Abstract
In the uterus, epithelial cell proliferation changes during the estrous cycle and pregnancy. Uncontrolled epithelial cell proliferation results in implantation failure and/or cancer development. Transforming growth factor-β (TGF-β) signaling is a fundamental regulator of diverse biological processes and is indispensable for multiple reproductive functions. However, the in vivo role of TGF-β signaling in uterine epithelial cells remains poorly defined. We have shown that in the uterus, conditional deletion of the Type 1 receptor for TGF-β (Tgfbr1) using anti-Müllerian hormone receptor type 2 (Amhr2) Cre leads to myometrial defects. Here, we describe enhanced epithelial cell proliferation by immunostaining of Ki67 in the uteri of these mice. The aberration culminated in endometrial hyperplasia in aged females. To exclude the potential influence of ovarian steroid hormones, the proliferative status of uterine epithelial cells was assessed following ovariectomy. Increased uterine epithelial cell proliferation was also revealed in ovariectomized Tgfbr1 Amhr2-Cre conditional knockout mice. We further demonstrated that transcript levels for fibroblast growth factor 10 (Fgf10) were markedly up-regulated in Tgfbr1 Amhr2-Cre conditional knockout uteri. Consistently, treatment of primary uterine stromal cells with TGF-β1 significantly reduced Fgf10 mRNA expression. Thus, our findings suggest a potential involvement of TGFBR1-mediated signaling in the regulation of uterine epithelial cell proliferation, and provide genetic evidence supporting the role of uterine epithelial cell proliferation in the pathogenesis of endometrial hyperplasia.
Keywords: transforming growth factor β, uterus, epithelial cell, proliferation, endometrial hyperplasia
Introduction
Endometrial hyperplasia is a pathological condition where endometrial cells undergo excessive proliferation (Mills and Longacre, 2010). As a premalignant lesion of endometrial carcinoma (Montgomery et al., 2004), endometrial hyperplasia causes multiple symptoms including abnormal uterine bleeding, fertility disorders and, in severe cases, morbidity and mortality. Therefore, understanding the pathophysiology and mechanisms of endometrial hyperplasia is of critical importance. In the uterus, epithelial cell proliferation changes during the estrous cycle and pregnancy (Wood et al., 2007; Li et al., 2011b). As a prerequisite for embryo implantation, luminal epithelial cells cease proliferation during early pregnancy (Dey et al., 2004). Uncontrolled epithelial cell proliferation results in implantation failure and/or cancer development.
The transforming growth factor-β (TGF-β) superfamily signaling plays an instrumental role in cell proliferation, differentiation and migration (Massague, 1990, 1998, 2000; Chang et al., 2002). The canonical TGF-β signaling is initiated by ligand-induced heteroligomerization of cell surface receptors. Transduction of TGF-β signaling is mediated by intracellular SMAD proteins, which comprise receptor-regulated SMADs (SMAD2/3 and SMAD1/5/8) and a common SMAD (i.e. SMAD4). Activation of SMAD2/3 and SMAD1/5/8 is generally believed to mediate the respective TGF-β/activin signaling and bone morphogenetic protein (BMP) signaling in a contextually dependent manner (Attisano and Wrana, 2002; Shi and Massague, 2003; Massague, 2012). TGF-β superfamily ligands also signal through noncanonical pathways, which include MAP kinase, phosphatidylinositol-3-kinase/AKT and microRNA pathways (Davis et al., 2008, 2010).
Accumulating evidence, including our own work, demonstrates an obligatory role for TGFβ superfamily signaling in female reproduction (Dong et al., 1996; Galloway et al., 2000; Tomic et al., 2004; Hashimoto et al., 2005; Juengel and McNatty, 2005; Diaz et al., 2007; Dragovic et al., 2007; Lee et al., 2007; Li et al., 2008, 2011a; Pangas et al., 2008; Gong and McGee, 2009; Edson et al., 2010; Nagashima et al., 2013). TGF-β ligands (TGF-βs 1-3) signal through their Type 2 (TGFBR2) and Type 1 (TGFBR1 or ALK5) receptors. Circumstantial evidence suggests the involvement of TGF-β isoforms in uterine function. As such evidence, TGF-βs are produced and regulated by hormones in the uterus (Takahashi et al., 1994). However, the spatial and temporal expression of TGFBR1 in the mouse uterus has not been well described, despite the demonstration of its predominant localization to the myometrial compartment in the post-natal uterus (Li et al., 2011a; Gao et al., 2014). The in vivo function of TGFβ signaling in female reproductive tract was elusive until the development of Tgfbr1 anti-Müllerian hormone receptor type 2 (Amhr2)-Cre conditional knockout (cKO) mice, in which sterility and prominent smooth muscle defects occur in the oviduct and uterus (Li et al., 2011a). Despite the above knowledge, little is known about the in vivo role of TGF-β signaling in uterine epithelial cells.
In this study, using the Amhr2-Cre conditional knockout mouse model of Tgfbr1, we provide evidence supporting the association of enhanced uterine epithelial cell proliferation and the development of endometrial hyperplasia. These results will help to understand the potential function of TGFβ signaling in this disease and in other conditions that involve altered uterine epithelial cell properties, such as endometrial cancer.
Materials and Methods
Animals
Experimental procedures using the laboratory mouse were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Mice were maintained on a mixed C57BL/6/129 genetic background. The Amhr2cre allele (Jamin et al., 2002), Tgfbr1flox allele (Larsson et al., 2001) and Tgfbr1 null allele (i.e. Tgfbr1Lacz; The Jackson Laboratory) were utilized to generate the conditional deletion of Tgfbr1 in the mensenchyme-derived tissues of the uterus as described (Li et al., 2011a).
Immunofluorescence microscopy and immunohistochemistry
Immunofluorescence was conducted as described (Li et al., 2011a). Briefly, serial paraffin sections (5 μm) were cut from paraffin-embedded tissues. Antigen retrieval was performed by boiling the sections in citrate buffer (10 mM; pH 6.0) for 20 min. After blocking with bovine serum albumin (BSA), sections were incubated with primary antibodies at 4°C overnight. Appropriate Alexa Fluor 488 or 594 conjugated secondary antibodies (Invitrogen) were then utilized to detect the expression/localization of antigens that were bound to the antibodies. The sections were mounted using ProLong Gold SlowFade media containing DAPI (4′,6-diamidino-2-phenylindole, Invitrogen) and examined under a fluorescence microscope.
