Abstract
Background
High-density lipoprotein (HDL) confers protection against atherosclerosis by several different mechanisms. Although in the general population, increasing levels of HDL are associated with reduced cardiovascular (CV) mortality, this association is not well known in patients with chronic disease states such as end-stage renal disease. We hypothesize that the association of serum HDL concentration and its ratio to total cholesterol with all-cause and CV mortality in hemodialysis patients is different from the general population.
Methods
A 3-year (July 2004 to June 2007) cohort of 33 109 chronic hemodialysis patients was studied in the USA in the dialysis clinics where lipid profile was measured in at least 50% of all outpatients of the clinic during a given calendar quarter. Cox proportional hazard models were adjusted for demographics and case–mix variables and cubic splines were plotted.
Results
Higher HDL concentrations up to 50 mg/dL were associated with better overall survival, while HDL at 60 mg/dL and above was associated with a rise in all-cause and CV mortality. All-cause and CV mortality hazard ratio was 1.28 (1.20–1.38) and 1.08 (1.01–1.16) for HDL <30 mg/dL and 1.05 (1.00–1.10) and 1.08 (1.00–1.16) for HDL ≥ 60 mg/dL, respectively (reference: HDL: 30–<60 mg/dL).
Conclusions
In contrast to the general population, low total cholesterol to HDL ratio was associated with higher mortality in hemodialysis patients. A U-shaped association between HDL cholesterol level and all-cause and CV mortality exists in hemodialysis patients with HDL between 50 and <60 mg/dL exhibiting the best survival. The underlying mechanisms responsible for these seemingly paradoxical associations await further investigation.
Keywords: cardiovascular disease, end-stage renal disease, hemodialysis, high-density lipoprotein, mortality
INTRODUCTION
End-stage renal disease (ESRD) is associated with accelerated atherosclerosis and a marked increase in cardiovascular (CV) mortality [1–4]. Several factors are involved in the pathogenesis of atherosclerosis and CV disease in chronic kidney disease (CKD). These include oxidative stress, inflammation, hypertension, endothelial dysfunction, vascular calcification and dyslipidemia [5–9]. Monocyte adhesion, infiltration and differentiation into macrophages and their ultimate conversion to foam cells are the primary steps in plaque formation [10, 11]. Foam cell formation is the result of increased uptake of oxidized or otherwise modified low-density lipoprotein (LDL) and remnant lipoproteins by macrophages in the artery wall [12–14]. High-density lipoprotein (HDL) protects against plaque formation and progression via reverse cholesterol transport (RCT) and prevention or reversal of LDL oxidation [15–20]. Therefore, it is not surprising that in the general population, increasing HDL cholesterol concentrations are associated with decreased atherosclerosis burden and reduced CV mortality [21–24]. In addition to its role in RCT, HDL serves as a potent antioxidant, anti-inflammatory and antithrombotic factor [15–20]. Indeed, recent studies have found that HDL function and activity are equally important in preventing CV disease and predicting CV mortality [15–20].
Plasma apolipoprotein A-I, the principal apolipoprotein constituent of HDL and HDL cholesterol content are commonly reduced in ESRD [25–29]. The reduction in plasma HDL can clearly lead to increased cholesterol influx and diminished RCT and thereby promote atherosclerosis. The available data on the role of plasma HDL concentration in predicting CV mortality in patients with ESRD is limited [30–32]. The present study was therefore designed to determine the relationship between plasma HDL concentration and CV and overall mortality in the ESRD patients maintained on hemodialysis. To this end, we examined a large national database of maintenance hemodialysis (MHD) patients of the contemporary origin with somewhat uniform practice patterns and with highly standardized laboratory values that were all measured in a single laboratory.
MATERIALS AND METHODS
Study population
We extracted, refined and analyzed data from all individuals with CKD Stage 5 who underwent hemodialysis treatments from July 2004 to June 2007 in one of the DaVita Inc. outpatient dialysis facilities. The study was approved by the Institutional Review Committees of the Los Angeles Biomedical Research Institute at Harbor-University of California, Los Angeles and DaVita Clinical Research. Given the large sample size, anonymity of the patients studied and non-intrusive nature of the research, the requirement for written consent was exempted.
The first (baseline) studied quarter for each patient was the calendar quarter in which the patient's dialysis vintage was ≥90 days and HDL data were available. Patients who were ≥18 years old, received hemodialysis in the baseline quarter and had available HDL data were included in the study.
