Abstract
Auxin is associated with the regulation of virtually every aspect of plant growth and development. Many previous genetic and biochemical studies revealed that, among the proposed routes for the production of auxin, the so-called indole-3-pyruvic acid (IPA) pathway is the main source for indole-3-acetic acid (IAA) in plants. The IPA pathway involves the action of 2 classes of enzymes, tryptophan-pyruvate aminotransferases (TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1(TAA1)/TRYPTOPHAN AMINOTRANSFERASE RELATED (TAR)) and flavin monooxygenases (YUCCA). Both enzyme classes appear to be encoded by small gene families in Arabidopsis consisting of 5 and 11 members, respectively. We recently showed that it is possible to induce transcript accumulation of 2 YUCCA genes, YUC8 and YUC9, by methyl jasmonate treatment. Both gene products were demonstrated to contribute to auxin biosynthesis in planta.1 Here we report that the overexpression of YUC8 as well as YUC9 led to strong lignification of plant aerial tissues. Furthermore, new evidence indicates that this abnormally strong secondary growth is linked to increased levels of ethylene production.
Keywords: YUCCA, auxin, ethylene, plant hormone interaction, lignin biosynthesis, Arabidopsis
Auxin is associated with the regulation of an incredible wealth of different processes related to plant growth and development. These processes range from the promotion of cell elongation, induction of cell division activity of cambia, and initiation of adventitious and lateral roots, to contributions to photo- and gravitropic responses and fruit development.2 Accumulation of auxin in plants is known to produce a number of auxin-related phenotypes. Elevated auxin levels translate into, for example, increased apical dominance, elongated hypocotyls and petioles, as well as epinastic cotyledons.3,4 Very recently, we were able to disclose direct and intimate crosstalk between jasmonate signaling and auxin homeostasis.1 With the conducted experiments we were able to demonstrate that the transcription of 2 YUCCA genes, YUC8 and YUC9, is significantly induced by oxylipins.
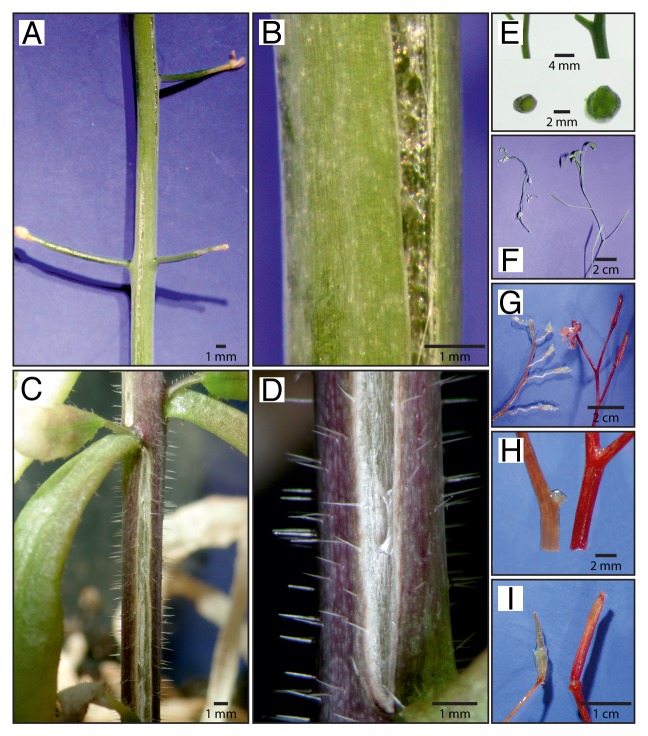
Jasmonate, either applied exogenously or produced endogenously as a response to wounding, has been shown to substantially trigger YUC8 and YUC9 transcript accumulation. In order to address the question whether these 2 YUCCA isoenzymes also contribute to auxin biosynthesis in planta, we took a genetic approach and generated several independent 35S-driven gain-of-function lines for both, YUC8 and YUC9 (YUC8ox and YUC9ox).1 When we analyzed the chemo- and phenotype of the overexpression lines, we discovered significantly increased free IAA levels in the overexpressors when compared with wild-type plants and clearly auxin-related phenotypes. Consistent with other auxin overproducer lines, YUC8ox and YUC9ox are characterized by elongated hypocotyls and petioles, as well as epinastic growing cotyledons. In addition, the lines showed longer and narrower leave blades than wild-type Arabidopsis. However, not all of the observed phenotypes could be directly attributed to the detected significantly increased amount of endogenous IAA. Intriguingly, some of the strong overexpression lines showed aberrant secondary growth of the stem (Fig. 1). Indeed, in some cases the secondary growth was so pronounced that the stem was no longer able to follow the increased growth and the epidermis cracked open from the bottom to the top (Fig. 1A-D). As can be estimated from Figure 1E, the stem diameter of overexpressor lines reached about twice the size of wild-type stems. In fact, this can be attributed to a more pronounced cell expansion growth, particularly of the cortex, vascular bundle, and parenchyma cells, in the overexpressor lines, which confirms the previous finding that the transient overexpression both of YUC8 and YUC9 results in an induction of cell expansion by 2- to 2.5-fold.1 In addition, we observed that the overexpressors lost much less of their size when slowly dried at ambient temperatures (Fig. 1F). Comparing weight loss after drying, we did not observe significant differences between overexpressor lines and wild type, pointing out that the examined lines lost the same amount of water by vaporization. Remarkably, the fresh weight of the YUC8ox and YUC9ox lines (2.51 ± 0.05 g) was only slightly higher than that of the wild type (2.24 ± 0.03 g). Nevertheless, shrinkage of the YUC8ox and YUC9ox lines was apparently reduced relative to wild-type Arabidopsis.