The immunohistochemistry procedure has been detailed elsewhere (Li et al., 2011a). The signals were developed using a Diaminobenzidine (DAB) kit (Vector Labs) and sections were counterstained with hematoxylin. To monitor the background levels of the staining, controls using normal IgGs were included for both types of the aforementioned analyses. Primary antibodies used in this study included rabbit anti-Ki67 (Abcam; 1 : 200), mouse anti-smooth muscle actin α (ACTA2; Abcam; 1 : 2000), rabbit anti-forkhead box A2 (FOXA2; Abcam;1 : 250), rabbit anti-vimentin (VIM; Cell signaling; 1 : 200) and rat anti-cytokeratin 8 (KRT8; Developmental Studies Hybridoma Bank; 1 : 100). All antibodies were validated and optimal dilutions were determined in a pilot experiment. Quantification of Ki67-positive cells in the uterus of ovariectomized mice was performed using high power images (40×) of Ki67 and KRT8 immunostained sections. Inclusion of KRT8 staining facilitates epithelial cell counting. A total of six independent samples (n = 3 per group) were used in this study. The mean value of Ki67-positive cells in the controls was set to 100% and results of experimental group are presented as the percentage of the controls.
X-gal staining
X-gal staining was performed using uterine samples from mice harboring a Tgfbr1Lacz allele (Li et al., 2011a). Briefly, uterine samples were collected and fixed in fixation buffer (2% paraformaldehyde, 0.2% glutaraldehyde, and 0.1 M phosphate; pH 7.4). The samples were washed and then stained with a buffer containing 1 mg/ml X-gal, 5 mM potassium ferricyanide and 5 mM potassium ferrocyanide. After staining, the samples were embedded, sectioned, and counterstained with fast red to visualize the nucleus (Vector Lab). All samples for histological studies were processed by the Histology Lab of Veterinary Integrative Biosciences at Texas A&M University.
Ovariectomy
Ovaries were removed from adult virgin control mice and Tgfbr1 Amhr2-Cre cKO mice under Avertin anesthesia. Two weeks after surgery, uterine samples were collected from control and Tgfbr1 Amhr2-Cre cKO mice (n = 5 per group). The samples were then processed for RNA isolation or immunofluorescence microscopy.
Mouse uterine stromal cell isolation, culture and treatment
Mouse uterine stromal cell isolation was performed based on previous reports with slight modifications (Daikoku et al., 2005; Chen et al., 2009). In brief, uterine horns of adult virgin mice were collected, cut into 3–5 mm pieces, and washed with calcium and magnesium free Hank's buffered salt solution (HBSS; Lonza) containing 100 U/ml penicillin and streptomycin (Gibco). The tissues were digested with 0.5% trypsin (AMRESCO; 100 U/ml) in HBSS for 1 h at 4°C, 1 h at room temperature, and 10 min at 37°C. After being washed with HBSS, the tissues were further digested with 0.05% collagenase (Sigma) for 45 min at 37°C and vortexed. The digestion mixture was then passed through a 70-μm cell strainer (Becton, Dickinson, and Company). Cells were collected by centrifugation and resuspended in Dulbecco's modified eagle medium-F12 growth media supplemented with 10% fetal bovine serum (FBS). The cells were incubated at 37°C with 5% CO2 for 90 min, washed with HBSS and then cultured in growth media. For TGF-β1 treatment, uterine stromal cells were serum-starved overnight and then treated with TGF-β1 (0.1, 1, 2.5, 5 and 10 ng/ml). Cells were collected after 20 h incubation and total RNA was isolated as described below.
Isolation of mouse uterine smooth muscle cells
Isolation of mouse uterine smooth muscle cells was based on previously described protocols with modifications (Shynlova et al., 2002; Renthal et al., 2010). Briefly, uteri from 2- to 3-month-old adult virgin mice were collected. The tissue was dissected to remove endometrium and kept in HBSS (pH 7.4) supplemented with penicillin–streptomycin (Gibco; 100 U/ml) and amphotericin B (Sigma; 2.5 µg/ml). Uterine tissues were then washed, cut into 2–3 mm pieces and digested at 37°C in a buffer containing 1 mg/ml collagenase Type II (Sigma), 0.15 mg/ml DNase I (Roche), 1 mg/ml BSA (Sigma), 0.1 mg/ml soybean trypsin inhibitor (Sigma) and 10% FBS (Gibco). After 30 min digestion, the mixture was triturated for 3 min before being passed through a cell strainer. The first digestion mixture was discarded. The tissues were digested five more times, and the cell suspension from each digestion was pooled and centrifuged to collect the cells.
RNA isolation
Total RNA from mouse uteri/stromal cells/smooth muscle cells was isolated using RNeasy Mini Kit (Qiagen) with on-column DNase digestion following the manufacturer's instructions. RNA was quantified using a NanoDrop Spectrophotometer ND 1000 (NanoDrop Technologies) and stored at −80°C until use.
Conventional RT–PCR
Fibroblast growth factor 10 (Fgf10) mRNA level was analyzed using stromal/smooth muscle cell cDNA by reverse transcription polymerase chain reaction (RT–PCR) using JumpStart Taq polymerase (Sigma) and gene-specific primers (forward: 5′-GGATACTGACACATTGTGCCTCAG-3′; reverse: 5′-TGTTTTTTGTCCTCTCCTGGGAG-3′) (Mailleux et al., 2005). PCR was performed using the following condition: 95°C for 5 min, 40 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 1 min, and an additional extension of 10 min at 72°C. Hypoxanthine guanine phosphoribosyl transferase (Hprt) was used as an internal control. The resultant amplicon was separated and visualized on 1% agarose gel containing ethidium bromide. The image of the gel was digitally captured under UV light and then reversed for presentation.
Quantitative real-time polymerase chain reaction
Samples of 200 ng total RNA with superscript III reverse transcriptase (Invitrogen) were used to generate cDNAs. The qPCR was performed on CFX384/CFX Connect Real-time PCR Detection System (Bio-Rad) using iTaq Universal SYBR Green Supermix (Bio-Rad) and primers for various Fgfs (Table I). The reaction was carried out at 95°C for 30 s, 40 cycles of 95°C for 5 s and 60°C for 30 s. Ribosomal protein L19 (Rpl19) was included as an internal control. Relative abundance of gene expression was calculated as previously described (Livak and Schmittgen, 2001).
Table I.
