Abstract
Background
The study aim was, for the first time, to conduct a multicenter randomized controlled trial to evaluate the effect of tonsillectomy in patients with IgA nephropathy (IgAN).
Methods
Patients with biopsy-proven IgAN, proteinuria and low serum creatinine were randomly allocated to receive tonsillectomy combined with steroid pulses (Group A; n = 33) or steroid pulses alone (Group B; n = 39). The primary end points were urinary protein excretion and the disappearance of proteinuria and/or hematuria.
Results
During 12 months from baseline, the percentage decrease in urinary protein excretion was significantly larger in Group A than that in Group B (P < 0.05). However, the frequency of the disappearance of proteinuria, hematuria, or both (clinical remission) at 12 months was not statistically different between the groups. Logistic regression analyses revealed the assigned treatment was a significant, independent factor contributing to the disappearance of proteinuria (odds ratio 2.98, 95% CI 1.01–8.83, P = 0.049), but did not identify an independent factor in achieving the disappearance of hematuria or clinical remission.
Conclusions
The results indicate tonsillectomy combined with steroid pulse therapy has no beneficial effect over steroid pulses alone to attenuate hematuria and to increase the incidence of clinical remission. Although the antiproteinuric effect was significantly greater in combined therapy, the difference was marginal, and its impact on the renal functional outcome remains to be clarified.
Keywords: clinical remission, estimated glomerular filtration rate, hematuria, proteinuria
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis worldwide, with primary IgAN characterized by the deposition of IgA antibodies in the glomerulus. The renal outcome of IgAN varies among individual patients [1–4]. Although ∼20% of patients remain stable in their renal function [1], 30–40% develop end-stage renal disease (ESRD) within 20 years from its onset [1–4]. The most important determinant of renal outcome in IgAN is the severity and duration of proteinuria, and a decreased severity in proteinuria is distinctly associated with a better renal outcome [1–10].
A number of studies have shown that corticosteroid therapy is effective for progressive IgAN [11–15]. A randomized controlled trial and its 10-year follow-up examined whether steroid pulse therapy is more effective than conventional therapy for long-term preservation of renal function [14, 15]. The risk of a doubling in serum creatinine after 10 years was found to be significantly lower in the group receiving steroid pulses than in the one receiving supportive therapy alone. However, steroid pulse therapy is associated with several problems. For instance, only a small fraction of the treated patients achieved remission of proteinuria after 1 year [15], and severe proteinuria relapsed in a sizable fraction of the patients after the cessation of treatment [15].
Several studies have examined the therapeutic efficacy of tonsillectomy in IgAN. A retrospective study by Rasche et al. [16] reported that tonsillectomy had no impact on renal outcome 10 years after biopsy. By contrast, in a retrospective study of 329 IgAN patients, Hotta et al. [17] found that tonsillectomy was an independent predictor of the remission of urine abnormalities and a lack of progression in renal injury. Xie et al. [18] followed-up 118 patients for an average of 20 years, and found that renal survival was better in the group with prior tonsillectomy than in the one without tonsillectomy at 240 months. More recently, in a non-randomized prospective study, Komatsu et al. [19] found that tonsillectomy combined with steroid pulse treatment had a significant impact on the disappearance of both proteinuria and hematuria, when compared with steroid pulse treatment alone. A recent meta-analysis has also reported that tonsillectomy combined with either conventional steroid or steroid pulse treatment resulted in higher remission rates with favorable long-term efficacy [20]. Thus, tonsillectomy combined with steroid pulses has become one of the most widely used therapy protocols in the treatment of active IgAN, and is now being performed in ∼50% of the institutions in Japan [21]. However, none of the previous analyses were randomized controlled studies, and there is growing concern that the evidence to date is insufficient for recommending tonsillectomy to IgAN patients [22, 23]. Importantly, the recent Kidney Disease: Improving Global Outcomes clinical guideline for glomerulonephritis suggests that tonsillectomy not be performed for IgAN, because no randomized controlled trial of tonsillectomy has been performed [24].
Here, we report the results of a multicenter, randomized, controlled trial of tonsillectomy combined with steroid pulse therapy in patients with IgAN conducted by the Special IgAN Study Group of the Progressive Glomerular Diseases Study Committee organized by the Ministry of Health, Labour and Welfare of Japan.
