Abstract
Diabetes mellitus is a major public health problem with tremendous medical and economic burdens. It is the seventh leading cause of death and the number one cause of end-stage renal disease, adult blindness, impotence, and nontraumatic lower-limb amputation in the United States. People with diabetes are 2 to 4 times more likely to suffer from stroke or from cardiovascular disease, and are twice as likely to die compared with age-matched individuals without diabetes. Diabetes cost the United States around $174 billion in 2007, $58 billion of which was related to disability, work loss, and early mortality. Although there is currently no known cure for diabetes, much progress has been made over the past 2 decades to improve the diagnosis and management of diabetes. Evidence has shown that applying aggressive interventions early can prevent or delay progression to microvascular complications that increase the mortality rate in diabetes. The authors review the guidelines for optimal evaluation of diabetes mellitus and discuss the current and emerging therapeutic options available in the United States.
Diabetes mellitus is a chronic and devastating disease, affecting 8% of the US population.1 Despite recent advances in diagnostic and therapeutic options, the incidence of diabetes continued to rise in 2007. According to the Centers for Disease Control and Prevention, approximately 24 million Americans are currently diagnosed with diabetes, an increase of 3 million over the past 2 years, and another 57 million are classified as having prediabetes.1 About one third of the people with diabetes remain undiagnosed.
Worldwide, the prevalence of diabetes is projected to reach 366 million people by the year 2030.2 Major increases in both macrovascular and microvascular complications can be projected on the basis of this growing prevalence. Indeed, recent studies have reported that life expectancy is reduced in patients with diabetes, with an estimated risk of death about twice that of the general population of similar age.1,3 Men and women who are diagnosed with diabetes before the age of 40 have an average life expectancy reduction of 12 and 19 years, respectively.4
The cost of diabetes to the US healthcare system is staggering; it was estimated to be around $174 billion in direct ($116 billion) and indirect ($58 billion) costs in 2007.1
Data from the United Kingdom Prospective Diabetes Study Group, the Diabetes Control and Complications Trial, and the Kumamoto study have clearly shown that early and aggressive glycemic interventions can reduce the risk of microvascular complications—retinopathy, nephropathy, and neuropathy—of diabetes.5–7 Despite this knowledge, studies continue to show that most Americans with diabetes are not achieving the recommended treatment goals. Recent data from the National Health and Nutrition Examination Survey reported that only one third of diabetic patients are at goal with regard to glycemic and blood pressure control, and only half are meeting their cholesterol goals.8 It is hopeful that the availability of published guidelines and algorithms will aid physicians to bring more patients to the desired glycemic, blood pressure, and lipid goals.
Diagnosis
The American Diabetes Association (ADA) currently recognizes 4 classifications of diabetes: type 1 diabetes, type 2 diabetes, other specific types of diabetes due to other causes, and gestational diabetes. Diagnostic criteria for diabetes mellitus are listed in Table 1. Fasting plasma glucose (FPG) continues to be the diagnostic test of choice for diabetes.9 Recent studies, however, have indicated that hemoglobin (Hb) A1C may be a more stable,10 convenient (ie, does not require fasting), and noninferior test compared with FPG.11 An HbA1C screening review panel has recently evaluated the current literature on this topic and concluded that HbA1C should be “incorporated into criteria for screening and diagnosing diabetes.”12 As assays for HbA1C testing become more reliable and less expensive, it is possible that HbA1C will be accepted as an alternative screening and diagnostic test in the future.
Table 1.
Diagnostic Criteria for Diabetes Mellitus
| Classification | Fasting plasma glucose, mg/dL | 2-h oral glucose tolerance test, m | Random plasma g/dL* glucose, mg/dL |
|---|---|---|---|
| Normal | <100 | <140 | |
| Prediabetes | |||
| Impaired fasting glucose | ≥100–125 | ||
| Impaired glucose tolerance | ≥140–199 | ||
| Diabetes | ≥126† | ≥200† | >200‡ |
75-g glucose dose.
Abnormal test must be repeated on subsequent day.
Must occur with hyperglycemic symptoms: polydipsia, polyphagia, polyuria, blurred vision.
Source: Executive summary: standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S5-S54.
KEY POINTS
-
▴
Approximately 24 million Americans are currently diagnosed with diabetes, an increase of 3 million over the past 2 years; 57 million have prediabetes.
-
▴
The cost of diabetes to the US healthcare system in 2007 was estimated to be $174 billion, which included direct ($116 billion) and indirect ($58 billion) costs.
-
▴
Early, aggressive glycemic interventions can reduce progression to retinopathy, nephropathy, and neuropathy; nevertheless, most Americans with diabetes are not reaching treatment goals.
-
▴
Patients with diabetes should receive aggressive blood pressure lowering to reduce cardiovascular events.