Primers for quantitative real-time PCR.
| Name | Sequence (5′–3′) | Reference |
|---|---|---|
| Fgf1 | ||
| Forward | CAGCTCAGTGCGGAAAGTG | PrimerBank ID 6753850a1 |
| Reverse | TGTCTGCGAGCCGTATAAAAG | |
| Fgf2 | ||
| Forward | TTGTGTCTATCAAGGGAGTGTGT | Takase et al., (2013) |
| Reverse | TGCCACATACCAACTGGAGTATT | |
| Fgf7 | ||
| Forward | TTTGGAAAGAGCGACGACTT | Takase et al., (2013) |
| Reverse | GGCAGGATCCGTGTCAGTAT | |
| Fgf9 | ||
| Forward | ATGGCTCCCTTAGGTGAAGTT | PrimerBank ID 7305057a1 |
| Reverse | TCATTTAGCAACACCGGACTG | |
| Fgf10 | ||
| Forward | GTTGCTCTTTTTGGTGTCTTCGT | N/A |
| Reverse | GGCCTCCTGTGACACCATGT | |
| Fgf12 | ||
| Forward | ACAGCTCAGATGTTTTTACCCC | PrimerBank ID 118129945c2 |
| Reverse | CTGGCGATACAGGGTTGAGG | |
| Fgf18 | ||
| Forward | CCTGCACTTGCCTGTGTTTAC | PrimerBank ID 6679781a1 |
| Reverse | TGCTTCCGACTCACATCATCT | |
| Rpl19 | ||
| Forward | ATGAGTATGCTCAGGCTACAGA | PrimerBank ID 6677773a1 |
| Reverse | GCATTGGCGATTTCATTGGTC | |
Statistical analysis
The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS; Version 21). A one-way analysis of variance (ANOVA) followed by a post hoc Dunnett test was performed to determine the effect of TGFβ1 treatment on Fgf10 mRNA expression. Comparisons of two means were made using Student's t-test. Data are presented as mean ± standard error of the mean (SEM). Statistical significance was defined at P< 0.05.
Results
Enhanced uterine epithelial cell proliferation in Tgfbr1 conditionally ablated mice
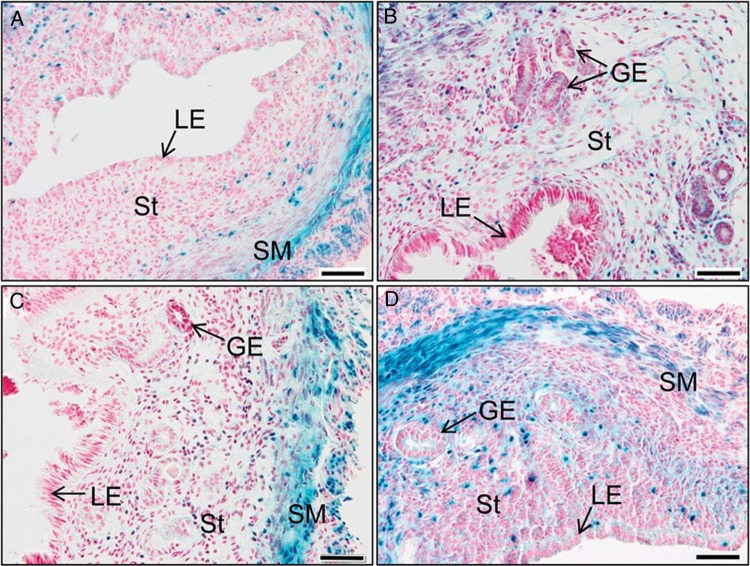
In an early study, we generated Tgfbr1 conditional knockout mice using Amhr2-Cre (Li et al., 2011a). These mice are sterile due to the development of oviductal diverticula. The primary defect of the uterus is the disruption of myometrium. However, uterine glandular defects and increased uterine size were observed in aged Tgfbr1 cKO mice (Li et al., 2011a). These results raised the question of how loss of TGFBR1 promoted uterine glandular pathology. Our initial analysis using random cycling mice showed that TGFBR1 was predominantly localized to uterine smooth muscle layers (Li et al., 2011a). Further X-gal staining using uterine tissues from different estrous cycle stages confirmed the predominant expression of TGFBR1 in the myometrium. X-gal staining in the endometrium seemed to be variable during estrous stages (Fig. 1). Notably, the staining was negligible in the uterine epithelium across all stages (Fig. 1). These results suggest that the glandular pathology in Tgfbr1 Amhr2-Cre cKO mice is likely attributed to loss of TGFBR1 in the endometrium.
Figure 1.
TGFBR1 localization in mouse uterus during estrous cycle. (A) Diestrus; (B) pro-estrus; (C) estrus and (D) metestrus. The uteri from 2- to 3-month-old mice containing a Tgfbr1lacZ allele were processed for X-gal staining. The sections were counterstained with fast red to visualize the nuclei. Note the predominant X-gal staining in the smooth muscle layers and variable staining in the stromal compartment. SM, smooth muscle; LE, luminal epithelium; GE, glandular epithelium; St, stroma. Scale bar = 50 µm.
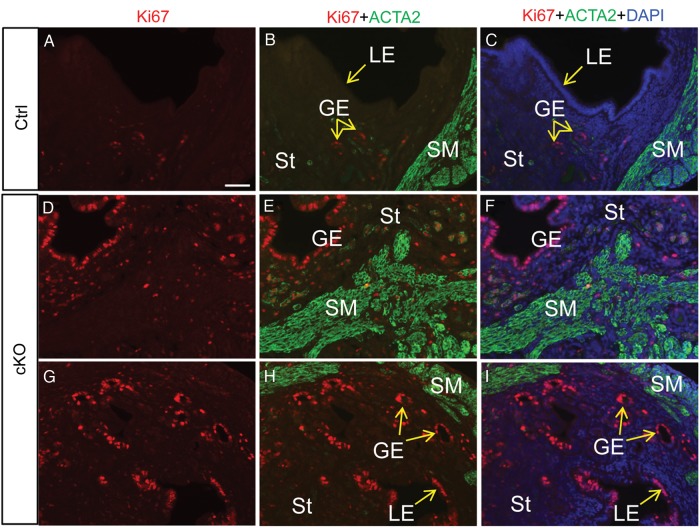
Next, to determine the proliferation status of uterine epithelial cells, we peformed immunofluorescence microscopy using Ki67. Because uterine epithelial cell proliferation in adult mice is affected by estrous stages and hormones (Wood et al., 2007), we first compared the Ki67 expression in Tgfbr1 Amhr2-Cre cKO and control mice at diestrous stage when the proliferative activity of uterine glandular epithelium is low (Wood et al., 2007). While low levels of Ki67 staining were found in the controls (Fig. 2A–C), extensive immunofluorescence signals for Ki67 were observed in the uterine epithelia of Tgfbr1 Amhr2-Cre cKO mice (Fig. 2D–I).
Figure 2.
Uterine epithelial cell proliferation in control and Tgfbr1 Amhr2-Cre cKO uteri. Immunofluorescence of Ki67 (A; red) and Ki67 and ACTA2 (green) (B and C) using uterine sections from wild-type control (Ctrl) mice. Immunostaining of Ki67 (D and G; red) and Ki67 and ACTA2 (green) (E, F, H and I) using uterine sections from Tgfbr1 Amhr2-Cre cKO mice. Uterine samples were collected from adult mice (∼3 months of age) at the diestrous stage. Note the extensive Ki67 immunostaining in epithelial cells of Tgfbr1 Amhr2-Cre cKO mice compared with the controls. LE, luminal epithelium; GE, glandular epithelium; St, stroma; SM, smooth muscle. Scale bar (50 μm) is representatively shown in (A).
