers between vaccinated and control group did not achieve statistical significance. Only two contact ferrets seroconverted at the end of the experiment, one from the 15 μg (the non-shedder, which showed a titer of 320) and the other from the 50 μg vaccine groups (Table 2). None of the contact ferrets showed significant changes in body weight or temperature during the study period. In summary, vaccination did not prevent H7N9 virus infection by natural exposure.
Discussion
Our data described here highlight two important key points. First, a single dose of inactivated whole-virus vaccine, even at 50 μg, induced low levels of virus-binding antibodies as measured by the conventional HI assay [21, 22]. Second, despite the low-HI antibody titers, vaccination protected from severe disease, as evidenced by the mild clinical signs, shorter duration of virus shedding, and reduced infections of the lower respiratory systems in vaccinated animals. A similar phenomenon was reported for an inactivated whole-virus H5 vaccine, whereby vaccinated ferrets did not show high HI titer but shed less virus than controls [23]. Since the sensitivity of the HI assay maybe dependent on the individual virus strain [24] it is unknown if the current HI titers reflected the true immunogenicity of the vaccine strain. Furthermore, other aspects of the immune response that were not measured in this study, including cell-mediated immunity and anti-NA antibodies, may have also played a role in mediating the protective effect observed. Although anti-NA antibodies do not provide sterilizing immunity, they have shown to contribute to protection [25, 26]. Thus conventional measures of HI titers as a correlate of protection in vaccine trials may be insufficient and need to be reassessed carefully for avian viruses.
Thus far, only one other published study has examined the protective efficacy of an H7N9-based vaccine. That study used a baculovirus-derived virus-like particle vaccine with the HA and NA from A/Anhui/1/2013 and internal genes from A/Indonesia/05/2005 (H5N1) in mice. The vaccine protected mice from lethal challenge, even against an H7N3 strain, which suggests that the immunogenicity derived from H7N9 vaccine strains can afford some cross-protection against other H7 strains [27]. A vaccine capable of inducing cross-reactive antibodies would have great utility as H7 outbreaks continue to occur in poultry (http://www.oie.int/animal-health-in-the-world/update-on-avian-influenza/2013/) and have caused substantial human infections in Europe and Mexico [28, 29].
In humans, there have only been three phase I clinical trials evaluating the immunogenicity of H7 vaccines to date. Inactivated virus, protein-based vaccines were poorly immunogenic, resulting in very low rates of seroconversion even after high doses of vaccine [13] and only marginally better response in the presence of an alum adjuvant [30]. On the other hand, a live attenuated H7N3 vaccine (LAIV) was able to induce mucosal immune responses and a better response rate [31]. However, as with other avian LAIVs, the inability of avian viruses to replicate in the human respiratory tract may limit its efficacy as a vaccine [31-33]. It should be noted, however, that the correlates for protection by LAIVs in humans are not well established, and thus what really indicates protection is as yet unknown.
Despite the importance of vaccines in the field of influenza, their use is not without its problems. The major issues related to influenza vaccinations include their variable efficacy in different populations and the uncertain availability of a timely supply. Various strategies have been adopted to improve their efficacy, such as high and multiple doses, use of adjuvants, and different routes of administration, while the timely supply of vaccine is largely contingent on good virus growth during production. Therefore, an ideal vaccine needs to be immunogenic even at low doses, so as to reduce manufacturing burden and ensure an adequate supply of vaccine during the time of greatest need. Even though higher doses of vaccine resulted in better outcomes, the minimum dose tested, 15 μg, was sufficient to prevent lower respiratory tract infections. Although a whole-virus vaccine is less likely to be widely used in humans, our findings provide justification and argues well for H7N9 vaccination since there was a protective effect observed, despite not achieving the conventional standards of immunogenicity. It is possible then that increasing the vaccine immunogenicity may even be able to prevent transmission events. Approaches that have shown promising results in humans for other avian influenza vaccines include the use of oil-in-water adjuvants and different routes of inoculation. Oil-in-water adjuvanted H5N1 split-virus vaccines were shown to be immunogenic in humans [34, 35] and reduced viral burden in vaccinated animals [36]. Alternatively, adjuvanted-intranasal delivery, which targets the mucosal compartment, whilst not tested for avian viruses, have shown some success in improving immunogenicity of seasonal influenza vaccines in animal models [37-39]. These alternative approaches may represent feasible solutions to the development of an immunogenic and efficacious H7N9 vaccine.
Supplementary Material
Highlights.
Inactivated whole virus H7N9 influenza vaccine is poorly immunogenic in ferrets.
Vaccinated ferrets were protected from severe disease but not infection.
Correlates of protection for avian influenza vaccines should be reassessed.
Study findings support the development of H7N9 vaccine.
ACKNOWLEDGMENTS
This work was supported by the ALSAC and NIH/NIAID Center of Excellence for Influenza Research and Surveillance (HHSN266200700005C). We would like to thank the staff of the Animal Resource Center, especially Beth Little and David Carey, for excellent technical assistance. We would also like to acknowledge Dr. Yue Long Shu from the Chinese CDC for the wild-type virus and the US Center for Disease Control and Prevention for the vaccine seed strain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization [1/21/2014];Number of confirmed human cases of avian influenza A(H7N9) reported to WHO. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/10u_ReportWebH7N9Number.pdf.