Figure 1. Phenotype of 10- to 12-week-old YUC8 and YUC9 overexpression lines. (A-D) Both the YUC8ox and YUC9ox lines show strong secondary growth. In some cases, the epidermis cannot follow the increased radial growth and cracks open. (E) Compared with wild-type Arabidopsis (left), stem cross sections of YUC8ox and YUC9ox (right) show a substantially bigger diameter. (F) Dried at ambient temperatures, the overexpressors (right) lose less size than the wild type (left). (G-I) Qualitative phloroglucinol stain for lignin levels in wild-type (left) and YUC8 and YUC9 overexpression lines (right). The images show either YUC8ox or YUC9ox lines, since the phenotypes evoked by the 35S-driven constitutive overexpression of either of the genes are similar.
Mechanical strength and plasticity of plants is to a great extent attributed to their cell walls. Cell walls are multilayered structures unique to plants that surround every cell providing sufficient rigidity to counteract the turgor pressure.5 The biosynthesis of this extracellular matrix that constitutes the boundary of the cell is a highly complex process that requires multiple coordinated enzymatic reaction steps.6 With respect to the described findings, we hypothesized that increased disposal of stabilizing biopolymers, e.g., lignin and embedded cellulosic compounds, in the secondary cell walls is likely to be the causal link for the abnormal phenotype of the overexpression lines relative to the wild type. Staining of various plant parts using phloroglucinol in the presence of alcohol and HCl,7 in fact, confirmed this hypothesis and revealed a substantially increased degree of lignification in YUC8ox and YUC9ox lines in comparison to the wild-type controls (Fig. 1G-I).
Both plant development and plant stress responses can be regulated by the mutual interoperability and congruence of plant hormones, a concept that is widely accepted. Various examples for the crosstalk between phytohormones and the underlying molecular bases can be found in the literature.8-11 For example, secondary plant growth can be stimulated by the interplay of auxin and strigolactone signaling, which seem to steer cambial activity.12 However, ethylene signaling is also assumed to contribute to radial (horizontal) growth by modulating vascular cell division.13
The stimulation of ethylene emission from plant tissues by exogenously applied as well as endogenously produced auxin is a well-established phenomenon.14,15 Ethylene, for its part, also affects auxin biosynthesis and transport-dependent local auxin distribution.16 Remarkably, recent findings significantly augmented the current insight into this intimate relationship between auxin and ethylene biosynthesis. Auxin and ethylene production are metabolically linked by a pyridoxal-phosphate-dependent aminotransferase, REVERSAL OF sav3 PHENOTYPE (VAS1), that catalyzes the transamination of IPA to l-tryptophan and 2-oxo-4-methylthiobutyric acid, specifically using methionine as the amino donor. Given that vas1 accumulates more auxin and 1-aminocyclopropane-1-carboxylate (ACC) under normal growth conditions, VAS1 seemingly controls the amounts of these 2 plant hormones.17 Overall, it seems as if there is a circle of mutual activation and a tight metabolic link between the 2 plant hormonal pathways. Most relevant for our experiments, however, was the discovery that overproduction of IAA in transgenic plants induces the concomitant overproduction of ethylene.15 To examine whether YUC8- and YUC9-mediated IAA overproduction also affects ethylene biosynthesis, we tested the primary root growth inhibition by an ethylene biosynthesis inhibitor, 2-aminoisobutyric acid (AIB), in wild type and YUC8ox and YUC9ox lines (Fig. 2). Although higher concentrations of AIB ultimately suppressed primary root growth in wild-type Arabidopsis as well as YUC8ox and YUC9ox, the 2 overexpression lines clearly showed hyposensitivity toward AIB, pointing toward an increased resistance that is mediated by the stimulated formation of ethylene. This result was confirmed by quantitative transcript analyses, which highlighted transcript accumulation of a number of ethylene biosynthesis- and signaling-related genes in YUC9ox (Table 1). Hence, we conclude that YUC8 and YUC9 overexpression-mediated overproduction of IAA, in turn, triggers the induction of ethylene production and signaling, which in combination stimulates secondary growth and deposition of lignin into the cell walls.