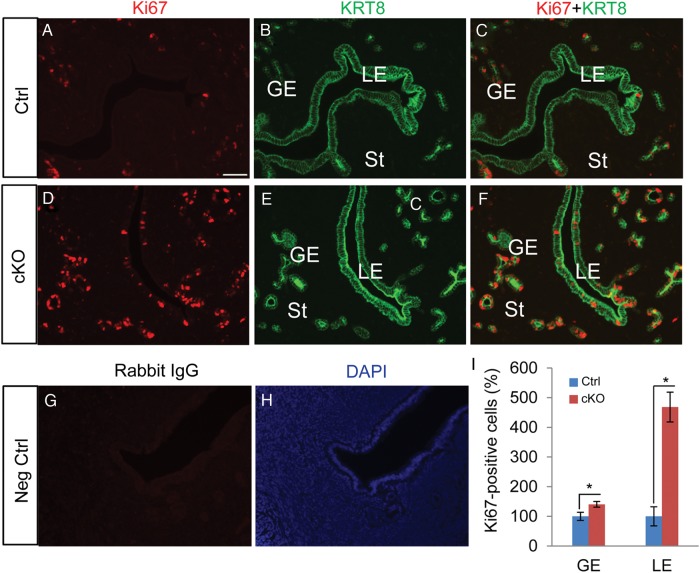
To exclude the potential effect of steroid hormones, uterine epithelial cell proliferation was further assessed using mice in which ovaries were removed 2 weeks before the experiment. To better visualize the epithelial compartment, double immunofluorescence using Ki67 and KRT8, an epithelial marker, was conducted. We found enhanced proliferation of cells from both glandular and luminal epithelia of the ovariectomized Tgfbr1 Amhr2-Cre cKO mice compared with the respective controls (Fig. 3A–F and I). Negative controls where Ki67 antibody was replaced by normal IgGs generated background staining (Fig. 3G and H).
Figure 3.
Increased epithelial cell proliferation in ovariectomized Tgfbr1 Amhr2-Cre cKO uteri. (A–F) Immunofluorescence of Ki67 (red) using uterine sections from ovariectomized control and Tgfbr1 Amhr2-Cre cKO mice. Uterine epithelia were labeled with anti-KRT8 antibody (green). Ovariectomy was performed on adult female mice from control and Tgfbr1 Amhr2-Cre cKO groups (∼3 month of age). Three independent samples were examined from each group and representative images were depicted. LE, luminal epithelium; GE, glandular epithelium; St, stroma. (G and H) Negative controls where primary antibodies were replaced with rabbit IgG. Scale bar (50 μm) is representatively shown in (A). (I) Quantification of Ki67-positive cells in glandular and luminal epithelia of control and Tgfbr1 Amhr2-Cre cKO uteri. Results are presented as the percentage of controls which is set to 100% (n = 3 per group). Data are mean ± SEM. *P < 0.05 versus corresponding controls.
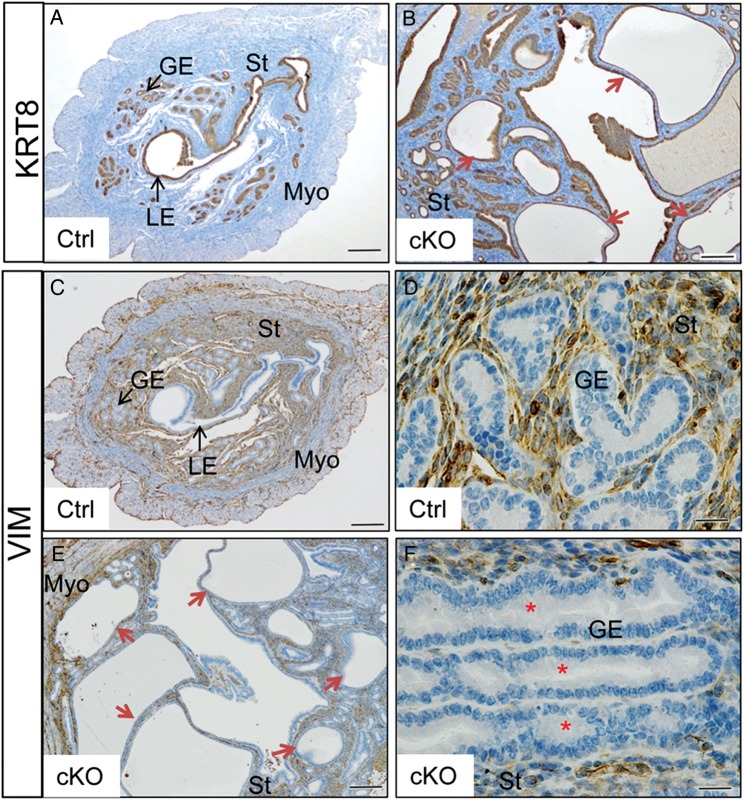
Cystic dilation of uterine glands in aged Tgfbr1 Amhr2-Cre cKO females
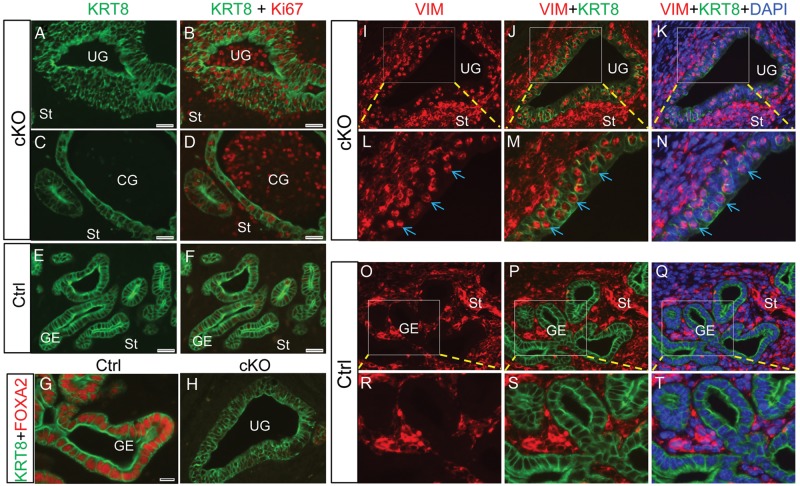
Consistent with the enhanced proliferation of uterine epithelial cells observed in Tgfbr1 Amhr2-Cre cKO mice, cystic dilation of uterine glands was prominent in 8-month-old Tgfbr1 Amhr2-Cre cKO females (Fig. 4). In contrast to controls (Fig. 4A), Tgfbr1 Amhr2-Cre cKO uteri comprised abundant cystic and irregular glands (Fig. 4B), which consisted of single layers of flattened epithelial cells. Compared with uterine glands within control uteri (Fig. 4C and D), loss of intervening stroma among glands was occasionally detected in the uteri of Tgfbr1 Amhr2-Cre cKO mice (Fig. 4E and F). In severe cases, the proliferation of epithelial cells was accompanied by loss of normal cell morphology, nuclear polarity and basement membrane boundary (Fig. 5A and B). Within some uterine glands, Ki67-positive cells could be identified, the identity of which is unknown (Fig. 5B and D). Another interesting finding is that vimentin was localized to some epithelial cells within a subset of endometrial glands (Fig. 5I–N), which was not observed in the control uterus (Fig. 5O–T). Double immunofluorescence studies using KRT8 and vimentin revealed the nuclear localization of vimentin within some epithelial cells (Fig. 5I–N), but the implication of which is unknown.