-
▴
Evidence has shown that metformin, orlistat, acarbose, and troglitazone successfully reduce the progression of impaired glucose tolerance and impaired fasting glucose to diabetes, although to a lesser degree than diet and exercise.
-
▴
Lifestyle modifications remain the best treatment for type 2 diabetes, but most patients will not be able to follow a rigid diet and exercise regimen and will eventually require antihyperglycemic medications.
Screening
Routine screening for type 1 diabetes is currently not recommended by any organization. Due to the low incidence and the lack of known preventive measures for type 1 diabetes, screening for this disease in healthy people is not cost-effective. In contrast, many experts believe that screening for type 2 diabetes is indicated because of the long—7 years—presymptomatic phase often seen in this population.12 Although microvascular complications may take years to develop, macrovascular morbidity and mortality are significantly elevated in individuals before the diagnosis of diabetes is made.13 The ADA currently recommends that screening be considered in all individuals ≥45 years of age. If normal, the screening test should be repeated every 3 years. For individuals with 1 or more risk factors for type 2 diabetes (Table 2), screening should be done at any age.9
Table 2.
Risk Factors for Type 2 Diabetes
| First-degree relative with diabetes |
| High-density lipoprotein cholesterol <35 mg/dL and/or triglyceride ≥250 mg/dL |
| History of gestational diabetes or delivery of a baby weighing >9 lb |
| History of vascular disease |
| Hypertension (≥140/90 mm Hg in adults) |
| IGT or IFG on previous testing |
| Member of a high-risk ethnic group (ie, African American, Native American, Hispanic, Asian American, Pacific Islander) |
| Obesity (body mass index >25 kg/m2) |
| Physical inactivity |
| Women with polycystic ovarian syndrome |
IGT indicates impaired glucose tolerance; IFG, impaired fasting glucose.Source: Executive summary: standards of medical care in diabetes—2008.Diabetes Care. 2008;31(suppl 1):S5-S54.
FPG is the screening test of choice for the ADA and the American Heart Association,14 whereas the 2-hour oral glucose tolerance is the preferred test for the American Association of Clinical Endocrinologists (AACE).15 In contrast, the US Preventive Services Task Force (USPSTF) has recently updated their diabetes screening recommendations and concluded that the evidence is still insufficient to recommend for or against routine screening of asymptomatic adults for prediabetes or type 2 diabetes. The only population where screening is indicated, according to the USPSTF, is people whose blood pressure is >135/80 mm Hg. Convincing evidence has demonstrated that aggressive lowering of blood pressure in patients with diabetes reduces the incidence of cardiovascular events and mortality.16
Prevention
Prevention of progression to diabetes is essential, considering the serious medical and economic consequences of the disease and the lack of cure. Several clinical trials have demonstrated that lifestyle modifications alone or in conjunction with pharmacologic therapies can prevent or delay the development of type 2 diabetes. Individuals who lose 7% of their body weight and maintain 150 minutes of exercise weekly can reduce the rate of progression from impaired glucose tolerance (IGT) to diabetes by 58%.17 Studies have demonstrated that the use of several pharmacologic agents—metformin,17 orlistat,18 acarbose,19 and troglitazone20—can successfully reduce the progression of IGT and impaired fasting glucose (IFG)9 to diabetes, although to a lesser degree than diet and exercise.
The ADA endorses lifestyle modifications as the initial treatment for prediabetes. If pharmacologic intervention is required, metformin is the only drug that is recommended in conjunction with diet and exercise in high-risk patients (those with both IGT and IFG).9
Glycemic Control
Glycemic goals as recommended by the ADA and AACE are summarized in Table 3. At an HbA1C level of <7%, long-term microvascular and macrovascular risks from hyperglycemia are minimized. Recognizing that there is no threshold to the benefits of lowering HbA1C, experts now stress that HbA1C be reduced to the lowest possible level at which frequent hypoglycemic episodes do not occur. Every 1% reduction in HbA1C is associated with a 40% reduction in microvascular complications.9
Table 3.
Targets for Glycemic Control
| Targets | ADA | AACE |
|---|---|---|
| Hemoglobin A1C | <7% | <6.5% |
| Preprandial glucose | 70–130 mg/dL | <110 mg/dL |
| Postprandial glucose | <180 mg/dL (peak) | <140 mg/dL at2 h from start of meal |
ADA indicates American Diabetes Association; AACE, American Association of Clinical Endocrinologists.
Sources: Executive summary: standards of medical care in diabetes—2008.Diabetes Care. 2008;31(suppl 1):S5-S54; American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(suppl 1):1–68.
Methods to achieve glycemic goals are lifestyle modifications and pharmacologic treatments. Although lifestyle modifications (ie, diet, exercise, and/or behavioral interventions) remain the best and first-line treatment, most patients will not be able to follow a rigid diet and exercise regimen and will eventually require the use of antihyperglycemic medications.