MATERIALS AND METHODS
Patients
This multicenter study was conducted between 1 April 2005 and 31 March 2010 in 18 university or community hospitals located in major cities across Japan. The participating institutions routinely performed tonsillectomy combined with steroid pulses to treat IgAN. The study was approved by the local ethics committees and was regulated by an independent data safety and monitoring board.
The inclusion criteria were established primarily according to the previous trial by Pozzi et al. [14, 15], and were biopsy-proven IgAN, an age ranging from 10 to 69 years, urinary protein excretion ranging from 1.0 to 3.5 g/day, serum creatinine of ≤1.5 mg/dL, a histological grade diagnosed as a relatively good prognosis, a relatively poor prognosis, or a poor prognosis in the classification proposed in 2004 [25], and systolic and diastolic blood pressures of <140 and <90 mmHg, respectively, regardless of the use or non-use of antihypertensive drugs. Exclusion criteria were nephrotic syndrome, serum creatinine of >1.5 mg/dL, recent treatment with corticosteroids and/or immunosuppressive agents, and contraindications for general anesthesia and/or tonsillectomy as assessed by otolaryngologists. Informed consent was obtained from individual patients following the confirmation of eligibility.
We estimated the frequency of the disappearance of proteinuria at 12 months after the initiation of the treatment would be 40% in patients treated with tonsillectomy plus steroid pulses [21, 26] and 10% in those with steroid pulses alone [14, 15]. Based on the power of 80% for detecting a significant difference (P < 0.05, two-sided), 38 patients were required for each study group. To compensate for non-evaluable patients, we planned to enroll 40 patients per group.
Randomization and masking
The profiles of patients with informed consent were sent to the registration center located at Jikei University School of Medicine. Randomization was done by a technical assistant in the registration center using a computer-based allocation program with a minimization method, which was developed by an outside company (East Asia Trading Corporation, Hyogo) independent of this study. Immediately after the input of patient information, including the date of enrollment, gender, histological grade, the severity of proteinuria (<2.0 g/day or ≥2.0 g/day), serum creatinine (male, <1.2 mg/dL or ≥1.2 mg/dL; female, <0.9 mg/dL or ≥0.9 mg/dL) and the use or non-use of renin-angiotensin system (RAS) inhibitors, the participants were randomly assigned to receiving tonsillectomy combined with steroid pulses (Group A) or steroid pulses alone (Group B). Since the allocation was based on the presence or absence of tonsillectomy, neither the patients nor the physicians were blinded to the group assignment. Although those assessing the outcomes were not blinded, they assessed the data regarding the pre-defined outcomes using pre-specified statistical analyses.
Study protocol
After the random allocation to Group A or Group B, the center sent the enrollment certificate with the results of randomization to the participating institutions. The patients assigned to Group A underwent tonsillectomy and subsequently received 0.5 g/day of methylprednisolone intravenously for 3 consecutive days at 1–3 weeks later and then at 2 and 4 months later. The patients were also given oral prednisolone at a dose of 0.5 mg/kg every other day for 6 months. The patients assigned to Group B received only the steroid pulse therapy, and were also given oral prednisolone in a manner identical to that in Group A. The protocol of steroid pulse therapy was essentially the same as the one in the trial by Pozzi et al. [14, 15], with the exception that a half dose of intravenous methylprednisolone was provided in the current study. The entire trial period (treatment + follow-up) was 12 months. If needed, the patients in both groups were given antihypertensive drugs to control blood pressure to <125/75 mmHg during the trial period. RAS inhibitors were the primary antihypertensive drugs recommended during the study.