Figure 4.
Glandular defects observed in aged Tgfbr1 Amhr2-Cre cKO mice. (A and B) Tgfbr1 Amhr2-Cre cKO mice develop cystic glands (B; red arrows) compared with age-matched controls (A). (C–F) Loss of intervening stroma among uterine glands (red asterisks) in Tgfbr1 Amhr2-Cre cKO mice (E and F) in contrast to controls (C and D). Uterine samples were from 8-month- old random cycling control and Tgfbr1 Amhr2-Cre cKO mice (n = 3 per group). Anti-KRT8 (A and B) and anti-VIM antibodies (C–F) were used to label uterine epithelial cells and mesenchymal cells, respectively. (D) and (F) are higher power images for (C) and (E), respectively. LE, luminal epithelium; GE, glandular epithelium; St, stroma; Myo, myometrium. Scale bar = 20 µm (D and F) and 200 µm (A, B, C and E).
Figure 5.
Epithelial cell lesions in Tgfbr1 Amhr2-Cre cKO mice. (A–D) Immunofluorescence staining of uterine sections using antibodies directed against KRT8 (green) and Ki67 (red) in aged control and Tgfbr1 Amhr2-Cre cKO mice. (E and F) Representative control uteri stained with KRT8 and Ki67. Scale bar = 25 µm (A–F). (G–T) Immunofluorescence of KRT8 (green) and FOXA2 (red; G and H) or vimentin (red; I–T) in the uterus of aged Tgfbr1 Amhr2-Cre cKO mice. Six 9- to 10.5-month-old Tgfbr1 Amhr2-Cre cKO mice were analyzed using immunofluorescence or immunohistochemistry. Epithelial cell defects were observed in all mice, and localization of vimentin in epithelial cells was observed in three mice. Representative images from a 10.5-month Tgfbr1 Amhr2-Cre cKO uterus are presented. (L–N) and (R–T) are higher power images for selected regions of (I–K) and (O–Q), respectively. Arrows (L–N) indicate localization of vimentin to KRT8-positive epithelial cells. UG, uterine gland; St, stroma; CG, cystic endometrial gland; GE, glandular epithelium. Scale bar is representatively shown in (G) and equals 10 µm (G and H, L–N and R–T) and 20 µm (I–K and O–Q).
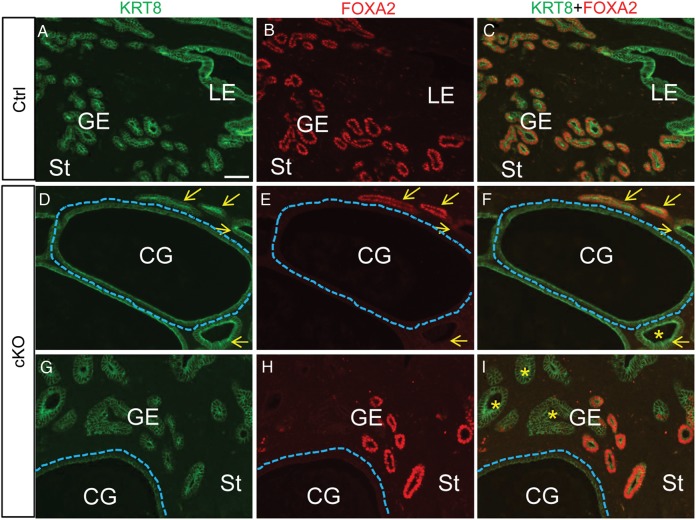
Additionally, we found that expression of FOXA2, a key regulator of uterine gland development and female fertility (Bazer, 2010; Jeong et al., 2010), was absent in a subset of endometrial glands (Fig. 5G and H and Fig. 6), especially cystic dilated glands in the uteri of aged mice (8–10.5 month old; Fig. 6D–I), compared with controls (Fig. 6A–C).
Figure 6.
Alteration of FOXA2 expression in endometrial glands of Tgfbr1 Amhr2-Cre cKO mice. (A–I) Immunofluorescence staining of KRT8 (green) and FOXA2 (red) in control (A–C; 8-month-old) and Tgfbr1 Amhr2-Cre cKO mice at the age of 8 (D–F) and 10.5 (G–I) months. Yellow arrows indicate glandular epithelium. Dotted lines in (D–I) outline cystic dilated glands. Note the absence of FOXA2 in the cystic glands (D–I) and endometrial glands marked with asterisks (F and I). GE, glandular epithelium; LE, luminal epithelium; St, stroma; CG, cystic endometrial gland. Scale bar (50 μm) is representatively shown in (A).
Potential involvement of TGFBR1-mediated paracrine signaling in uterine epithelial cell proliferation
Because X-gal staining was observed in the endometrium and Amhr2-Cre is also expressed in uterine stroma (Petit et al., 2007), the enhanced uterine epithelial cell proliferation in Tgfbr1 Amhr2-Cre cKO mice is likely caused by altered epithelial-mesenchymal interactions resulting from loss of TGFBR1 in the uterine mesenchymal compartment. In support of this hypothesis, we found that the expression of Fgf10 was significantly increased in the Tgfbr1 Amhr2-Cre cKO uteri (Fig. 7). FGFs are known mitogens for uterine epithelial cells (Li et al., 2011b). RT–PCR showed that the Fgf10 transcript was expressed in uterine stromal cells, with negligible expression in smooth muscle cells (Fig. 7A). Negative controls without reverse transcriptase failed to amplify Fgf10 target band (Fig. 7A). Further analysis performed using ovariectomized mice confirmed that Fgf10 mRNA levels were higher in Tgfbr1 Amhr2-Cre cKO mice compared with controls (Fig. 7B). Significant changes of transcript levels for a number of other Fgfs including Fgf1, Fgf2, Fgf7, Fgf9, Fgf12, and Fgf18 were not found (Fig. 7B). To substantiate this finding, we treated primary uterine stromal cells with TGF-β1 (0.1, 1, 2.5, 5, and 10 ng/ml) and determined its effect on Fgf10 mRNA expression. qPCR demonstrated that Fgf10 mRNA levels were significantly reduced following the treatment with TGF-β1 (Fig. 7C). These results collectively suggest a potential involvement of TGFBR1-mediated signaling in the regulation of uterine epithelial cell function.
Figure 7.