Treatment Options
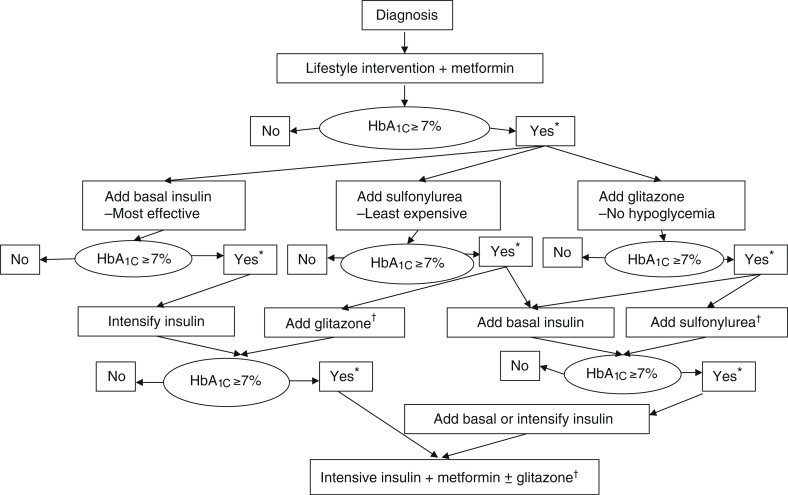
The ADA and the European Association for the Study of Diabetes have copublished an algorithm to guide practitioners in the use of oral and/or parenteral antihyperglycemic agents available for the treatment of type 2 diabetes (Figure). A “road map to achieve glycemic goals” has also been developed by the AACE and the American College of Endocrinology and was revised in April 2008.21
Figure. Algorithm for the Metabolic Management of Type 2 Diabetes.
Reinforce lifestyle intervention at every visit.
*Check HbA1c every 3 months until <7% and then at least every 6 months.
†Although 3 oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
Hb indicates hemoglobin.
Reprinted with permission from Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2006;29:1963–1972.
Oral Antihyperglycemic Agents
Six major classes of oral drugs are currently available for management of type 2 diabetes in the United States (Table 4). These medications can be divided into 4 broad categories based on their mechanisms of action—secretagogues, sensitizers, carbohydrate absorption inhibitors, and dipeptidyl peptidase-4 (DPP-4) inhibitors. These drugs are indicated in patients who do not respond to 3 months of lifestyle modifications.
Table 4.
Oral Antihyperglycemic Agents
| Drug | Mechanism of action | Hemoglobin A1C reduction, % | Dose range | Adverse events | Comments | Cost for 30-day supply (range)* | |
|---|---|---|---|---|---|---|---|
| Alpha-glucosidase inhibitors | |||||||
| Acarbose(Precose) | Delay complex carbohydrate absorption | 0.5–0.8 | 25–100 mg tid w/meals | Flatulence, diarrhea, abdominal pain | Titrate slowly to minimize GI effects | $73.79-$80.99 | |
| Miglitol(Glyset) | 25–100 mg tid w/meals | $79.99-$99.99 | |||||
| Biguanides | |||||||
| Metformin (Glucophage) | Decrease hepatic glucose output; increase peripheral glucose uptake | 1–2 | IR: 1000–2550 mg/d divided bid or tidER: 500–2000 mg/d | Nausea, vomiting, diarrhea, flatulence | Take w/meals; avoid use in patients w/renal or hepatic impairment or CHF due to increased risk of lactic acidosis |
IR: $24.99-$77.99 ER: $6.33-$25.32 |
|
| Dipeptidyl peptidase-4 inhibitors | |||||||
| Sitagliptin (Januvia) | Slow inactivation of incretin hormones | 0.5–0.8 | 25–100 mg/d | Not clinically significant | Renal dose: 50 mg/d for CrCl ≥30 to <50 mL/min; 25 mg/d for CrCl <30 mL/min | $45.27-$181.09 | |
| Nonsulfonylurea secretagogues | |||||||
| Nateglinide (Starlix) | Stimulate insulin secretion from the pancreas | 1–1.5 | 60–120 mg tid | Hypoglycemia, weight gain | Take w/meals due to rapid onset | $154.11-$164.85 | |
| Repaglinide (Prandin) | 0.5–4 mg tid to qid | $164.82-$396.08 | |||||
| Sulfonylureas | |||||||
| First-generation | |||||||
| Chlorpropamide (Diabinese) | Stimulate insulin secretion from the pancreas | 1–2 | 100–750 mg/d | Hypoglycemia, weight gain | Use of these agents has declined due to side effects and unpredictable results | $8.62-$31.49 | |
| Tolazamide (Tolinase) | 100–1000 mg/d given as a single dose or divided bid if dose is >500 mg/d | $12-$69.98 | |||||
| Tolbutamide (Orinase) | 250–3000 mg/d in divided doses | $5-$59.97 | |||||
| Second-generation | |||||||
| Glimepiride (Amaryl) | 1–8 mg/d | Hypoglycemia, weight gain | $12.99-$29.98 | ||||
| Glipizide (Glucotrol) | IR: 2.5–40 mg/d as a single dose or divided bid ER: 2.5–20 mg/d | IR: $3-$25.99 ER: $18.99$39.98 | |||||
| Glyburide (Micronase, Diabeta, Glynase) | 1.25–20 mg/d as a single dose or divided bid (Glynase: 0.7512 mg/d as a single dose or divided bid if dose >6 mg/d) | $12.99-$47.96 (Glynase: $4.33$109.42) |