Data collection
During the trial, the patients were examined every other month for blood pressure, urinary protein excretion, serum creatinine and urinary sediment with red blood cells. Other data including the incidence of adverse effects and the prescription of additional drugs were also obtained. Urinary protein was measured primarily by the Pyrogaroll Red method. Urinary protein at baseline was represented by a mean value from three consecutive data points prior to the treatment (i.e. tonsillectomy in Group A, first steroid pulses in Group B). The disappearance of proteinuria was defined as urinary protein excretion of <0.3 g/g creatinine in a 24-h urine collection or in urine samples at visits as described previously [15, 27], although the distinct value for disappearance of proteinuria had not been specified at pre-registration. Urinary creatinine concentration was not available in three patients (one patient, Group A; two, Group B). In these patients, the disappearance of proteinuria was defined as levels of <0.3 g/day. Disappearance of hematuria was defined as a number of red blood cells in urinary sediments of <5 per high power field. Clinical remission was defined as the disappearance of both proteinuria and hematuria. eGFR was computed using the following equation [28] developed as a modification of the modified MDRD equation [29]: eGFR (mL/min/1.73 m2) = 194 ×(serum creatinine in mg/dL)−1.094 × (age in years)−0.287 (×0.739 if female).
Outcome definitions
The primary end points were the percentage decrease in urinary protein excretion from baseline and the frequency of the disappearance of proteinuria and/or hematuria 12 months after the initial treatment. The secondary end points were a change in eGFR from baseline, the frequencies of a 100% increase in serum creatinine from baseline, a 50% decrease in eGFR from baseline, indications for renal replacement therapy and adverse effects.
Statistical analysis
Data were subjected to intention-to-treat analysis. All patients, physicians and those assessing the outcomes were not blinded to group assignment. Stata version 11 for Windows (StataCorp LP, College station, TX, USA) was used for the analysis. The percent reduction of proteinuria from baseline was compared between Groups A and B by analyzing the values from six fixed time points (2, 4, 6, 8, 10 and 12 months after randomization) using a mixed effects model. We included as fixed effects group allocation, time, baseline eGFR, mean arterial pressure and the use of RAS inhibitors at baseline. Time was coded as months after the randomization and was given the values of 0, 2, 4, 6, 8, 10 and 12. The patients were included as a random effect. For comparing the parameters between the two groups, the unpaired t-test and non-parametric Wilcoxon rank-sum test were used for normally and non-normally distributed variables, respectively. The difference in frequency between the two groups was evaluated using Pearson's chi-square test. Logistic regression analysis was used to evaluate the impact of tonsillectomy, eGFR, mean arterial pressure, urinary protein excretion and the use of RAS inhibitors at baseline on the disappearance of proteinuria, hematuria or both after adjusting for the other covariates. The results were presented as odds ratios with 95% confidence intervals and P-values; P < 0.05 was considered statistically significance in all analyses.
RESULTS
Characteristics of the study subjects
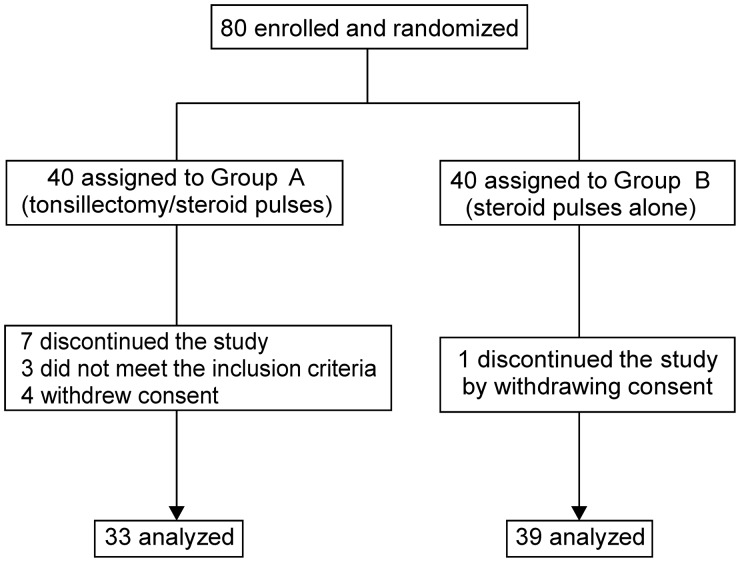
Eighty eligible patients were enrolled and randomly allocated to receive tonsillectomy with steroid pulses (Group A) or steroid pulses alone (Group B) (Figure 1). In Group A, three and four patients failed to meet the inclusion criteria and withdrew consent, respectively. In Group B, one patient withdrew consent. One patient in Group A did not undergo tonsillectomy after randomization but was analyzed within this group according to the policy of intention-to-treat analysis. Likewise, two patients in Group B who underwent tonsillectomy after randomization were analyzed as part of Group B. We therefore analyzed 33 and 39 patients in Groups A and B, respectively. The two groups did not differ in age, gender distribution, estimated glomerular eGFR, urinary protein excretion, blood pressure, the proportion of patients given RAS inhibitors or histological grades (Table 1).