Race/ethnicity, other demographic and comorbidity measures
Creation of the cohort has previously been described [33, 34]. Information on race/ethnicity, primary insurance, marital status and the presence of diabetes at baseline were obtained from the DaVita database. In the DaVita database, race/ethnicity is self-categorized and patients select the race and/or ethnicity with which they most closely identified according to the US Census Bureau [1].
To minimize measurement variability, all repeated laboratory and clinical measurements for each patient during any given 13-week calendar quarter interval were averaged to create a summary estimate that was used in all models. Values were obtained for the baseline quarter (from 01 July 2004 through 30 June 2006) for each patient, and patients' outcomes were followed for four additional quarters up to 30 June 2007. Dialysis vintage was defined as the duration of time between the first day of dialysis treatment and the day that the patient entered the cohort study. In addition to laboratory values, posthemodialysis dry weight and baseline height were used to calculate body mass index (BMI). Data on baseline comorbidities, active tobacco smoking, drug and alcohol dependence (current or within the past 10 years) were obtained by linking the DaVita database to data from the US Renal Data System (USRDS) Medical Evidence Form 2728. Baseline comorbidities include and were categorized into 11 conditions: atherosclerotic heart disease (AHD), cardiac failure, hypertension, cerebrovascular disease, other cardiac disease, peripheral vascular disease (PVD), chronic obstructive pulmonary disease, malignancy, HIV, AIDS and non-ambulatory state.
Laboratory measures
Blood samples were drawn using standardized techniques in all DaVita dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida typically within 24 h. All laboratory values were measured using automated and standardized methods in the DaVita laboratory. Most laboratory parameters were measured monthly, including urea nitrogen, albumin, creatinine, total iron-binding capacity (TIBC), bicarbonate, phosphorous and calcium. Serum ferritin and intact parathyroid hormone were measured at least quarterly. Lipid panels were measured quarterly to annually in select dialysis facilities and in at least 50% of all outpatients of the clinic during a given calendar quarter. Hemoglobin was measured at least monthly in all patients. The normalized protein catabolic rate was measured monthly as an indicator of daily protein intake. Most blood samples were collected before dialysis, except for postdialysis serum urea nitrogen to calculate urea kinetics.
Outcome ascertainment
The primary and secondary outcomes of interest were time to all-cause death and time to CV death, respectively, which were ascertained from the DaVita database as well as through linkage to the USRDS database. CV death data were obtained by identifying primary cause of death from the USRDS. For mortality analyses, patients remained at-risk until death, censoring for renal transplantation or end of the study period (30 June 2007).
Statistical methods
Using cox proportional-hazards regression models with fractional polynomials, we examined the relation of all-cause or CV mortality to continuous baseline HDL or baseline cholesterol/HDL ratio. For each analysis, two models were examined based on the level of multivariable adjustment: (i) a minimally adjusted model that included mortality as the outcome, HDL (in either continuous or ordinal format), and entry calendar quarter as covariates and (ii) case–mix adjusted models that included all of the above plus age, gender, race, diabetes mellitus, dialysis vintage, primary insurance, marital status, dialysis dose and residual renal function during the baseline quarter, the 11 baseline comorbid conditions, active tobacco smoking, drug and alcohol dependence.
Missing covariate data (less than 1% for most laboratory and demographic variables) were imputed by multiple imputation methods. Plots of log [−log (survival rate)] against log (survival time) were used to check the proportionality assumption. Most analyses were carried out with SAS version 9.1 (SAS Institute, Inc., Cary, NC). Fractional polynomial graphs were carried out using Stata version 10.1 (Stata Corporation, College Station, TX).