|||||
| Thiazolidinediones | |||||||
| Pioglitazone (Actos) | Increase peripheral tissue insulin sensitivity | 0.5–1.4 | 15–45 mg/d | Edema, weight gain | Black box warning: these agents can cause or exacerbate CHF; contraindicated in patients with NYHA class III or IV heart failure | $134.07-$195.99 | |
| Rosiglitazone (Avandia) | 4–8 mg/d as a single dose or divided bid | $120.10-$223.49 | |||||
Cost calculated from generic, if available, and lowest bottle size available. GI indicates gastrointestinal; IR, immediate release; ER, extended release; CHF, congestive heart failure; CrCl, creatinine clearance; NYHA, New York Heart Association.
Sources: Lacy CF, Armstrong LL, Goldman MP, Lance LL, eds. Drug Information Handbook. 17th ed. Hudson, OH: Lexi-Comp; 2008. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2008. Cost: www.drugstore.com.
Biguanides (sensitizers). Metformin is the only biguanide available in the United States. Its main action is to decrease hepatic glucose production and enhance insulin sensitivity. As monotherapy, metformin is very effective in decreasing plasma glucose level, reducing HbA1C by approximately 1% to 2% compared with diet or placebo.22 Aside from its glycemic effect, metformin also lowers triglycerides and low-density lipoprotein cholesterol.23 By itself, it does not cause weight gain nor hypoglycemia.24 Gastrointestinal (GI) discomfort, which usually resolves over time with continuation of the medication or dose reduction, is the most common side effect of metformin. To minimize the risk of lactic acidosis, metformin should not be given to patients with renal dysfunction (serum creatinine ≥1.5 mg/dL in men, ≥1.4 mg/dL in women). Because of its cost and benign side-effect profile, metformin is considered a first-line drug in the treatment of diabetes. It can be used concurrently with lifestyle modifications at the time of diagnosis or even for diabetes prophylaxis.9,22 To date, it remains 1 of only 2 oral antihyperglycemic medications that have been shown to reduce macrovascular complications.23
Sulfonylureas (secretagogues). Sulfonylureas are the oldest class of antihyperglycemic agents, first introduced to the US market in 1946. Their primary action is to promote insulin secretion from the pancreas. Similar to metformin, sulfonylureas are effective at decreasing plasma glucose level, reducing HbA1C by approximately 1% to 2%.22 Hypoglycemia and weight gain are the 2 major side effects of this class. Because they are the least expensive of all antihyperglycemic medications, sulfonylureas—which are second-line agents after lifestyle modifications and metformin—are the drugs of choice for patients with financial considerations.
Nonsulfonylurea secretagogues (secretagogues). Repaglinide (a meglitinide) and nateglinide (a phenylalanine) function in the same manner as sulfonylureas, by increasing insulin secretion from the pancreas, and they reduce HbA1C at the same level (−1.5%).22 They are structurally different from the sulfonylureas and have a faster onset of action; thus, they should be taken at mealtime to reduce the risk of hypoglycemia.23 Because these agents are metabolized exclusively by the liver, they can be used in patients with renal insufficiency.25
In head-to-head trials against sulfonylureas, meglitinides and phenylalanines have not been found to be superior in terms of improving postprandial control.26,27 At a cost of 3 to 4 times more than sulfonylureas,23 it is unlikely that these drugs will be widely used.