FIGURE 1:
Trial profile.
Table 1.
Baseline patient characteristics
| Group A Tonsillectomy/steroid pulse therapy (n = 33) |
Group B Steroid pulse therapy alone (n = 39) |
|
|---|---|---|
| Age (years) | 36 (13) | 40 (13) |
| Gender | ||
| Male | 17* (52) | 18* (46) |
| Female | 16* (48) | 21* (54) |
| eGFR (mL/min/1.73 m2) | 75 (24) | 69 (22) |
| Proteinuria (g/day) | 1.6 (0.5) | 1.6 (0.6) |
| Proteinuria (g/g creatinine) | 1.7 (1.0) | 1.7 (1.0) |
| Systolic blood pressure (mmHg) | 117 (12) | 121 (10) |
| Diastolic blood pressure (mmHg) | 69 (9) | 73 (8) |
| Mean arterial pressure (mmHg) | 85 (9) | 89 (8) |
| Patients receiving RASi (%) | 16* (48) | 18* (46) |
| Histological grade | ||
| Good prognosis | 0* | 0* |
| Relatively good prognosis | 2* (6) | 3* (8) |
| Relatively poor prognosis | 20* (61) | 23* (59) |
| Poor prognosis | 11* (33) | 13* (33) |
Data are mean (SD) or *number of patients (%). Histological grade was assessed by the classification proposed by the Special IgAN Study Group in 2004 [30].
eGFR, estimated glomerular filtration rate; RASi, renin-angiotensin system inhibitors.
Impact of steroid pulses and tonsillectomy on proteinuria
Figure 2 shows the percent changes in urinary protein excretion from baseline during the trial period. As revealed by a mixed effect model employing six fixed effects (group allocation, eGFR, mean arterial pressure, the use of RAS inhibitors at baseline, time and the interaction of group and time; Supplementary Table S1), the percentage decrease in urinary protein excretion during the 12 months from baseline was significantly larger in Group A than that in Group B (coefficient estimate −1.316, 95% CI −2.617 to −0.015, P = 0.047).
FIGURE 2:
Urinary protein excretion during the trial period. Mean values and standard errors are presented. The rate of decrease in urinary protein excretion was significantly higher in Group A than in Group B using a mixed effect model. The numbers of patients analyzed at each time point are shown below the figure for each group.
The percentage of patients with the disappearance of proteinuria (<0.3 g/gCr) was significantly higher in Group A than in Group B after 10 months (P = 0.029; Figure 3). However, at 12 months, the difference was not statistically significant (Group A, 63%; Group B, 39%; P = 0.052).
FIGURE 3:
Patient distribution of the severity of proteinuria during the trial period. The severity of proteinuria was divided into the four grades shown below the figure according to the level of urinary protein (UP) in g/g creatinine (Cr). The patient distribution in the four grades is shown as a percentage. *The rate of the disappearance of proteinuria (UP level of <0.3 g/g Cr) was significantly higher in Group A than in Group B (Pearson's chi-square test).
Impact of steroid pulses and tonsillectomy on hematuria
The severity of microscopic hematuria gradually decreased following the initiation of therapy in both groups (Figure 4). However, the proportion of patients with the disappearance of hematuria was not different between the two groups at any time point (e.g. at 12 months, Group A, 68%; Group B, 64%, P = 0.672).
FIGURE 4:
Patient distribution of the severity of hematuria during the trial period. The severity of hematuria was divided into the five grades according to the number of red blood cells per high power field (HPF). The patient distribution in the five grades is shown as a percentage. The rate of the disappearance of hematuria, defined as the number of red blood cells <5/HPF, was not different between both groups at any time point (Pearson's chi-square test).
Impact of steroid pulses and tonsillectomy on clinical remission
The disappearance of both proteinuria and hematuria (i.e. clinical remission) did not occur at a higher rate in Group A than in Group B at any time point (P = 0.160 at 10 months, P = 0.103 at 12 months; Figure 5).