RESULTS
General data
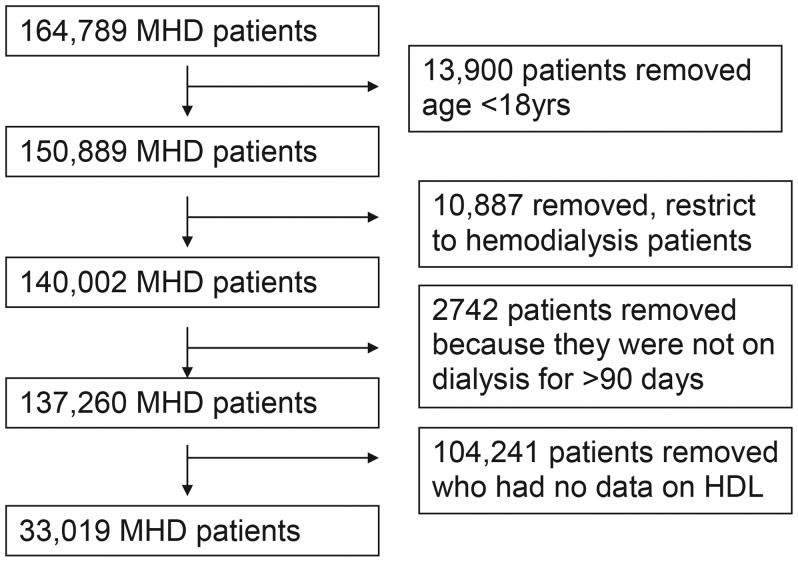
The original 3-year national database of all DaVita dialysis patients included 137 620 hemodialysis patients, aged 18 years or older and had a vintage ≥90 days. After excluding patients with no HDL information, the final study population consists of 33 109 patients (Figure 1).
FIGURE 1:
Selection process of the study population.
Tables 1 and 2 show baseline demographic, clinical and laboratory characteristics of the main cohort as well as the subset of patients with total and HDL serum cholesterol measurements. As expected, MHD patients have a higher incidence of low HDL cholesterol (HDL < 40 mg/dL) when compared with the general population (34% versus 26.5%) [35]. However, surprisingly there are a substantial number of MHD patients whose serum HDL cholesterol concentration is at and beyond 60 mg/dL. The majority of patients with HDL cholesterol at and beyond 60 mg/dL were female and of African-American racial background. Furthermore, they had a slightly lower incidence of diabetes mellitus. On laboratory evaluation, patients in the highest range of HDL cholesterol also had the highest level of total cholesterol and lowest level of triglycerides.
Table 1.
Demographic and clinical characteristics of 137 260 MHD patients, including 33 109 MHD patients who had available HDL measurements
| HDL (mg/dL) |
||||||
|---|---|---|---|---|---|---|
| Characteristics | All MHD patients | HDL measured | <30 | 30–<60 | 60+ | |
| n = 137 260 | n = 33 109 | n = 2354 | n = 24 376 | n = 6289 | REG | |
| 61 ± 15 | 60 ± 15 | 58 ± 15 | 60 ± 15 | 62 ± 15 | <0.0001 | |
| Age (years) | ||||||
| Gender (% women) | 45 | 45 | 24 | 43 | 64 | <0.0001 |
| Diabetes mellitus (%) | 57 | 61 | 64 | 61 | 57 | <0.0001 |
| Race (%) | ||||||
| White | 43 | 41 | 54 | 42 | 30 | <0.0001 |
| African American | 32 | 35 | 19 | 32 | 49 | <0.0001 |
| Hispanic | 14 | 17 | 19 | 17 | 13 | <0.0001 |