Alpha-glucosidase inhibitors (carbohydrate absorption inhibitors). Alpha-glucosidase inhibitors delay carbohydrate absorption in the small intestine, thereby lowering postprandial blood glucose without causing hypoglycemia.28 They do not cause weight gain but are associated with significant GI side effects (ie, diarrhea, abdominal pain, flatulence)24; thus, long-term compliance is a significant issue with these drugs. Unlike the biguanides, alpha-glucosidase inhibitors are not considered first-line therapy because of their minimal glycemic control (HbA1C −0.5%)22 and higher costs.23
Thiazolidinediones (sensitizers). Thiazolidinediones (TZDs; rosiglitazone and pioglitazone) reduce insulin resistance by binding to peroxisome proliferator-activated receptors-γ (PPAR-γ) in the peripheral muscles, liver, and adipose tissues. This, in turn, alters the transcription of genes that positively regulate glucose uptake. As monotherapy, they lower HbA1C by 0.5% to 1.4%.22 Their onset of action is slower than that of the sulfonylureas, but they do not cause hypoglycemia.28 In addition to lowering blood glucose, these agents exert beneficial effects on lipid metabolism, as well as improve surrogate markers for pancreatic beta-cell regeneration and vascular inflammation.15,28
A recent meta-analysis has raised the possibility that rosiglitazone may increase the risk of myocardial infarction (not seen with pioglitazone).29 Because of the methodological limitations of this analysis, numerous studies, including one from the US Food and Drug Administration (FDA),30 were subsequently initiated to reevaluate these findings. Results from these studies were mixed and inconclusive,30–32 prompting the FDA to recommend caution in the use of TZDs and to mandate the inclusion of a black box warning in the package inserts of TZDs relaying the 2-fold increased risk of fluid retention and heart failure with this class.33 The future of TZDs remains strong. But at a cost of 2 to 3 times more than the cost of metformin, TZDs should only be considered after failure of lifestyle modifications and metformin.9
DPP-4 inhibitors. These medications represent the newest class of oral antihyperglycemic medications, introduced to the US market in 2006. This class inhibits the breakdown of endogenous glucagon-like peptide 1 (GLP-1),15 a compound that can control or even reverse some of the metabolic derangements seen in type 2 diabetes. Sitagliptin, the only FDA-approved drug in this class to date, has been shown to lower HbA1C by 0.5% to 0.8%.22 One advantage of this DPP-4 is that it only works when blood glucose is elevated; thus, the risk for hypoglycemia is minimal. Other advantages include once-daily administration, oral availability, and, more important, weight neutrality.34 Sitagliptin is effective and safe in patients with renal insufficiency, including those on hemodialysis; however, dose adjustments will be necessary based on the severity of renal impairment.35 Although the appropriate role of DPP-4 inhibitors is still unclear, sitagliptin's efficacy and safety profile suggests that this class is a reasonable treatment option for type 2 diabetes.
Parenteral Antihyperglycemic Agents
The 3 parenteral antihyperglycemic classes currently available in the United States are insulins (Table 5), and amylin analogs and incretin mimetics (Table 6).
Table 5.
Insulin Preparations
| Drug | Onset | Peak | Duration | Comments | Cost (10-mL vial) |
|---|---|---|---|---|---|
| Rapid-acting | |||||
| Insulin Aspart (NovoLog) | 10–20 min | 1–3 h | 3–5 h | Administer within 15 min before or immediately after meals | $94.25 |
| Insulin Glulisine (Apidra) | 25 min | 45–48 min | 4–5 h | $90.68 | |
| Insulin Lispro (Humalog) | 15–30 min | 0.5–2.5 h | 3–6.5 h | $89.99 | |
| Short-acting | |||||
| Insulin Regular (Novolin R, Humulin R) | 30–60 min | 1–5 h | 6–10 h | Administer 30 min before meals | $45.99-$49.99 |
| Intermediate-acting | |||||
| Insulin NPH (Novolin N, Humulin N) | 1–2 h | 6–14 h | 16–24+ h | Cloudy appearance | $46.99-$49.99 |
| Long-acting | |||||
| Insulin Detemir (Levemir) | 1.1–2 h | 3.2–9.3 h | 5.7–24 h (dose-dependent) | Don't mix with other insulins | $95.30 |
| Insulin Glargine (Lantus) | 1.1 h | None | 24 h | $97.73 | |
| Premixed | |||||
| 70% Insulin Aspart Protamine/30% Insulin Aspart (NovoLog Mix 70/30) |
10–20 min | 1–4 h | 24 h | Cloudy appearance; administer within 15 min before meals | $95.03 |
| 75% Insulin Lispro Protamine/25% Insulin Lispro Protamine (Humalog Mix 75/25) |
15–30 min | 2 h | 22 h | $96.89 | |
| 50% Insulin Lispro Protamine/50% Insulin Lispro Protamine (Humalog Mix 50/50) |
Not available | ||||
| 70% Insulin NPH/30% Insulin Regular (Humulin 70/30, Novolin 70/30) |
30 min | 1.5–12 h | 24 h | Cloudy appearance; administer within 30 min before meals | $46.26-$49.99 |
| 50% Insulin NPH/50% Insulin Regular (Humulin 50/50) |
30–60 min | 1.5–4.5 h | 7.5–24 h | $49.99 | |
Sources: Lacy CF, Armstrong LL, Goldman MP, Lance LL, eds. Drug Information Handbook. 17th ed. Hudson, OH: Lexi-Comp; 2008. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2008. Cost: www.drugstore.com.
