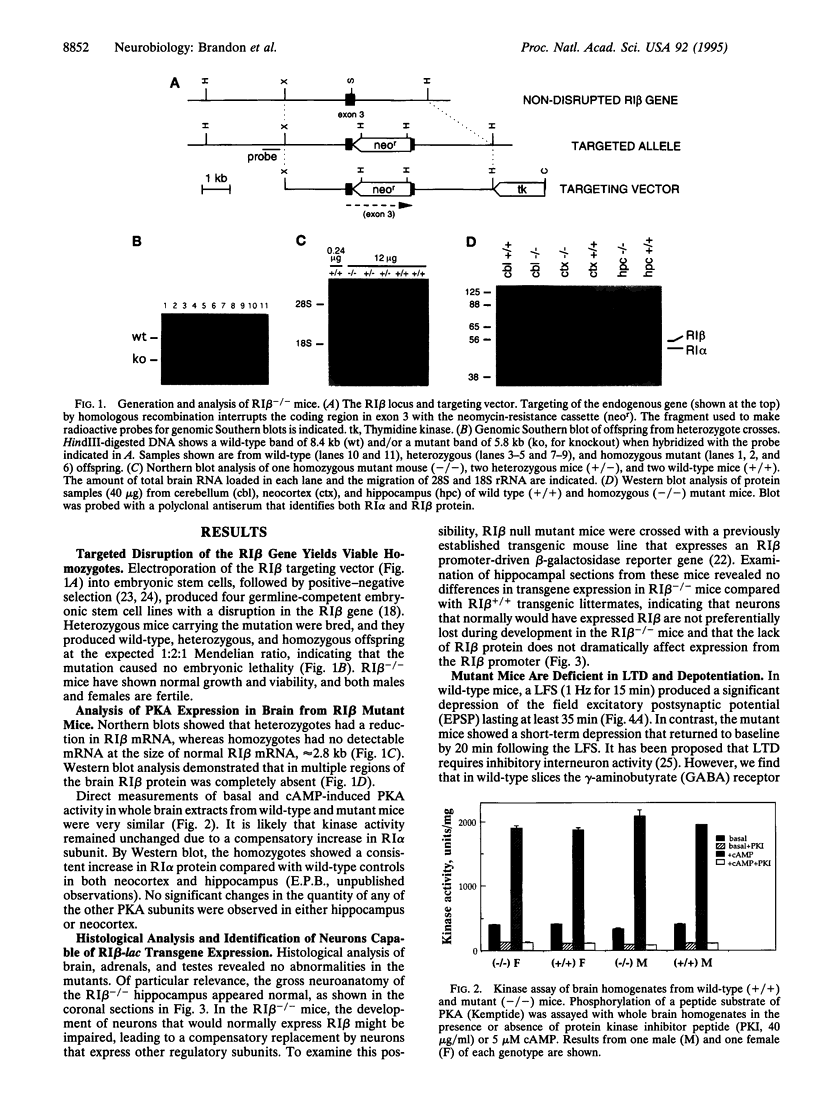
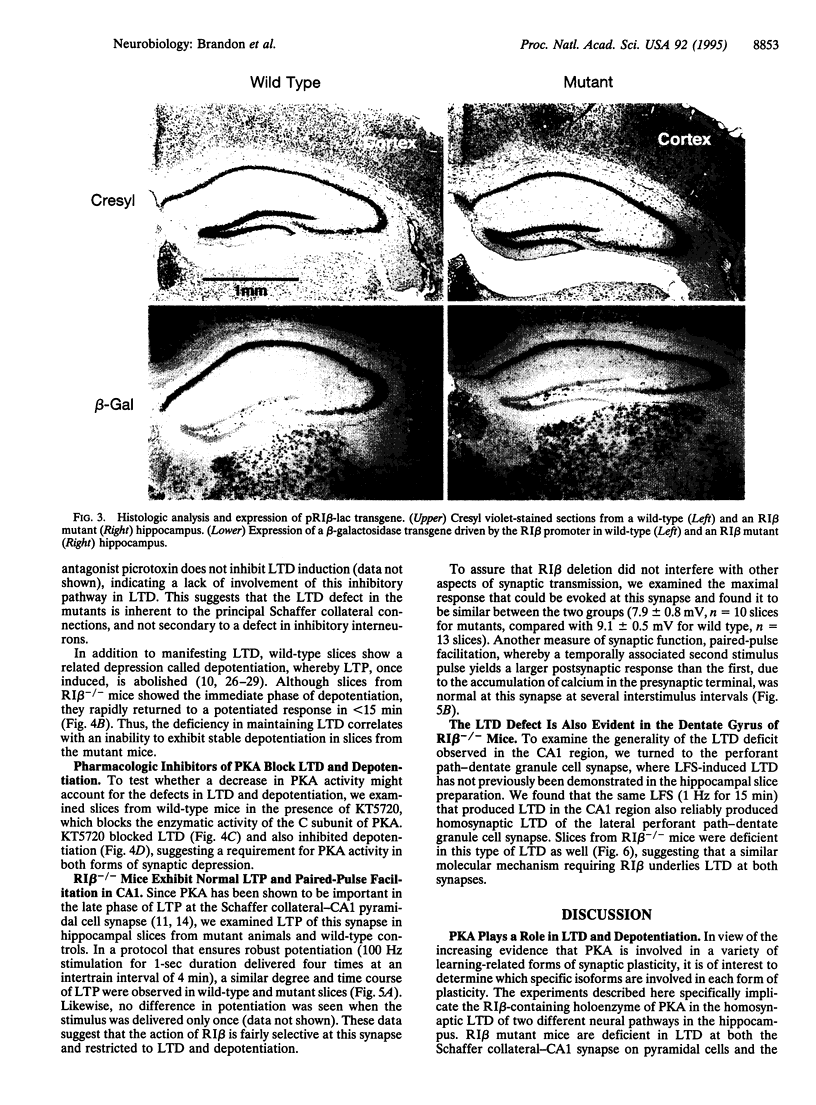
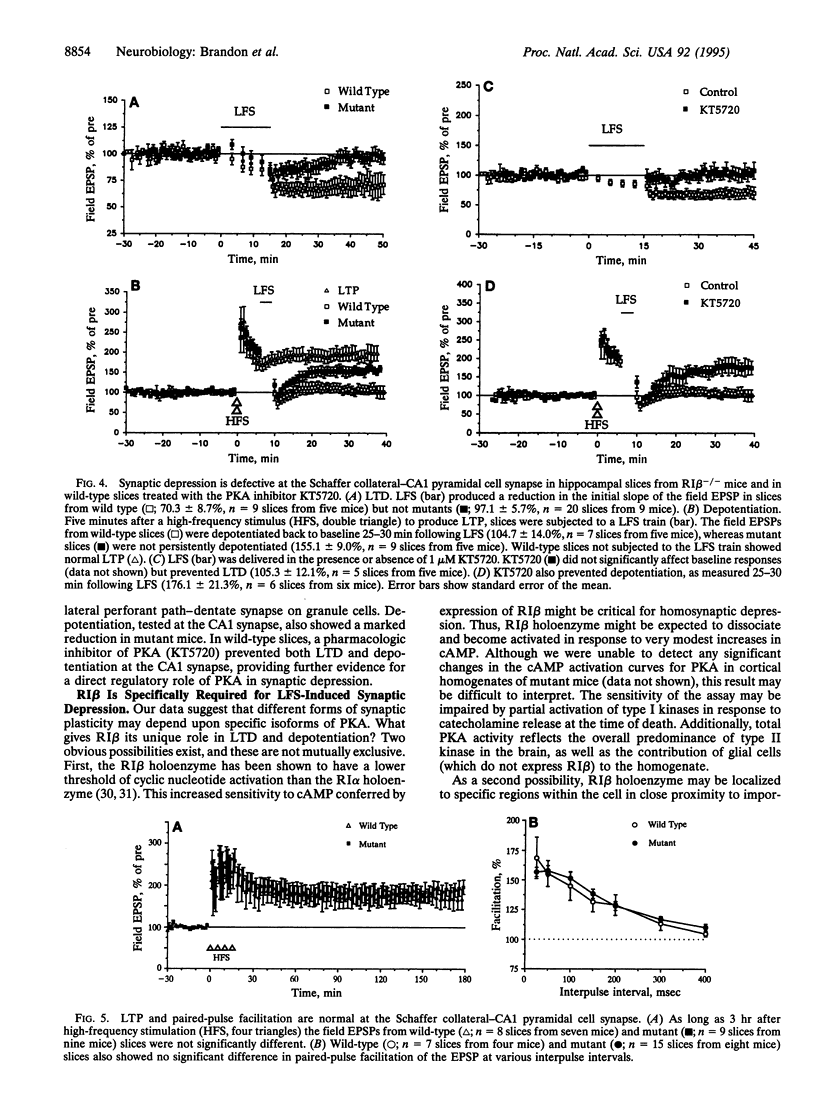
Abstract
The cAMP-dependent protein kinase (PKA) has been shown to play an important role in long-term potentiation (LTP) in the hippocampus, but little is known about the function of PKA in long-term depression (LTD). We have combined pharmacologic and genetic approaches to demonstrate that PKA activity is required for both homosynaptic LTD and depotentiation and that a specific neuronal isoform of type I regulatory subunit (RI beta) is essential. Mice carrying a null mutation in the gene encoding RI beta were established by use of gene targeting in embryonic stem cells. Hippocampal slices from mutant mice show a severe deficit in LTD and depotentiation at the Schaffer collateral-CA1 synapse. This defect is also evident at the lateral perforant path-dentate granule cell synapse in RI beta mutant mice. Despite a compensatory increase in the related RI alpha protein and a lack of detectable changes in total PKA activity, the hippocampal function in these mice is not rescued, suggesting a unique role for RI beta. Since the late phase of CA1 LTP also requires PKA but is normal in RI beta mutant mice, our data further suggest that different forms of synaptic plasticity are likely to employ different combinations of regulatory and catalytic subunits.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrionuevo G., Schottler F., Lynch G. The effects of repetitive low frequency stimulation on control and "potentiated" synaptic responses in the hippocampus. Life Sci. 1980 Dec 15;27(24):2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Bolshakov V. Y., Siegelbaum S. A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994 May 20;264(5162):1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- Brandon E. P., Gerhold K. A., Qi M., McKnight G. S., Idzerda R. L. Derivation of novel embryonic stem cell lines and targeting of cyclic AMP-dependent protein kinase genes. Recent Prog Horm Res. 1995;50:403–408. doi: 10.1016/b978-0-12-571150-0.50028-7. [DOI] [PubMed] [Google Scholar]