| Asian | 3 | 2 | 2 | 2 | 3 | 0.0238 |
| Other | 8 | 5 | 6 | 5 | 5 | 0.1823 |
| Primary insurance (%) | ||||||
| Medicare | 68 | 68 | 67 | 68 | 70 | 0.0023 |
| Medicaid | 6 | 6 | 7 | 6 | 6 | 0.5648 |
| Private insurance | 10 | 8 | 8 | 8 | 7 | 0.1989 |
| Other | 16 | 18 | 19 | 19 | 17 | 0.0146 |
| Vintage (time on dialysis) (%) | ||||||
| 3–<6 months | 52 | 64 | 67 | 65 | 64 | 0.0052 |
| 6–<24 months | 19 | 14 | 12 | 14 | 14 | 0.1691 |
| 2–<5 years | 18 | 13 | 14 | 13 | 14 | 0.7697 |
| ≥5 years | 11 | 8 | 8 | 8 | 9 | 0.0062 |
| Kt/V (dialysis dose) | 1.56 ± 0.36 | 1.57 ± 0.35 | 1.49 ± .35 | 1.56 ± 0.35 | 1.62 ± 0.36 | <0.0001 |
| KRU (residual renal function) (mL/min) | 1.34 ± 2.24 | 1.31 ± 2.16 | 1.37 ± 2.11 | 1.32 ± 2.19 | 1.24 ± 2.05 | 0.1314 |
| Comoridities (%) | ||||||
| AIDS | 1 | 1 | 2 | 1 | 1 | 0.0037 |
| HIV positive status | 2 | 2 | 3 | 1 | 1 | 0.0155 |
| Malignant neoplasm, cancer | 5 | 4 | 5 | 4 | 4 | 0.2947 |
| History of hypertension | 79 | 82 | 80 | 82 | 82 | 0.2059 |
| Congestive heart failure | 27 | 28 | 30 | 28 | 25 | <0.0001 |
| AHD | 21 | 21 | 24 | 22 | 18 | <0.0001 |
| PVD | 11 | 11 | 13 | 12 | 9 | <0.0001 |
| Cerebrovascular disease | 7 | 7 | 7 | 8 | 7 | 0.5613 |
| Other cardiovascular disease | 6 | 6 | 7 | 6 | 5 | <0.0001 |
| Chronic obstructive pulmonary disease | 6 | 6 | 6 | 6 | 5 | 0.7316 |
| Inability to ambulate | 3 | 3 | 4 | 3 | 3 | 0.0004 |
| Tobacco use (current smoker) | 5 | 5 | 6 | 5 | 5 | 0.7604 |
| Alcohol dependence | 1 | 1 | 1 | 1 | 1 | 0.0342 |
| Drug dependence | 1 | 1 | 1 | 1 | 2 | <0.0001 |
P-value compares those who had cholesterol data with the subset of patients who had available HDL measurements.
Table 2.
Laboratory characteristics of 137 260 MHD patients, including 33 109 who had available HDL measurements
| Characteristics | HDL (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|
| Total | Total | <30 | 30–<60 | 60+ | |||