Table 6.
Noninsulin Injectable Antihyperglycemic Agents
| Drug | Mechanism of action | Hemoglobin A1C reduction, % | Dose range | Adverse events | Comments | Cost for 30-day supply (range)* |
|---|---|---|---|---|---|---|
| Amylin analog | ||||||
| Pramlintide (Symlin) | Acts in conjunction with insulin to prolong gastric emptying, reduce postprandial glucose secretion, and promote appetite suppression | 0.5–1 |
Type 1 DM: 15–60 μg SC immediately before each meal Type 2 DM: 60–120 μg SC immediately before each meal |
Nausea, vomiting | Black box warning: coadministration w/insulin may induce severe hypoglycemia | $127.58/5-mL vial |
| Incretin mimetic | ||||||
| Exenatide (Byetta) | Stimulates insulin secretion; slows gastric emptying, suppresses glucagon release, induces satiety | 0.5–1 | 5–10 μg SC bid within 60 min before morning and evening meals | Nausea, vomiting, diarrhea | Acute pancreatitis has been reported during postmarketing experience | $213.03(5-μg cartridge)-$229.27(10-μg cartridge) |
Cost calculated from generic, if available, and lowest bottle size available.
DM indicates diabetes mellitus; SC, subcutaneous.
Sources: Lacy CF, Armstrong LL, Goldman MP, Lance LL, eds. Drug Information Handbook. 17th ed. Hudson, OH: Lexi-Comp; 2008. McEvoy GK, ed. AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists; 2008. Cost: www.drugstore.com.
Insulins. Insulin is the best agent for reducing blood glucose concentrations. It is mandatory in patients with type 1 diabetes but is a second-line therapy for patients with type 2 diabetes who have failed lifestyle modifications with or without oral antihyperglycemic therapy.22 Patients receiving insulin therapy are more likely to develop hypoglycemia and weight gain than patients taking oral medications.36 However, the recent advent of rapid-acting as well as long-acting insulins has significantly reduced these risks.24 Insulin detemir, the newest long-acting insulin analog in the United States, has consistently shown in clinical trials to be very effective in reducing blood glucose levels with less weight gain than other basal insulins.37
Incretin mimetics. Incretins are hormones that are secreted by cells in the small intestine during an oral nutrient load. GLP-1 is one incretin that has antihyperglycemic effects. In the presence of hyperglycemia, GLP-1 causes the release of insulin from the pancreas, shuts down glucagon secretion, slows down gastric emptying, and acts on the hypothalamus to increase satiety.38
Exenatide is the first synthetic GLP-1 to be introduced in the United States. Possessing many of the properties of endogenous GLP-1, exenatide can lower HbA1C by 0.5% to 1%.22 Aside from its antiglycemic effect, exenatide can also induce weight loss. Patients who were treated with exenatide for 30 weeks had an average weight loss of 4.4 kg,39 which was maintained when the study was extended for another 22 weeks using a longer-acting version of exenatide.40 Nausea is the most common side effect of exenatide, which usually subsides with continuation of this medication.38
The FDA has recently alerted physicians to 6 cases of necrotizing pancreatitis thought to be related to exenatide use41; this is in addition to the 30 cases of non-necrotizing pancreatitis reported by the FDA in October 2007. Although the exact relationship between pancreatitis and the drug is still unknown, it is recommended that patients receiving exenatide and presenting with abdominal pain be evaluated promptly.
Amylin analogs. Amylin is a neuropeptide that is cosecreted with insulin by pancreatic beta-cells in response to food intake. Amylin complements insulin's action by suppressing glucagon release, slowing gastric emptying, and inducing satiety.15,42
Pramlintide is the only amylin analog currently available in the United States. Approved as an adjunctive treatment for patients with type 1 or type 2 diabetes who are not optimally controlled with short-acting insulin (mealtime insulin), pramlintide can lower HbA1C by 0.5% to 1%.22 To lower the risk of hypoglycemia, the dose of premeal insulin should be reduced by 50% when pramlintide is initiated. In clinical trials, the use of pramlintide has been associated with modest weight loss.42 The most common side effect is nausea, which usually subsides with continuation of treatment. The availability of a pen formulation has made pramlintide more user-friendly; however, the future of this drug class is still uncertain because of its cost, need for multiple injections, and relatively modest therapeutic profile.
In the Pipeline
A multitude of new compounds with very distinct mechanisms of action are being developed in the United States to be added to the clinician's armamentarium for the treatment of diabetes. A selected number of these emerging compounds are outlined in Table 7.
Table 7.