FIGURE 5:
Frequency of clinical remission during the trial period. The frequency of patients with clinical remission (i.e. the disappearance of both proteinuria and hematuria) is shown for each time point. The frequency was not significantly higher in Group A than Group B at any time point (Pearson's chi-square test).
Impact of steroid pulse and tonsillectomy on renal functions
eGFR remained stable throughout the trial period and was comparable between the two groups at 12 months (Group A, 75 mL/min/1.73 m2; Group B, 69 mL/min/1.73 m2; Figure 6). No patient in either group showed a 100% increase in serum creatinine from baseline or a 50% decrease in eGFR from baseline, or had indications for renal replacement therapy. No adverse effect related to tonsillectomy or general anesthesia was reported. One patient in Group A and three in Group B developed diabetes during the trial period, with one of these Group B patients requiring insulin therapy during the treatment with corticosteroid. At the end of the study, blood sugar levels of all four patients were restored to the normal range without any medications. No patient had a new onset of hypertension.
FIGURE 6:
Renal function during the trial period. Mean values and standard errors of the estimated glomerular filtration rate (eGFR) are shown. The value of eGFR remained stable in both groups.
Logistic regression analysis
Logistic regression analysis was performed to evaluate the impact of multiple covariates on the disappearance of proteinuria or hematuria and the occurrence of clinical remission. Independent variables included the allocated treatment, eGFR, mean blood pressure, urinary protein excretion and the use of RAS inhibitors at baseline (Table 2). Only the allocated treatment had a significant and independent impact on the disappearance of proteinuria (hazard ratio, 2.98; 95% confidence interval, 1.01–8.83; P = 0.049). No independent factors were identified as achieving the disappearance of hematuria or clinical remission.
Table 2.
Logistic regression analysis of the impact of tonsillectomy, renal function, blood pressure and urinary protein excretion at baseline and after disappearance of proteinuria, hematuria or both at study completion
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Disappearance of proteinuria | |||
| Assigned treatment | 2.98 | 1.01–8.83 | 0.049 |
| eGFR (baseline) | 0.99 | 0.97–1.02 | 0.560 |
| Mean blood pressure (baseline) | 1.04 | 0.97–1.11 | 0.297 |
| Proteinuria (baseline) | 0.61 | 0.33–1.13 | 0.115 |
| RASi (baseline) | 0.51 | 0.16–1.68 | 0.270 |
| Disappearance of hematuria | |||
| Assigned treatment | 1.23 | 0.43–3.55 | 0.697 |
| eGFR (baseline) | 0.99 | 0.97–1.01 | 0.304 |
| Mean blood pressure (baseline) | 0.97 | 0.91–1.04 | 0.450 |
| Proteinuria (baseline) | 0.91 | 0.54–1.54 | 0.737 |
| RASi (baseline) | 0.95 | 0.29–3.13 | 0.930 |
| Clinical remission | |||
| Assigned treatment | 2.24 | 0.77–6.51 | 0.140 |
| eGFR (baseline) | 0.99 | 0.97–1.02 | 0.554 |
| Mean blood pressure (baseline) | 1.01 | 0.94–1.08 | 0.858 |
| Proteinuria (baseline) | 0.75 | 0.41–1.38 | 0.348 |
| RASi (baseline) | 0.63 | 0.19–2.06 | 0.445 |
Logistic regression analysis was used to determine the association of assigned treatment, eGFR, mean blood pressure or urinary protein excretion at baseline with the disappearance of proteinuria, hematuria or both (clinical remission) after 12 months of treatment with tonsillectomy plus steroid pulse therapy or steroid pulse therapy alone after adjusting for the other covariates.
CI, confidence interval; eGFR, estimated glomerular filtration rate; RASi, renin-angiotensin system inhibitors.
Use of RASi during the trial
RAS inhibitors were started after the initiation of treatment in three patients in Group A (losartan 50 mg, telmisartan 40 mg or valsartan 80 mg) and four patients in Group B (aliskiren 150 mg, losartan 50 mg, olmesartan 10 mg or valsartan 80 mg). The disappearance of proteinuria was achieved in two of these patients from each group after 12 months. No patient started other antihypertensive drugs during the period.