- Cadd G. G., Uhler M. D., McKnight G. S. Holoenzymes of cAMP-dependent protein kinase containing the neural form of type I regulatory subunit have an increased sensitivity to cyclic nucleotides. J Biol Chem. 1990 Nov 15;265(32):19502–19506. [PubMed] [Google Scholar]
- Cadd G., McKnight G. S. Distinct patterns of cAMP-dependent protein kinase gene expression in mouse brain. Neuron. 1989 Jul;3(1):71–79. doi: 10.1016/0896-6273(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Cadd G. G., McKnight G. S. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3703–3707. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C. H., Correll L. A., Cadd G. G., McKnight G. S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987 Sep 25;262(27):13111–13119. [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. A., Greenberg M. E. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994 Oct 7;79(1):5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Frey U., Huang Y. Y., Kandel E. R. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993 Jun 11;260(5114):1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Fujii S., Saito K., Miyakawa H., Ito K., Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991 Jul 26;555(1):112–122. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Hell J. W., Yokoyama C. T., Wong S. T., Warner C., Snutch T. P., Catterall W. A. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J Biol Chem. 1993 Sep 15;268(26):19451–19457. [PubMed] [Google Scholar]
- Huang Y. Y., Kandel E. R. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994 May-Jun;1(1):74–82. [PubMed] [Google Scholar]