| n = 137 260 | n = 33 109 | n = 2354 | n = 24 376 | n = 6289 | ANOVA | REG | |
| Serum levels | |||||||
| Albumin (g/dL) | 3.69 ± 0.50 | 3.72 ± 0.48 | 3.61 ± 0.57 | 3.72 ± 0.47 | 3.72 ± 0.49 | <0.0001 | <0.0001 |
| Creatinine (mg/dL) | 7.8 ± 3.1 | 7.9 ± 3.1 | 8.1 ± 3.4 | 8.0 ± 3.1 | 7.6 ± 2.8 | <0.0001 | <0.0001 |
| TIBC (mg/dL) | 209 ± 46 | 211 ± 45 | 206 ± 51 | 212 ± 44 | 211 ± 44 | <0.0001 | 0.0281 |
| Bicarbonate (mg/dL) | 22.2 ± 3.5 | 22.2 ± 3.4 | 22.1 ± 3.5 | 22.2 ± 3.4 | 22.3 ± 3.5 | 0.0264 | 0.0071 |
| Ferritin (ng/mL) | 496 ± 448 | 484 ± 421 | 532 ± 555 | 481 ± 407 | 477 ± 413 | <0.0001 | <0.0001 |
| White blood cell count (×103/μL) | 7.4 ± 2.6 | 7.3 ± 2.5 | 7.7 ± 3.3 | 7.4 ± 2.5 | 7.0 ± 2.3 | <0.0001 | <0.0001 |
| Protein catabolic rate (g/kg/day) | 0.94 ± 0.26 | 0.95 ± 0.25 | 0.94 ± 0.25 | 0.95 ± 0.25 | 0.94 ± 0.26 | <0.0001 | 0.1583 |
| Lymphocyte (% of total WBC) | 20.4 ± 8.1 | 20.9 ± 8.0 | 20.4 ± 8.2 | 20.8 ± 7.9 | 21.2 ± 8.1 | <0.0001 | <0.0001 |
| Blood hemoglobin (g/dL) | 11.88 ± 1.39 | 11.92 ± 1.37 | 11.65 ± 1.48 | 11.93 ± 1.36 | 12.0 ± 1.3 | <0.0001 | <0.0001 |
| BMI (kg/m2) | 27.2 ± 7.0 | 27.5 ± 7.0 | 28.7 ± 7.2 | 27.9 ± 7.0 | 25.6 ± 6.4 | <0.0001 | <0.0001 |
| Calcium (mg/dL) | 9.2 ± 0.7 | 9.2 ± 0.7 | 9.1 ± 0.7 | 9.2 ± 0.7 | 9.26 ± 0.7 | <0.0001 | <0.0001 |
| Phosphorus (mg/dL) | 5.4 ± 1.5 | 5.4 ± 1.4 | 5.5 ± 1.5 | 5.4 ± 1.4 | 5.3 ± 1.4 | <0.0001 | <0.0001 |
| Iron saturation ratio | 28.6 ± 12.1 | 28.7 ± 11.8 | 28.5 ± 12.9 | 28.6 ± 11.6 | 29.5 ± 12.2 | <0.0001 | <0.0001 |
| Alkaline phosphotase | 127 ± 93 | 126 ± 91 | 129 ± 110 | 122 ± 84 | 137 ± 109 | <0.0001 | <0.0001 |
| Intact parathyroid hormone | 413 ± 457 | 411 ± 463 | 400 ± 415 | 408 ± 459 | 428 ± 495 | 0.024 | 0.0088 |
| Cholesterol (mg/dL) | 154 ± 42 | 154 ± 42 | 128 ± 38 | 152 ± 41 | 173 ± 43 | <0.0001 | <0.0001 |
| Non-HDL cholesterol (mg/dL) | 106 ± 41 | 106 ± 41 | 109 ± 39 | 107 ± 41 | 100 ± 41 | <0.0001 | <0.0001 |