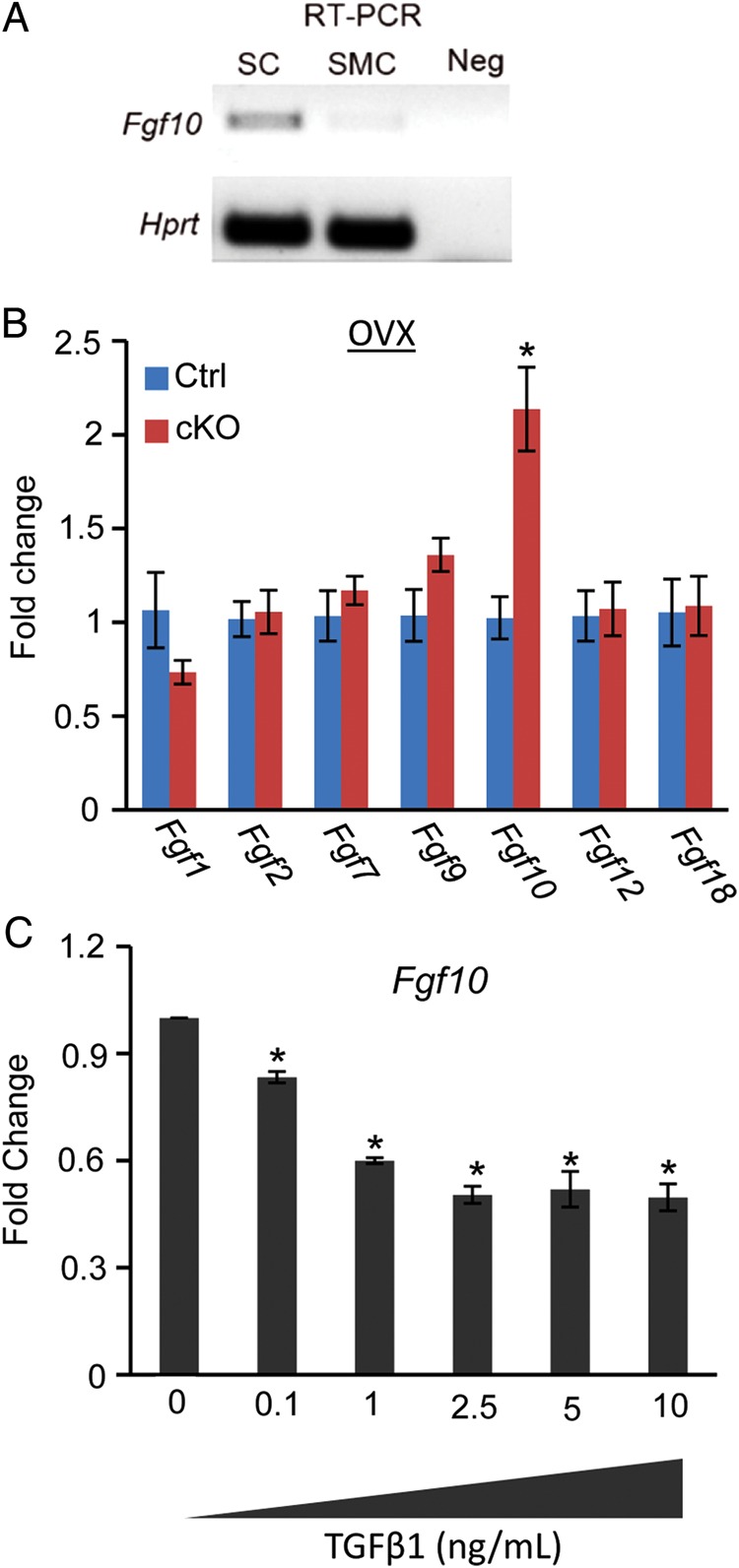
Elevated Fgf10 mRNA levels in the uterus of Tgfbr1 Amhr2-Cre cKO mice. (A) RT–PCR amplification of Fgf10 in uterine stromal cells and smooth muscle cells. Note that Fgf10 was readily detectable in uterine stromal cells. No target band was detected in the negative controls. Hprt was included as an internal control. SC, stromal cells; SMC, smooth muscle cells; Neg, negative control without reverse transcriptase. (B) Fgf10 mRNA expression was increased in ovariectomized Tgfbr1 Amhr2-Cre cKO uteri among the examined Fgfs compared with corresponding controls. n = 5 per group. OVX, ovariectomy. (C) Fgf10 mRNA abundance was reduced in uterine stromal cells treated with TGF-β1 (0.1–10 ng/ml) for 20 h versus controls. Three independent cell culture experiments were performed. Data are mean ± SEM. *P < 0.05.
Discussion
Endometrial hyperplasia, a premalignant lesion of endometrial cancer, is a casual cause of reproductive disorders. The pathogenesis of endometrial hyperplasia has not been fully understood. In the uterus, uncontrolled epithelial cell proliferation affects uterine function and pregnancy, leading to pathological conditions such as endometrial hyperplasia that adversely impact reproductive potential. TGFβ superfamily signaling regulates fundamental cellular functions (Massague, 1990, 1998, 2000; Chang et al., 2002) and is indispensable for female reproduction (Dong et al., 1996; Galloway et al., 2000; Tomic et al., 2004; Hashimoto et al., 2005; Juengel and McNatty, 2005; Diaz et al., 2007; Dragovic et al., 2007; Li et al., 2008, 2011a; Pangas et al., 2008; Gong and McGee, 2009; Edson et al., 2010; Gao et al., 2013; Nagashima et al., 2013). Dysregulation of TGFβ signaling results in impaired reproductive function and may cause cancer development (Matzuk et al., 1992; Pangas et al., 2008; Middlebrook et al., 2009; Edson et al., 2010). Using an established mouse model, this study provides new evidence supporting a potential involvement of TGFBR1 in the regulation of uterine epithelial cell function. These results will aid in understanding the complex tissue-/cell-specific functions of TGFβ signaling in one of the most important female reproductive organs, the uterus.
Since the initiation of uterine gland development (i.e. adenogenesis) appears to occur normally in Tgfbr1 Amhr2-Cre cKO mice during early post-natal uterine development (Gao et al., 2014), the manifestation of epithelial abnormality in Tgfbr1 Amhr2-Cre cKO females is likely a secondary effect, which is not due to a direct disruption of TGFβ signaling machinery in the epithelial compartment. This postulation was based on the following evidence: (i) X-gal staining showed minimal TGFBR1 expression in uterine epithelia during estrous cycles, and (ii) Amhr2-Cre is not expected to be expressed in the uterine epithelial compartment based on findings using Amhr2-lacZ mice (Arango et al., 2008). It is thus conceivable that conditional deletion of Tgfbr1 might increase production of stromal cell-derived factors that promote uterine epithelial cell proliferation or impair the expression of factors inhibitory to cell proliferation. In support of the former possibility, we found that Fgf10 mRNA abundance was significantly up-regulated in Tgfbr1 Amhr2-Cre cKO uteri. FGF10 regulates cell migration (Tao et al., 2005; Nomura et al., 2008) and proliferation, and is expressed in the ovine endometrium (Satterfield et al., 2008). Expression of Fgf10 transcripts in mouse uterine stromal cells was verified in the current study. Of note, since the primary phenotype observed in Tgfbr1 Amhr2-Cre cKO mice is disrupted smooth muscle formation, the potential contribution of the myometrial defect, hormone signaling, as well as other growth factors to the development of this uterine pathology needs further investigation.