- Huang Y. Y., Li X. C., Kandel E. R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994 Oct 7;79(1):69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A., Bear M. F. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994 May;14(5 Pt 2):3404–3412. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J., Xiao P., Lynch G. Reversal of LTP by theta frequency stimulation. Brain Res. 1993 Jan 8;600(1):97–102. doi: 10.1016/0006-8993(93)90406-d. [DOI] [PubMed] [Google Scholar]
- Linden D. J. Long-term synaptic depression in the mammalian brain. Neuron. 1994 Mar;12(3):457–472. doi: 10.1016/0896-6273(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka R. C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994 Aug 26;78(4):535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Massa J. S., Walker P. S., Moser D. R., Fellows R. E., Maurer R. A. Fetal development and neuronal/glial cell specificity of cAMP-dependent protein kinase subunit mRNAs in rat brain. Dev Neurosci. 1991;13(1):47–53. doi: 10.1159/000112140. [DOI] [PubMed] [Google Scholar]
- Matthies H., Reymann K. G. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport. 1993 Jun;4(6):712–714. doi: 10.1097/00001756-199306000-00028. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Clegg C. H., Uhler M. D., Chrivia J. C., Cadd G. G., Correll L. A., Otten A. D. Analysis of the cAMP-dependent protein kinase system using molecular genetic approaches. Recent Prog Horm Res. 1988;44:307–335. doi: 10.1016/b978-0-12-571144-9.50014-4. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Endo S., Shenolikar S., Malenka R. C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994 Jun 9;369(6480):486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Herron C. E., Malenka R. C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993 Aug 20;261(5124):1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Malenka R. C. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992 Nov;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Kandel E. R. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994 Jul-Aug;1(2):129–139. [PubMed] [Google Scholar]