DISCUSSION
For the first time, we performed a multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with IgA nephropathy. The findings of the present study indicated that the decrease in urinary protein excretion during follow-up was significantly greater, albeit marginally, in patients receiving tonsillectomy combined with steroid pulse therapy than in those receiving steroid pulse therapy alone, as shown by a mixed effect model and logistic regression analysis. However, 12 months after the initial treatment, the frequency of the disappearance of microscopic hematuria and clinical remission was comparable between the two groups. Thus, we conclude that tonsillectomy has no impact on the disappearance of hematuria, but can have a beneficial effect on the decrease in proteinuria of IgAN patients, at least for those clinically comparable to the present patients. However, whether this subtle antiproteinuric effect by tonsillectomy indeed leads to better renal outcome remains to be elucidated.
Our patients had urinary protein excretion ranging from 1.0 to 3.5 g/day, and most patients showed moderate to severe histological damage (i.e. relatively poor prognosis or poor prognosis; Table 1), indicating that the present study excluded patients with mild IgAN. In view of the possible effectiveness of steroid pulses alone, as revealed in the present and previous studies [14, 15], a question remains as to whether the advantage of tonsillectomy seen in the present study is relevant to patients with milder IgAN than those in the present patients. Moreover, based on the randomized controlled trial by Pozzi et al. [15] demonstrating an ∼10% incidence in the disappearance of proteinuria following steroid pulses, it can be speculated that only a few patients with advanced IgAN, such as those with serum creatinine of >1.5 mg/dL, can achieve the disappearance of proteinuria following steroid pulses alone. In this regard, tonsillectomy combined with steroid pulse therapy can be more effective in patients with advanced IgAN, as suggested by a previous report [30], which found that renal outcome was better with tonsillectomy plus steroid pulses in IgAN patients, particularly in patients with serum creatinine of 1.5–2.0 mg/dL. Further studies are necessary to clarify the profiles of IgAN patients suited for treatment with tonsillectomy plus steroid pulses.
This study had several limitations. First, the follow-up period was too short to be able to assess several long-term outcomes, i.e. renal function, incidence of relapse/recurrence of proteinuria, frequency of patients who need additional therapies, etc. Indeed, none of the patients were found to reach the end points. In this regard, the secondary end points established in this trial appeared inadequate in view of a short follow-up period. The primary end points used in this study (e.g. the disappearance of proteinuria and/or hematuria after 12 months) were surrogate markers, since the real hard end points should have represented long-term renal survival, such as the progression of renal disease or the development of ESRD. Nevertheless, many previous studies indicate that a marked reduction of proteinuria as an early response to the initial treatment ensures stable renal function after the cessation of treatment [14, 15, 17, 31, 32]. In addition to those studies that examined the relationship between the level of proteinuria after 12 months and the final renal outcome, Hirano et al. recently reported that, in the IgAN patients receiving 6 months of steroid therapy (Pozzi's protocol), the achievement of proteinuria <0.4 g/day after 12 months could be a therapeutic indicator for a favorable renal outcome [27]. Therefore, a superior antiproteinuric effect of tonsillectomy plus steroid pulses compared with steroid pulses alone could lead to better preservation of renal function in the long-term. Since it is crucially important whether tonsillectomy can protect IgAN patients from the progressive deterioration of renal function or the relapse/recurrence of proteinuria during a long-term follow-up, we are now in the process of a study to follow-up the present patients for 3 years.