- 2.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. The New England journal of medicine. 2013;368:2277–85. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, Lu S, Song Z, Wang W, Hao P, Li J, et al. Association between adverse clinical outcome in human disease caused by novel influenza A H7N9 virus and sustained viral shedding and emergence of antiviral resistance. Lancet. 2013;381:2273–9. doi: 10.1016/S0140-6736(13)61125-3. [DOI] [PubMed] [Google Scholar]
- 4.Yen HL, McKimm-Breschkin JL, Choy KT, Wong DD, Cheung PP, Zhou J, et al. Resistance to neuraminidase inhibitors conferred by an R292K mutation in a human influenza virus H7N9 isolate can be masked by a mixed R/K viral population. mBio. 2013:4. doi: 10.1128/mBio.00396-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam TT, Wang J, Shen Y, Zhou B, Duan L, Cheung CL, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013;502:241–4. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization [10/14/2013];Vaccine response to the avian influenza A(H7N9) outbreak. http://www.who.int/influenza/vaccines/virus/CandidateVaccineVirusesH7N9_02May13.
- 7.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, Wood J, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–64. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 8.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. The New England journal of medicine. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 9.Reber AJ, Chirkova T, Kim JH, Cao W, Biber R, Shay DK, et al. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging and disease. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Gross PA, Ennis FA, Gaerlan PF, Denson LJ, Denning CR, Schiffman D. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. The Journal of infectious diseases. 1977;136:623–32. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- 11.Wright PF, Dolin R, La Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines--II. The Journal of infectious diseases. 1976;134:633–8. doi: 10.1093/infdis/134.6.633. [DOI] [PubMed] [Google Scholar]
- 12.Couch RB, Decker WK, Utama B, Atmar RL, Nino D, Feng JQ, et al. Evaluations for in vitro correlates of immunogenicity of inactivated influenza a H5, H7 and H9 vaccines in humans. PloS one. 2012;7:e50830. doi: 10.1371/journal.pone.0050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PloS one. 2012;7:e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groot AS, Ardito M, Terry F, Levitz L, Ross T, Moise L, et al. Low immunogenicity predicted for emerging avian-origin H7N9: Implication for influenza vaccine design. Human vaccines & immunotherapeutics. 2013;9:950–6. doi: 10.4161/hv.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, Govorkova EA, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization [1/21/2014];WHO provisional recommendation on influenza A(H7N9) vaccine virus. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/ProvisionalRecommendation_H7N9_31May13.pdf.
- 17.World Health Organization [10/15/2013];Summary of status of development and availability of avian influenza A (H7N9) candidate vaccine viruses. http://www.who.int/influenza/vaccines/virus/candidates_reagents/summary_a_h7n9_cvv_20130712.pdf.
- 18.Liu M, Wood JM, Ellis T, Krauss S, Seiler P, Johnson C, et al. Preparation of a standardized, efficacious agricultural H5N3 vaccine by reverse genetics. Virology. 2003;314:580–90. doi: 10.1016/s0042-6822(03)00458-6. [DOI] [PubMed] [Google Scholar]
- 19.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:493–7. [Google Scholar]
- 20.Network WHOGIS . Manual for the laboratory diagnosis and virological surveillance of influenza. WHO Press; 2011. Serological diagnosis of influenza by hemagglutination inhibition testing. pp. 59–62. [Google Scholar]
- 21.European Agency for the Evaluation of Medicinal Product [1/21/2014];Note for guidance on harmonisation of requirements for influenza vaccines. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf.
- 22.Wood JM, Levandowski RA. The influenza vaccine licensing process. Vaccine. 2003;21:1786–8. doi: 10.1016/s0264-410x(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 23.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. The Journal of infectious diseases. 2006;194:1040–3. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann E, Lipatov AS, Webby RJ, Govorkova EA, Webster RG. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12915–20. doi: 10.1073/pnas.0506416102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson BE, Bucher DJ, Kilbourne ED. Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. Journal of virology. 1989;63:1239–46. doi: 10.1128/jvi.63.3.1239-1246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS medicine. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, et al. Development of influenza H7N9 virus like particle (VLP) vaccine: Homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31:4305–13. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–93. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Martinez I, Balish A, Barrera-Badillo G, Jones J, Nunez-Garcia TE, Jang Y, et al. Highly Pathogenic Avian Influenza A(H7N3) Virus in Poultry Workers, Mexico, 2012. Emerging infectious diseases. 2013:19. doi: 10.3201/eid1909.130087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009;27:1889–97. doi: 10.1016/j.vaccine.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 31.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27:3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. The Journal of infectious diseases. 2009;199:711–6. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaat KR, Karron RA, Luke CJ, Thumar B, McMahon BA, Chen GL, et al. An open label Phase I trial of a live attenuated H6N1 influenza virus vaccine in healthy adults. Vaccine. 2011;29:3144–8. doi: 10.1016/j.vaccine.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. The Journal of infectious diseases. 2008;197:667–75. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 35.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–9. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 36.Baras B, Stittelaar KJ, Simon JH, Thoolen RJ, Mossman SP, Pistoor FH, et al. Cross-protection against lethal H5N1 challenge in ferrets with an adjuvanted pandemic influenza vaccine. PloS one. 2008;3:e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das SC, Hatta M, Wilker PR, Myc A, Hamouda T, Neumann G, et al. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine. 2012;30:6871–7. doi: 10.1016/j.vaccine.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamouda T, Sutcliffe JA, Ciotti S, Baker JR., Jr Intranasal immunization of ferrets with commercial trivalent influenza vaccines formulated in a nanoemulsion-based adjuvant. Clinical and vaccine immunology : CVI. 2011;18:1167–75. doi: 10.1128/CVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maltais AK, Stittelaar KJ, Kroeze EJ, van Amerongen G, Dijkshoorn ML, Krestin GP, et al. Intranasally administered Endocine formulated 2009 pandemic influenza H1N1 vaccine induces broad specific antibody responses and confers protection in ferrets. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.03.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.