- Ramírez-Solis R., Davis A. C., Bradley A. Gene targeting in embryonic stem cells. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Rogers K. V., Boring L. F., McKnight G. S., Clegg C. H. Promoter for the regulatory type I beta subunit of the 3',5'-cyclic adenosine monophosphate-dependent protein kinase directs transgene expression in the central nervous system. Mol Endocrinol. 1992 Oct;6(10):1756–1765. doi: 10.1210/mend.6.10.1448119. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Carr D. W., Bergeson S. E., Nilaver G., Scott J. D., Westbrook G. L. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994 Apr 28;368(6474):853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- Skålhegg B. S., Taskén K., Hansson V., Huitfeldt H. S., Jahnsen T., Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994 Jan 7;263(5143):84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- Solberg R., Taskén K., Wen W., Coghlan V. M., Meinkoth J. L., Scott J. D., Jahnsen T., Taylor S. S. Human regulatory subunit RI beta of cAMP-dependent protein kinases: expression, holoenzyme formation and microinjection into living cells. Exp Cell Res. 1994 Oct;214(2):595–605. doi: 10.1006/excr.1994.1297. [DOI] [PubMed] [Google Scholar]
- Staubli U., Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1-5 Hz stimulation. Brain Res. 1990 Apr 9;513(1):113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Yan C., Bentley J. K., Sonnenburg W. K., Beavo J. A. Differential expression of the 61 kDa and 63 kDa calmodulin-dependent phosphodiesterases in the mouse brain. J Neurosci. 1994 Mar;14(3 Pt 1):973–984. doi: 10.1523/JNEUROSCI.14-03-00973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. D., Connor J. A., Faber D. S. Weak excitation and simultaneous inhibition induce long-term depression in hippocampal CA1 neurons. J Neurophysiol. 1994 Apr;71(4):1586–1590. doi: 10.1152/jn.1994.71.4.1586. [DOI] [PubMed] [Google Scholar]