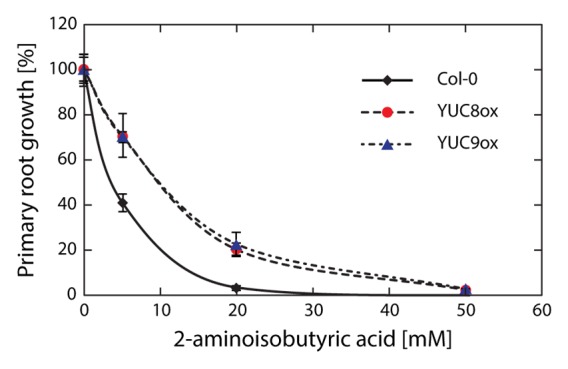
Figure 2. Root growth assay in presence of the ethylene biosynthesis inhibitor, 2-aminoisobutyric acid (AIB). Wild-type control plants as well as YUC8ox and YUC9ox plants were grown on vertical plates containing varying amounts of AIB. Depicted is the relative root growth inhibition in percent of untreated control plants of each genotype. Means are given with their SE (n = 10–12 seedlings).
Table 1. Ethylene biosynthesis and signaling components significantly affected in their expression in the YUC9 overexpression line.
| Name | Gene ID | p-value | Fold |
|---|---|---|---|
| Ethylene biosynthesis-related genes | |||
| ACS4 | At2g22810 | 6.18E-03 | +1.5 |
| ACS8 | At4g37770 | 3.37E-06 | +1.9 |
| ACS11 | At4g08040 | 4.47E-02 | +1.6 |
| ACO1 | At2g19590 | 4.10E-02 | +1.3 |
| ACO4 | At1g05010 | 5.89E-03 | +1.3 |
| ACO-like | At5g43440 | 1.01E-03 | +1.5 |
| Ethylene signaling-related genes | |||
| EIL1 | At2g27050 | 2.72E-03 | +1.4 |
| EIN3 | At3g20770 | 1.19E-02 | +1.3 |
| ETR2 | At3g23150 | 1.61E-03 | +1.5 |
| ESE3 | At5g25190 | 4.50E-04 | +2.1 |
| ERF-like | At5g61590 | 2.50E-07 | +2.8 |
Having in mind that jasmonates are capable of inducing the accumulation of YUC8 and YUC9 transcripts,1 thus being probably the initiator of a jasmonate/auxin/ethylene cascade, there is already evidence that coaction of ethylene and jasmonate is integrated through the ethylene-stabilized transcription factors EIN3 and EIL1, which physically interact with JASMONATE-ZIM-domain (JAZ) proteins to repress EIN3/EIL1.18 This leads to the emergence of a picture in which all 3 plant hormone signaling pathways may contribute to the stimulation of lignification in the overexpression lines.
There is mounting evidence that changes in lignification are linked to various plant hormone actions.19 For instances, the cellulose synthase mutant cev1 and the V-type ATPase mutant vha-a3 show ectopic lignification alongside with increased levels of JA-regulated genes and JA-precursors.20,21 In vha-a3, the AtMYB61 transcription factor22 appears to be misexpressed and suppression of AtMYB61 can restore the mutant phenotype. It is therefore possible that JA signaling is linked to lignin biosynthesis through the transcriptional regulation of AtMYB61. In addition, it has been shown that another MYB-type transcription factor, AtMYB32, is largely upregulated by IAA,23 while the KNOX gene family transcription factor BREVIPEDICELLUS (BP) that negatively regulates lignin biosynthesis is effectively repressed by IAA.24 Finally, also ethylene has been associated with the regulation of lignin biosynthesis. Characterization of a mutant in the chitinase-like protein AtCTL1, elp1, revealed that the phenotype of the mutant was due to the ectopic deposition of lignin and increased ethylene production.25 A similar lignin deposition phenotype has also been found in mutants of 2 leucine-rich-repeat receptor-like kinases, which seemingly link cell wall biosynthesis with ACC activity in Arabidopsis.26 Consistent with these circumstantial evidences, the transcription of AtMYB61 and AtMYB32 responds to both IAA and methyl jasmonate treatment, while BP additionally also responds to ACC treatment in a transient manner (http://jsp.weigelworld.org/expviz/expviz.jsp). Although the underlying molecular mechanisms for the cross-regulation of these transcription factors is largely unknown, it may be reasonable to think that their coordinated expression, orchestrated by the 3 plant hormones, is a possible determinant to regulate lignin formation. How AtCTL1 and the 2 receptor-like kinases feed into this picture remains, however, uncertain. So far, there is only indication that their mutation translates into altered lignin deposition and the concomitant increase of ethylene and ACC levels, respectively. Changes in the transcript profiles of both YUC8ox and YUC9ox have not yet been assessed, and nothing is known about the differential regulation of lignin synthesis-related genes in these mutants. It will be interesting to determine the molecular bases for the increased strong secondary growth and aberrant lignin deposition in YUC8ox and YUC9ox.