| LDL (mg/dL) | 73 ± 33 | 73 ± 33 | 59 ± 31 | 74 ± 32 | 76 ± 35 | <0.0001 | <0.0001 |
| HDL (mg/dL) | 48 ± 16 | 48 ± 16 | 25 ± 4 | 43 ± 8 | 73 ± 13 | <0.0001 | <0.0001 |
| Triglycerides (mg/dL) | 167 ± 114 | 167 ± 115 | 221 ± 139 | 174 ± 113 | 120 ± 93 | <0.0001 | <0.0001 |
P-value compares those who had cholesterol data with the subset of patients who had available HDL measurements.
HDL and mortality
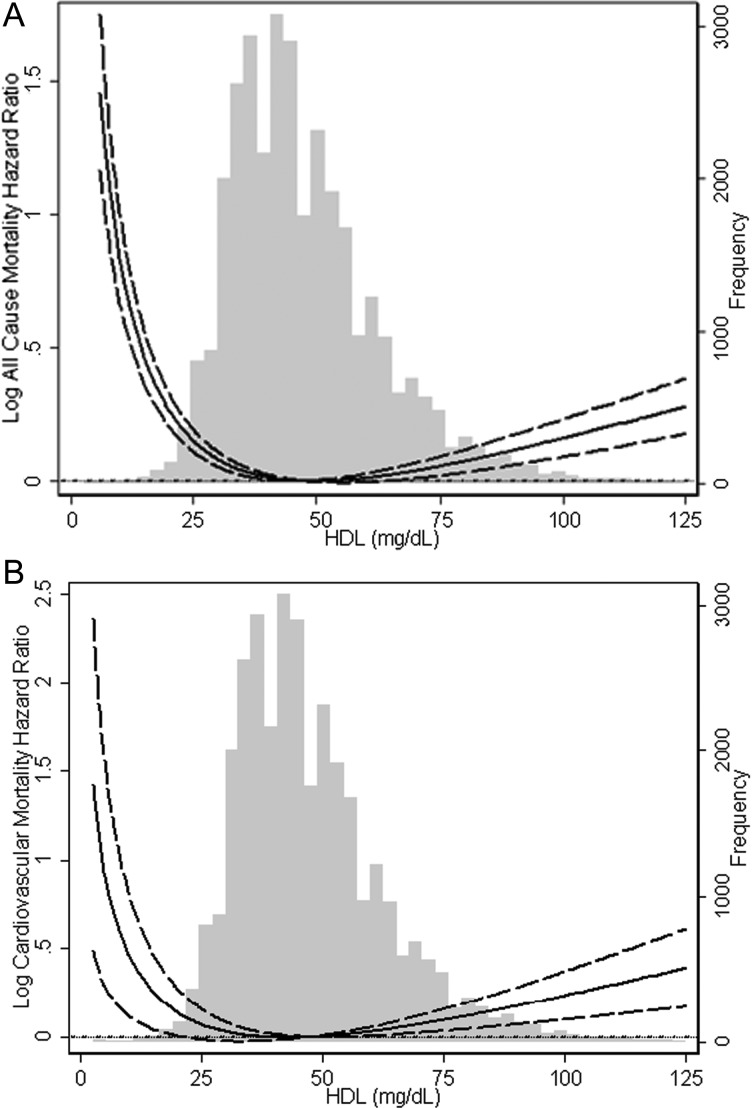
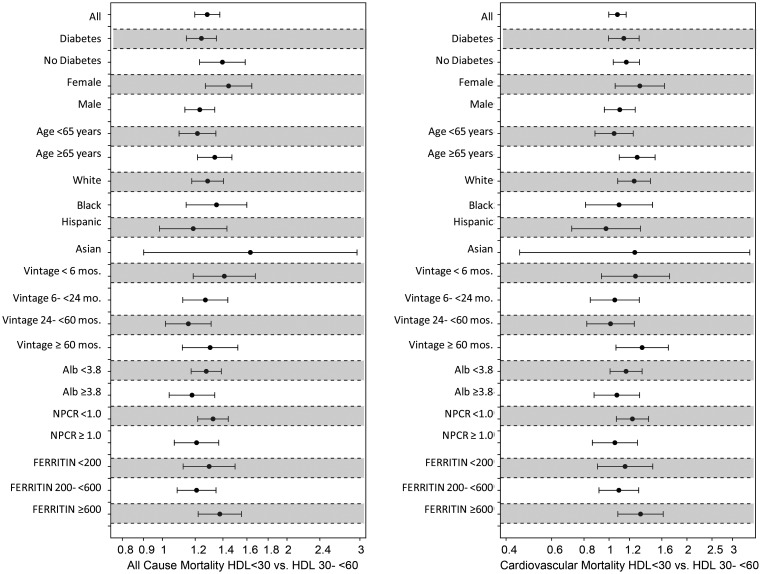
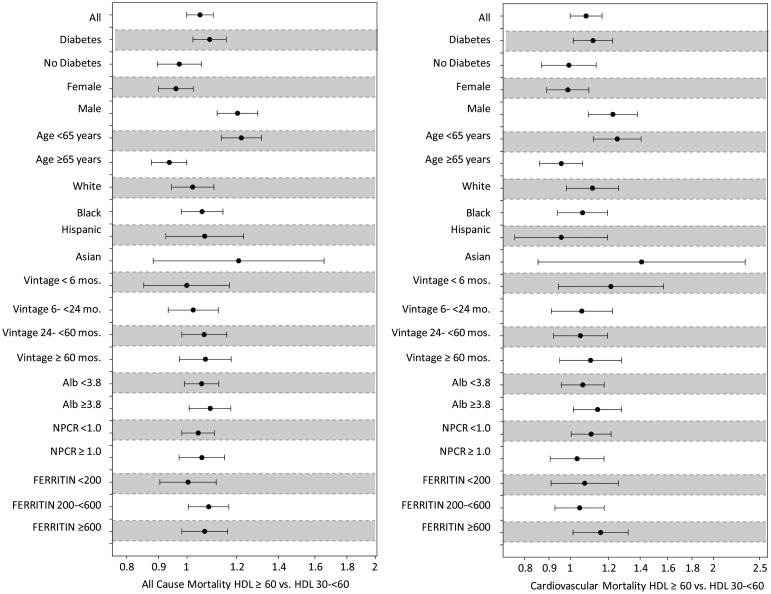
As expected, increasing serum HDL cholesterol concentrations were associated with reduced overall and CV mortality. However, this trend was reversed as serum HDL cholesterol concentration approached 50 mg/dL. Paradoxically, serum HDL cholesterol concentrations beyond 60 mg/dL were associated with a significant increase in overall and CV mortality (Figure 2). Therefore, the association of HDL cholesterol concentrations with mortality in MHD patients follows a U-shaped pattern. These findings remained robust even after adjustment for LDL cholesterol and across different levels of serum LDL concentrations (data not shown). Further subgroup analysis confirmed the strong association between low HDL cholesterol concentration and higher overall and CV mortality across subgroups of age, race, diabetes and markers of inflammation (Figure 3). In addition, subgroup analysis also confirmed that high HDL cholesterol concentrations (at and beyond 60 mg/dL) were associated with increased CV and overall mortality. These associations were strongest in patients of male gender, age <65 years, diabetics, those with elevated ferritin levels and albumin levels ≥3.8 g/dL (Figure 4). All-cause and CV mortality hazard ratio (and 95% confidence interval) was 1.28 (1.20–1.38) and 1.08 (1.01–1.16) for HDL <30 mg/dL and 1.05 (1.00–1.10) and 1.08 (1.00–1.16) for HDL ≥60 mg/dL, respectively (reference: HDL 30–<60 mg/dL).