Tgfbr1 Amhr2-Cre cKO mice develop oviductal diverticula which prevent the embryo from being transported to the uterus for implantation, leading to female sterility (Li et al., 2011a). However, it was not clear whether the uterus of Tgfbr1 Amhr2-Cre cKO mice could support pregnancy. The endometrium plays a key role in accepting and fostering embryos and is of paramount importance for female fertility. Normal function of luminal epithelium is critical for embryo attachment and implantation (Li et al., 2011b). Secretions from the uterine epithelium such as leukemia inhibitory factor (Stewart et al., 1992) and mucin 1 (Thathiah and Carson, 2002) play essential roles during pregnancy. Meanwhile, endometrial glands provide an important source of nutrients and growth factors for establishing a successful pregnancy (Spencer et al., 2012). Indeed, uterine epithelia are sites for the production of TGF-βs that could potentially act on the embryo and endometrium during pregnancy (Shooner et al., 2005; Jones et al., 2006). As further evidence of altered endometrial gland development/function in aged Tgfbr1 Amhr2-Cre cKO mice, we found that FOXA2 was absent in a subset of uterine glands including cystic endometrial glands. It has been well established that FOXA2 is an essential regulator of endometrial gland development and loss of FOXA2 during uterine development dramatically reduces endometrial gland formation, rendering female mice infertile (Bazer, 2010; Jeong et al., 2010; Filant et al., 2012). It seems counterintuitive that FOXA2 was absent in some endometrial glands of the aged Tgfbr1 Amhr2-Cre cKO mice since the expression of FOXA2 was positively associated with endometrial hyperplasia in a recent study (Villacorte et al., 2013). One plausible explanation is that some of the dilated glands were composed of luminal epithelia. Alternatively, absence of FOXA2 in some glandular epithelium might be an irreversible consequence of the development of certain cystic/hyperplastic glands. However, further studies are warranted to address these possibilities.
Based on the altered properties of uterine epithelial cells, we predicted that conditional deletion of Tgfbr1 in uterine mesenchymal compartment would be detrimental to pregnancy, potentially by affecting embryo implantation and/or development. Our preliminary embryo transfer experiment showed that Tgfbr1 Amhr2-Cre cKO mice were incapable of establishing a pregnancy with a successful outcome when wild-type embryos were transferred into the uteri of these mice (Gao and Li, unpublished observation). This finding suggests that although the infertility in Tgfbr1 Amhr2-Cre cKO mice is predisposed by the development of oviductal diverticula, uterine defects may also lead to reproductive failure even when the oviductal deficiency is bypassed.
Clinically, endometrial hyperplasia is a cause of abnormal uterine bleeding and fertility disorders (Kurman et al., 1985; Shutter and Wright, 2005; Lacey and Chia, 2009; Hahn et al., 2010). Genetic alterations, including mutations of phosphatase and tensin homolog (Pten) tumor suppressor, have been shown to be associated with endometrial hyperplasia (Stambolic et al., 2000; Milam et al., 2008). Endometrial hyperplasia is a premalignant lesion of endometrial carcinoma (Montgomery et al., 2004), a common malignancy that affected >49 000 women and caused over 8000 deaths in 2013 (Siegel et al., 2013). The etiology of endometrial carcinoma is not well defined. Elegant studies have demonstrated that TGFβ signaling is inactivated in endometrial cancers (Parekh et al., 2002; Lecanda et al., 2007). Although the putative role for TGFβ signaling in the pathogenesis of endometrial cancer was proposed more than a decade ago (Gold and Parekh, 1999), the function of TGFβ signaling in endometrial cancer has remained vague in many aspects; robust genetic evidence supporting TGFβ signaling in the etiology of endometrial cancer is lacking. In this study, we observed a uterine pathology resembling endometrial hyperplasia in aged Tgfbr1 Amhr2-Cre cKO mice.These results suggest that TGFBR1 is a potential component of the pathogenic network of endometrial hyperplasia, a risk factor of endometrial carcinoma. Thus, this model might be further exploited to understand the mechanisms of a broad spectrum of uterine conditions that bear the similar cellular and molecular alterations in epithelial cells revealed by the current study.
Conclusion
TGFBR1-mediated signaling is potentially involved in the regulation of uterine epithelial cell proliferation. This study provides genetic evidence supporting the role of uterine epithelial cell proliferation in the pathogenesis of endometrial hyperplasia.
Authors’ roles
Y.G. and S.L. performed the experiments. Y.G. and Q.L. designed the study and analyzed the data. All authors contributed to the manuscript preparation.
Funding
This research is supported by the National Institutes of Health grant (R21HD073756 to Q.L.) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the Ralph E. Powe Junior Faculty Enhancement Awards (to Q.L.) from Oak Ridge Associated Universities, and the New Faculty Start-up Funds (to Q.L.) and College of Veterinary Medicine graduate student research award (to Y.G.) from Texas A&M University. S.L. is partially supported by a fellowship from China Scholarship Council.
Acknowledgements
We thank Dr Martin Matzuk for generous support and help with importing the Tgfbr1 mice and Dr Richard Behringer for offering the Amhr2-Cre mice. We are grateful to Drs Robert Burghardt and Kayla Bayless for helpful discussions.