In the Pipeline: Selected Emerging Antihyperglycemic Compounds
| Drug category | Mechanism of action | Comments |
|---|---|---|
| Glucokinase activators | Activate key enzymes to increase hepatic glucose metabolism | Several drugs are currently in phase 2 clinical trials |
| Sodium-glucose cotransporter 2 inhibitors | Inhibit reabsorption of glucose at the proximal tubule of the kidney, thereby decreasing systemic hyperglycemia | Low potential for hypoglycemia |
| 11β-hydroxysteroid dehydrogenase 1 (11β-HSD 1) inhibitors | Inhibit an enzyme responsible for activating cortisone to cortisol, thus minimizing antiglycemic effects of cortisol | Low potential for hypoglycemia, may have beneficial effects on metabolic syndrome |
| Glucagon receptor antagonists | Block glucagon from binding to hepatic receptors, thereby decreasing gluconeogenesis | Low potential for hypoglycemia |
| Cannabinoid-1 (CB-1) receptor antagonists | Block CB-1 receptors systemically | Marketed as an obesity drug, may cause psychiatric symptoms |
Source: Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes mellitus: part II: incretin-based therapy and beyond. Circulation. 2008;117:574–584.
Conclusions
Lifestyle modification is recognized as the mainstay of therapy for diabetes. Metformin is considered the first-line oral antihyperglycemic drug. If immediate lowering of blood glucose level is required, insulin should be used. Exenatide and DPP-4 inhibitors are 2 new classes with favorable weight profiles. Optimal management should focus not only on glycemic control but also on comorbid conditions that often accompany this potentially life-threatening condition, including hypertension, dyslipidemia, and obesity.
Stakeholder Perspective
Evidence-Based Diabetes Management
PAYERS: At a time when the future of the US healthcare system is the subject of a great deal of debate, this excellent review of the current evidence guiding the screening, diagnosis, and treatment of diabetes is indeed timely.
Diabetes is one of the key disease states associated with controllable health impact in terms of future patient disability and system cost. Diabetes illustrates how over time we have been able to identify an etiology and understand the disease process and how it can be slowed, thereby mitigating its long-term effects, albeit without a “cure.”
By achieving “adequate control” of glucose, long-term microvascular complications may be delayed or prevented, resulting in a reduction in retinal and renal pathology, cardiac disease, and stroke. These negative end points are devastating in terms of patient disability and catastrophic in term of total downstream costs. Yet despite a robust treatment armamentarium, we fail to achieve adequate control in too many patients.
Through education and promotion of healthy lifestyle (ie, appropriate diet and exercise) we must slow the epidemic of obesity that will swell the future ranks of diabetic patients. We must be vigilant in screening and monitoring at-risk populations to ensure that secondary prevention and early treatment are initiated in a timely manner. Patients must understand their disease and the steps they can take to participate in their care to reduce the likelihood of complications. We must strive to achieve adequate glucose control and blood pressure while monitoring for early signs of renal, retinal, and neuropathic impact.
Because the disease onset and the eventual development of complications may be separated by years if not by decades, physicians and payers involved in early treatment may not benefit from the reductions in complications and may not be responsible for the consequences of poor control. Therefore, through monitoring “process indicators,” all payers and providers must be held responsible (using quality control and health plan monitoring measures) for achieving the best possible results to reduce long-term complications. We must allow neither the fractured system nor the misaligned incentives to serve as barriers to achieving the best short- and long-term outcomes for every diabetic patient.
Thomas McCarter, MD, FACP
Chief Clinical Officer, Executive Health Resources, West Chester, PA
Biography

Quang Nguyen
Disclosure Statement
Dr Felicetta is on the Speaker's Bureau of Merck and sanofi-aventis. Drs Nguyen and Nguyen have no potential or apparent conflict of interest to report.
Contributor Information
Quang Nguyen, Assistant Professor, Department of Endocrinology, Diabetes, and Metabolism, University of Nevada School of Medicine, Reno.
Loida Nguyen, Clinical Pharmacy Specialist, VA Sierra Nevada Health Care System, Reno.