Second, the incidence of the disappearance of proteinuria and/or hematuria after 12 months was not significantly different between the two groups. In our study, the disappearance of proteinuria with steroid pulses alone was more frequent than that extrapolated from the results of the previous reports [14, 15, 26]. This unexpectedly high incidence may have resulted in the failure to find statistical difference between the two groups. More patients should be included for a more definitive conclusion. Third, the pattern in the decrease of urinary protein excretion could not be analyzed using repeated ANOVA, because the data available at some time points were insufficient for analysis. Fourth, a few of the enrolled patients had to be excluded from the analysis, which may have reduced the effectiveness of randomization. Nevertheless, all the parameters at baseline were comparable between the two groups. This notion is supported by the results of the logistic regression analysis. Fifth, RAS inhibitors were administered only in nearly half of the patients in both groups at baseline. Therefore, some patients could show proteinuria <1 g/day at baseline, if all the patients were given RAS inhibitors prior to the trial. The mixed effect model revealed that a significantly greater antiproteinuric effect of tonsillectomy plus steroid pulses was independent from the use of RAS inhibitors at baseline. Nevertheless, the differential use of RAS inhibitors by different investigators could have potentially biased the results. Moreover, the impact of RAS inhibitors on patients who started RAS inhibitors during the trial was not clear. Finally, the study lacks sufficient information for the removed tonsil, such as, the frequency of presence of tonsils with infection as assessed by the presence of crypt abscesses or bacterial colonies in tonsillar tissues by macroscopic or microscopic inspection. It has been reported that the efficacy of tonsillectomy is difficult to predict on the basis of the appearance of tonsils or clinical episodes of recurrent tonsillitis [33]; thus, the relationship between the condition of removed tonsils and the outcome of proteinuria remains elusive.
In conclusion, tonsillectomy combined with steroid pulses had no additional benefit over steroid pulses alone in the disappearance of hematuria or the achievement of clinical remission. Although the antiproteinuric effect was significantly larger in the treatment with tonsillectomy plus steroid pulses than in steroid pulses alone, the difference was very subtle. Whether this marginal antiproteinuric effect improves renal outcome remains to be clarified.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
(See related article by Zand and Fervenza. Does tonsillectomy have a role in the treatment of patients with immunoglobulin A nephropathy? Nephrol Dial Transplant 2014; 29: 1456–1459.)
Supplementary Material
ACKNOWLEDGEMENTS
The study is registered with UMIN Clinical Trial Registry (registration number, C000000384), and was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research (Research on Intractable Disease) from the Ministry of Health, Labour and Welfare of Japan. We thank the study participants and the clinicians for their day-to-day clinical care. We thank Dr Homma for his valuable advice during manuscript preparation.
REFERENCES
- 1.Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis. 1997;29:526–532. doi: 10.1016/s0272-6386(97)90333-4. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico G. Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls KM, Fairley KF, Dowling JP, et al. The clinical course of mesangial IgA associated nephropathy in adults. Q J Med. 1984;53:227–250. [PubMed] [Google Scholar]
- 4.Beukhof JR, Kardaun O, Schaafsma W, et al. Toward individual prognosis of IgA nephropathy. Kindey Int. 1986;29:549–556. doi: 10.1038/ki.1986.33. [DOI] [PubMed] [Google Scholar]
- 5.Alamartine E, Sabatier JC, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: An extensive study with univariate and multivariate analysis. Am J Kidney Dis. 1991;18:12–19. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 6.Donadio JV, Bergstralh EJ, Offord KP, et al. Clinical and histopathological associations with impaired renal function in IgA nephropathy. Mayo Nephrology Collaborative Group. Clin Nephrol. 1994;41:65–71. [PubMed] [Google Scholar]
- 7.Bartosik LP, Lajoie G, Sugar L, et al. Predicting progression in IgA nephropathy. Am J Kidney Dis. 2001;38:728–735. doi: 10.1053/ajkd.2001.27689. [DOI] [PubMed] [Google Scholar]