References
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Bazer FW. Uterine adenogenesis and pregnancy: multiple roles for foxa2 in mice. Biol Reprod. 2010;83:319–321. doi: 10.1095/biolreprod.110.086694. [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Chen L, Belton RJ, Nowak RA. Basigin-mediated gene expression changes in mouse uterine stromal cells during implantation. Endocrinology. 2009;150:966–976. doi: 10.1210/en.2008-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Tranguch S, Friedman DB, Das SK, Smith DF, Dey SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–697. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. SMAD proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod. 2007;76:848–857. doi: 10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nalam RL, Clementi C, Franco HL, Demayo FJ, Lyons KM, Pangas SA, Matzuk MM. Granulosa cell-expressed BMPR1A and BMPR1B have unique functions in regulating fertility but act redundantly to suppress ovarian tumor development. Mol Endocrinol. 2010;24:1251–1266. doi: 10.1210/me.2009-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filant J, Zhou HJ, Spencer TE. Progesterone inhibits uterine gland development in the neonatal mouse uterus. Biol Reprod. 2012;86:1–9. doi: 10.1095/biolreprod.111.097089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25:279–283. doi: 10.1038/77033. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wen H, Wang C, Li Q. SMAD7 antagonizes key TGFβ superfamily signaling in mouse granulosa cells in vitro. Reproduction. 2013;146:1–11. doi: 10.1530/REP-13-0093. [DOI] [PubMed] [Google Scholar]
- Gao Y, Bayless KJ, Li Q. TGFBR1 is required for mouse myometrial development. Mol Endocrinol. 2014;28:380–394. doi: 10.1210/me.2013-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LI, Parekh TV. Loss of growth regulation by transforming growth factor-beta (TGF-beta) in human cancers: studies on endometrial carcinoma. Semin Reprod Endocr. 1999;17:73–92. doi: 10.1055/s-2007-1016214. [DOI] [PubMed] [Google Scholar]
- Gong X, McGee EA. Smad3 is required for normal follicular follicle-stimulating hormone responsiveness in the mouse. Biol Reprod. 2009;81:730–738. doi: 10.1095/biolreprod.108.070086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn HS, Chun YK, Kwon YI, Kim TJ, Lee KH, Shim JU, Mok JE, Lim KT. Concurrent endometrial carcinoma following hysterectomy for atypical endometrial hyperplasia. Eur J Obstet Gyn R B. 2010;150:80–83. doi: 10.1016/j.ejogrb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Moore RK, Shimasaki S. Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci USA. 2005;102:5426–5431. doi: 10.1073/pnas.0409533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83:396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–232. doi: 10.1530/rep.1.01076. [DOI] [PubMed] [Google Scholar]
- Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11:144–161. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lacey JV, Chia VM. Endometrial hyperplasia and the risk of progression to carcinoma. Maturitas. 2009;63:39–44. doi: 10.1016/j.maturitas.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, Leveen P, Xu X, ten Dijke P, Mummery CL, Karlsson S. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J. 2001;20:1663–1673. doi: 10.1093/emboj/20.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecanda J, Parekh TV, Gama P, Lin K, Liarski V, Uretsky S, Mittal K, Gold LI. Transforming growth factor-beta, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Cancer Res. 2007;67:1007–1018. doi: 10.1158/0008-5472.CAN-06-0235. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28:7001–7011. doi: 10.1128/MCB.00732-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Agno JE, Edson MA, Nagaraja AK, Nagashima T, Matzuk MM. Transforming growth factor beta receptor type 1 is essential for female reproductive tract integrity and function. PLoS Genet. 2011a;7:e1002320. doi: 10.1371/journal.pgen.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011b;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zalfran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132:2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth-factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- Middlebrook BS, Eldin K, Li X, Shivasankaran S, Pangas SA. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology. 2009;150:5208–5217. doi: 10.1210/en.2009-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam MR, Soliman PT, Chung LH, Schmeler KM, Bassett RL, Broaddus RR, Lu KH. Loss of phosphatase and tensin homologue deleted on chromosome 10 and phosphorylation of mammalian target of rapamycin are associated with progesterone refractory endometrial hyperplasia. Int J Gynecol Cancer. 2008;18:146–151. doi: 10.1111/j.1525-1438.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- Mills AM, Longacre TA. Endometrial hyperplasia. Semin Diagn Pathol. 2010;27:199–214. doi: 10.1053/j.semdp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–378. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Li Q, Clementi C, Lydon JP, Demayo FJ, Matzuk MM. BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest. 2013;123:2539–2550. doi: 10.1172/JCI65710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Yoshitomi H, Takano S, Shida T, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, et al. FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. Br J Cancer. 2008;99:305–313. doi: 10.1038/sj.bjc.6604473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, et al. Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol. 2008;28:248–257. doi: 10.1128/MCB.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh TV, Gama P, Wen X, Demopoulos R, Munger JS, Carcangiu ML, Reiss M, Gold LI. Transforming growth factor beta signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res. 2002;62:2778–2790. [PubMed] [Google Scholar]
- Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai M-J, Tsai SY. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA. 2007;104:6293–6298. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield MC, Hayashi K, Song G, Black SG, Bazer FW, Spencer TE. Progesterone regulates FGF10, MET, IGFBP1, and IGFBP3 in the endometrium of the ovine uterus. Biol Reprod. 2008;79:1226–1236. doi: 10.1095/biolreprod.108.071787. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shooner C, Caron PL, Frechette-Frigon G, Leblanc V, Dery MC, Asselin E. TGF-beta expression during rat pregnancy and activity on decidual cell survival. Reprod Biol Endocrinol. 2005;3:20. doi: 10.1186/1477-7827-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter J, Wright TC. Prevalence of underlying adenocarcinoma in women with atypical endometrial hyperplasia. Int J Gynecol Pathol. 2005;24:313–318. doi: 10.1097/01.pgp.0000164598.26969.c3. [DOI] [PubMed] [Google Scholar]
- Shynlova OP, Oldenhof AD, Liu MY, Langille L, Lye SJ. Regulation of c-fos expression by static stretch in rat myometrial smooth muscle cells. Am J Obstet Gynecol. 2002;186:1358–1365. doi: 10.1067/mob.2002.122415. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Dunlap KA, Filant J. Comparative developmental biology of the uterus: insights into mechanisms and developmental disruption. Mol Cell Endocrinol. 2012;354:34–53. doi: 10.1016/j.mce.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WR, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/- mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Eitzman B, Bossert NL, Walmer D, Sparrow K, Flanders KC, Mclachlan J, Nelson KG. Transforming growth-factors beta-1, beta-2, and beta-3 messenger-RNA and protein expression in mouse uterus and vagina during estrogen-induced growth—a comparison to other estrogen-regulated genes. Cell Growth Differ. 1994;5:919–935. [PubMed] [Google Scholar]
- Takase HM, Itoh T, Ino S, Wang T, Koji T, Akira S, Takikawa Y, Miyajima A. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Shimizu M, Kusumoto R, Ono K, Noji S, Ohuchi H. A dual role of FGF10 in proliferation and coordinated migration of epithelial leading edge cells during mouse eyelid development. Development. 2005;132:3217–3230. doi: 10.1242/dev.01892. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Carson DD. Mucins and blastocyst attachment. Rev Endocr Metab Disord. 2002;3:87–96. doi: 10.1023/a:1015446626671. [DOI] [PubMed] [Google Scholar]
- Tomic D, Miller KP, Kenny HA, Woodruff TK, Hoyer P, Flaws JA. Ovarian follicle development requires Smad3. Mol Endocrinol. 2004;18:2224–2240. doi: 10.1210/me.2003-0414. [DOI] [PubMed] [Google Scholar]
- Villacorte M, Suzuki K, Hirasawa A, Ohkawa Y, Suyama M, Maruyama T, Aoki D, Ogino Y, Miyagawa S, Terabayashi T, et al. β-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene. 2013;32:3477–3482. doi: 10.1038/onc.2012.376. [DOI] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]