Acknowledgments
Support for this research was provided to SP by DFG grant SFB480-A10, MICINN grant BFU2011–25925, Marie-Curie grant FP7-PEOPLE-CIG-2011–303744, and a grant of the Ruhr-University Bochum (RUB) to promote young academics. MH was supported by a fellowship from the German National Academic Foundation and a membership of the Research School of the RUB.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hentrich M, Böttcher C, Düchting P, Cheng Y, Zhao Y, Berkowitz O, Masle J, Medina J, Pollmann S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 2013;74:626–37. doi: 10.1111/tpj.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies PJ. Plant Hormones. Biosynthesis, Signal Transduction, Action! 2010; Revised 3rd ed.; Kluwer Academic Publishers; Dordrecht, Boston, London. [Google Scholar]
- 3.Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–9. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 2002;16:3100–12. doi: 10.1101/gad.1035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keegstra K. Plant cell walls. Plant Physiol. 2010;154:483–6. doi: 10.1104/pp.110.161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanholme R, Storme V, Vanholme B, Sundin L, Christensen JH, Goeminne G, Halpin C, Rohde A, Morreel K, Boerjan W. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell. 2012;24:3506–29. doi: 10.1105/tpc.112.102574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liljegren S. Phloroglucinol Stain for Lignin. Cold Spring Harbor Protocols 2010; 2010:pdb.prot4954 [DOI] [PubMed]
- 8.Hoffmann M, Hentrich M, Pollmann S. Auxin-oxylipin crosstalk: relationship of antagonists. J Integr Plant Biol. 2011;53:429–45. doi: 10.1111/j.1744-7909.2011.01053.x. [DOI] [PubMed] [Google Scholar]
- 9.Kazan K, Manners JM. Jasmonate signaling: toward an integrated view. Plant Physiol. 2008;146:1459–68. doi: 10.1104/pp.107.115717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolters H, Jürgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet. 2009;10:305–17. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- 11.Kazan K, Manners JM. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012;17:22–31. doi: 10.1016/j.tplants.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Agusti J, Herold S, Schwarz M, Sanchez P, Ljung K, Dun EA, Brewer PB, Beveridge CA, Sieberer T, Sehr EM, et al. Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc Natl Acad Sci U S A. 2011;108:20242–7. doi: 10.1073/pnas.1111902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etchells JP, Provost CM, Turner SR. Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genet. 2012;8:e1002997. doi: 10.1371/journal.pgen.1002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abeles FB, Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964;39:963–9. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitbon F, Hennion S, Little CHA, Sundberg B. Enhanced ethylene production and peroxidase activity in IAA-overproducing transgenic tobacco plants is associated with increased lignin content and altered lignin composition. Plant Sci. 1999;141:165–73. doi: 10.1016/S0168-9452(98)00236-2. [DOI] [Google Scholar]
- 16.Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Guo Y, Novák O, Dai X, Zhao Y, Ljung K, Noel JP, Chory J. Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol. 2013;9:244–6. doi: 10.1038/nchembio.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z, An F, Feng Y, Li P, Xue L, A M, Jiang Z, Kim JM, To TK, Li W, et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108:12539–44. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Dixon RA. Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 2011;16:227–33. doi: 10.1016/j.tplants.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell. 2008;20:1088–100. doi: 10.1105/tpc.108.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis C, Karafyllidis I, Wasternack C, Turner JG. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell. 2002;14:1557–66. doi: 10.1105/tpc.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman LJ, Perazza DE, Juda L, Campbell MM. Involvement of the R2R3-MYB, AtMYB61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant J. 2004;37:239–50. doi: 10.1046/j.1365-313X.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 23.Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW. AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J. 2004;40:979–95. doi: 10.1111/j.1365-313X.2004.02280.x. [DOI] [PubMed] [Google Scholar]
- 24.Mele G, Ori N, Sato Y, Hake S. The knotted1-like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes Dev. 2003;17:2088–93. doi: 10.1101/gad.1120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong R, Kays SJ, Schroeder BP, Ye ZH. Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. Plant Cell. 2002;14:165–79. doi: 10.1105/tpc.010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell. 2008;20:3065–79. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]