- 8.Syrjänen J, Mustonen J, Pasternack A. Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant. 2000;15:34–42. doi: 10.1093/ndt/15.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Li PK, Ho KK, Szeto CC, et al. Prognostic indicators of IgA nephropathy in the Chinese—clinical and pathological perspectives. Nephrol Dial Transplant. 2002;17:64–69. doi: 10.1093/ndt/17.1.64. [DOI] [PubMed] [Google Scholar]
- 10.Daniel L, Saingra Y, Giorgi R, et al. Tubular lesions determine prognosis of IgA nephropathy. Am J Kidney Dis. 2000;35:13–20. doi: 10.1016/S0272-6386(00)70295-2. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Hiki Y, Kokubo T, et al. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–242. doi: 10.1159/000188848. [DOI] [PubMed] [Google Scholar]
- 12.Julian B, Barker C. Alternate-day prednisone therapy in IgA nephropathy: preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol. 1993;104:198–206. [PubMed] [Google Scholar]
- 13.Lai KN, Lai FM, Ho CP, et al. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long-term controlled trial. Clin Nephrol. 1986;26:174–180. [PubMed] [Google Scholar]
- 14.Pozzi C, Bolasco PG, Fogazzi GB, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet. 1999;353:883–887. doi: 10.1016/s0140-6736(98)03563-6. [DOI] [PubMed] [Google Scholar]
- 15.Pozzi C, Andrulli S, Del Vecchio L, et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 16.Rasche FM, Schwarz A, Keller F. Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol. 1999;51:147–152. [PubMed] [Google Scholar]
- 17.Hotta O, Miyazaki M, Furuta T, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. Am J Kidney Dis. 2001;38:736–743. doi: 10.1053/ajkd.2001.27690. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Nishi S, Ueno M, et al. The efficacy of tonsillectomy on long-term renal survival in patients with IgA nephropathy. Kidney Int. 2003;63:1861–1867. doi: 10.1046/j.1523-1755.2003.00935.x. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu H, Fujimoto S, Hara S, et al. Effect of tonsillectomy plus steroid pulse therapy on clinical remission of IgA nephropathy: a controlled study. Clin J Am Soc Nephrol. 2008;3:1301–1307. doi: 10.2215/CJN.00310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Chen J, Wang Y, et al. A meta-analysis of the clinical remission rate and long-term efficacy of tonsillectomy in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:1923–1931. doi: 10.1093/ndt/gfq674. [DOI] [PubMed] [Google Scholar]
- 21.Miura N, Imai H, Kikuchi S, et al. Tonsillectomy and steroid pulse (TSP) therapy for patients with IgA nephropathy: a nationwide survey of TSP therapy in Japan and an analysis of the predictive factors for resistance to TSP therapy. Clin Exp Nephrol. 2009;13:460–466. doi: 10.1007/s10157-009-0179-1. [DOI] [PubMed] [Google Scholar]
- 22.Appel GB, Waldman M. The IgA nephropathy treatment dilemma. Kidney Int. 2006;69:1939–1944. doi: 10.1038/sj.ki.5000434. [DOI] [PubMed] [Google Scholar]
- 23.Locatelli F, Vecchio LD, Pozzi C. IgA glomerulonephritis: beyond angiotensin-converting enzyme inhibitors. Nat Clin Pract Nephrol. 2006;2:24–31. doi: 10.1038/ncpneph0055. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO Clinical Practice Guideline for Glomerulonephritis. Chapter 10: Immunoglobulin A nephropathy. Kidney Int Suppl. 2012;2:209–217. doi: 10.1038/kisup.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomino Y, Sakai H. Clinical guidelines for immunoglobulin A (IgA) nephropathy in Japan, second version. Clin Exp Nephrol. 2003;7:93–97. doi: 10.1007/s10157-003-0232-4. [DOI] [PubMed] [Google Scholar]
- 26.Hotta O. Use of corticosteroids, other immunosuppressive therapies, and tonsillectomy in the treatment of IgA nephropathy. Semin Nephrol. 2004;24:244–255. doi: 10.1016/j.semnephrol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hirano K, Kawamura T, Tsuboi N, et al. The predictive value of attenuated proteinuria at 1 year after steroid therapy for renal survival in patients with IgA nephropathy. Clin Exp Nephrol. 2012;17:555–562. doi: 10.1007/s10157-012-0744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 30.Sato M, Hotta O, Tomioka S, et al. Cohort study of advanced IgA nephropathy: efficacy and limitations of corticosteroids with tonsillectomy. Nephron Clin Pract. 2003;93:c137–c145. doi: 10.1159/000070233. [DOI] [PubMed] [Google Scholar]
- 31.Reich HN, Troyanov S, Scholey JW, et al. Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 32.Tatematsu M, Yasuda Y, Morita Y, et al. Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clin Exp Nephrol. 2012;16:883–891. doi: 10.1007/s10157-012-0644-0. [DOI] [PubMed] [Google Scholar]
- 33.Matutani S, Honma R, Adachi M, et al. Clinical observation of palatine tonsils with IgA nephropathy. Acta Otolaryngol Suppl. 2004;555:58–61. doi: 10.1080/036555230410003279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.