James Felicetta, Chairman, Department of Medicine, Carl T. Hayden VA Medical Center, and Professor of Clinical Medicine, University of Arizona, Tucson.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf Accessed August 16, 2008. [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27: 1047–1053 [DOI] [PubMed] [Google Scholar]
- 3.Franco OH, Steyerberg EW, Mackenbach J, et al. Association of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007; 167: 1145–1151 [DOI] [PubMed] [Google Scholar]
- 4.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003; 920: 1884–1890 [DOI] [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352: 837–853 [PubMed] [Google Scholar]
- 6.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 7.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28: 103–117 [DOI] [PubMed] [Google Scholar]
- 8.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004; 291: 335–342 [DOI] [PubMed] [Google Scholar]
- 9.Executive summary: standards of medical care in diabetes—2008. Diabetes Care. 2008; 31 (suppl 1): S5–S54 [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Crainiceanu CM, Brancati FL, et al. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007; 167: 1545–1551 [DOI] [PubMed] [Google Scholar]
- 11.Bennett CM, Guo M, Dharmage SC. HbA1c as a screening tool for detection of type 2 diabetes: Diabet Med. 2007; 24: 333–343 [DOI] [PubMed] [Google Scholar]
- 12.Saudek CD, Herman WH, Sacks DB, et al. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008; 93: 2447–2453 [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Stampfer MJ, Haffner SM, et al. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002; 25: 1129–1134 [DOI] [PubMed] [Google Scholar]
- 14.Pearson TA, Blair SN, Daniels SR. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. Circulation. 2002; 106: 388. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007; 13 (suppl 1): 1–68 [DOI] [PubMed] [Google Scholar]
- 16.US Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008; 148: 846–855 [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346: 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L.XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004; 27: 155–161 [DOI] [PubMed] [Google Scholar]
- 19.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for the prevention of type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: facts and interpretations concerning the critical analysis of the STOP-NIDDM trial data. Diabetologia. 2004; 47: 969–975 [DOI] [PubMed] [Google Scholar]
- 20.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002; 51: 2796–2803 [DOI] [PubMed] [Google Scholar]
- 21.Jellingere PS, Davidson JA, Blonde L, et al. for the ACE/ACCE Diabetes Road Map Task Force. Road Map to Achieve Glycemic Control: Naïve to Therapy (Type 2). Revision April 2008. http://www.aace.com/meetings/consensus/odimplementation/roadmap.pdf Accessed September 20, 2008.
- 22.Nathan D, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. Update regarding the thiazolidinediones. Diabetologia. 2008; 51: 8–11 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999; 131: 281–303 [DOI] [PubMed] [Google Scholar]
- 24.Fonseca V. Drug Therapy. In: Cefalu WT, Gerich JE, LeRoith D, eds. The CADRE Handbook of Diabetes Management. 1st ed.New York, NY: Medical Information Press; 2004 [Google Scholar]
- 25.Sheehan MT. Current therapeutic options in type 2 diabetes mellitus: a practical approach. Clin Med Res. 2003; 1: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll MF, Izard A, Riboni K, et al. Control of postprandial hyperglycemia: optimal use of short-acting insulin secretagogues. Diabetes Care. 2002; 25: 2147–2152 [DOI] [PubMed] [Google Scholar]
- 27.Cozma LS, Luzio SD, Dunseath GJ, et al. Comparison of the effects of three insulinotropic drugs on plasma insulin levels after a standard meal. Diabetes Care. 2002; 25: 1271–1276 [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006; 29: 1963–1972 [DOI] [PubMed] [Google Scholar]
- 29.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356: 2457–2471 [DOI] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. FDA briefing document. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4308b1-02-fda-backgrounder.pdf Accessed August 20, 2008.
- 31.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007; 298: 1189–1195 [DOI] [PubMed] [Google Scholar]
- 32.Home PD, Jones NP, Pocock SJ, et al. Rosiglitazone RECORD study: glucose control outcomes at 18 months. Diabet Med. 2007; 24: 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: Lancet. 2007; 370: 1129–1136 [DOI] [PubMed] [Google Scholar]
- 34.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: JAMA. 2007; 298: 194–206 [DOI] [PubMed] [Google Scholar]
- 35.Bergman AJ, Cote J, Yi B, et al. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidylpeptidase-4 inhibitor. Diabetes Care. 2007; 30: 1862–1864 [DOI] [PubMed] [Google Scholar]
- 36.Barnett AH, Bowen JD, Burden AC, et al. Multicentre study to assess quality of life and glycaemic control of type 2 diabetic patients treated with insulin compared with oral hypoglycemic agents. Pract Diabetes lnt. 1996; 13: 179–183 [Google Scholar]
- 37.Fritsche A, Häring H. At last, a weight neutral insulin? lnt J Obes Metab Disord. 2004; 28 (suppl 2): S41–S46 [DOI] [PubMed] [Google Scholar]
- 38.Exenatide (Byetta) for type 2 diabetes. Med Lett Drug Ther. 2005; 47: 45–46 [PubMed] [Google Scholar]
- 39.DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005; 28: 1092–1100 [DOI] [PubMed] [Google Scholar]
- 40.Buse JB. Late-breaking clinical trial: exenatide once weekly elicits sustained glycemic control and weight loss over 52 weeks. Presented at the American Diabetes Association 68th Scientific Sessions June 9, 2008San Francisco, CA [Google Scholar]
- 41.US Food and Drug Administration. Information for health care professionals: exenatide. http://www.fda.gov/cder/drug/InfoSheets/HCP/exenatide2008HCP.htm Accessed August 20, 2008.
- 42.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002; 25: 724–730 [DOI] [PubMed] [Google Scholar]