FIGURE 2:
Cubic spline models of the Cox proportional regression analyses reflecting case–mix-adjusted overall (A) and cardiovascular (B) mortality predictability (with 95% confidence intervals) according to increasing serum HDL cholesterol concentrations.
FIGURE 3:
Hazard ratio of overall and cardiovascular mortality for two selected dichotomized levels of serum HDL concentrations (left: <30 mg/dL; right: 30–60 mg/dL) in selected subgroups of 33 109 MHD patients who were observed for up to 3 years.
FIGURE 4:
Hazard ratio of overall and cardiovascular mortality for two selected dichotomized levels of serum HDL cholesterol concentrations (left: 30–<60 mg/dL; right: ≥60 mg/dL) in selected subgroups of 33 109 MHD patients who were observed for up to 3 years.
Total cholesterol to HDL ratio and mortality
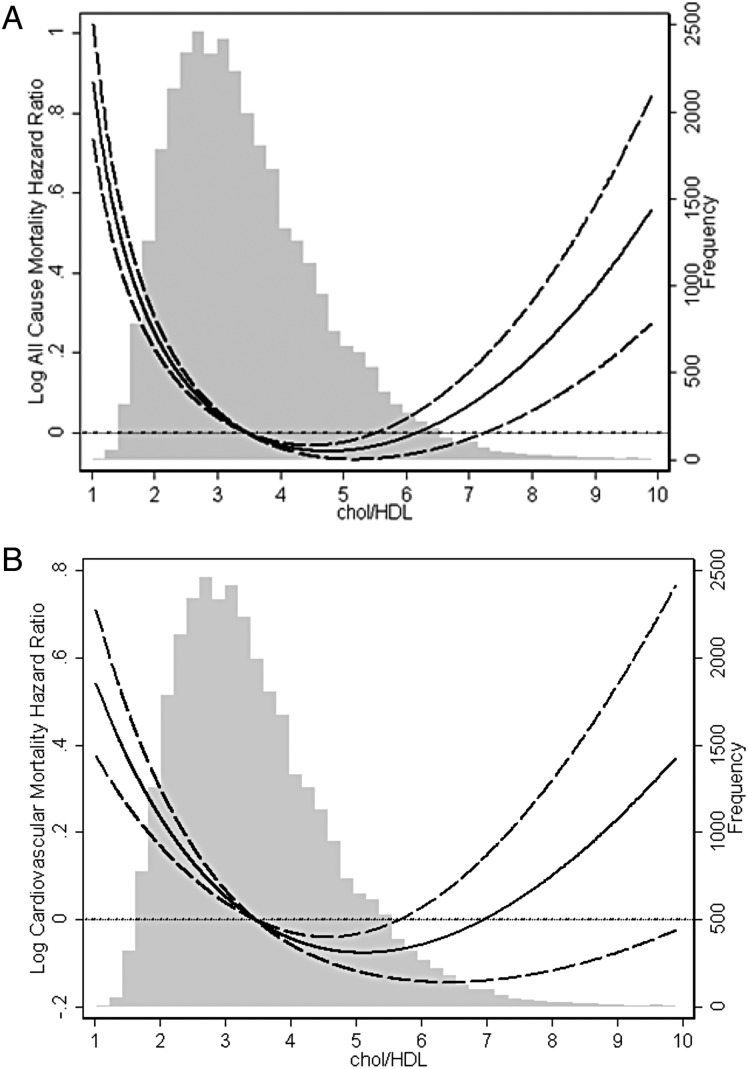
In contrast to the general population, increasing total cholesterol to HDL ratio was associated with improved CV and overall survival in patients with ESRD on MHD. However, this association also followed a U-shaped trend and increasing total cholesterol to HDL ratio beyond a level of 6 was associated with increasing overall and CV mortality (Figure 5).
FIGURE 5:
Cubic spline models of the Cox proportional regression analyses reflecting case–mix-adjusted overall (A) and cardiovascular (B) mortality predictability (with 95% confidence intervals) according to increasing total cholesterol to HDL cholesterol ratios.
DISCUSSION
As expected, low plasma HDL cholesterol concentrations were associated with increased CV and overall mortality and increasing HDL cholesterol levels up to 50 mg/dL were associated with improved survival in patients with ESRD receiving MHD. However, increments in HDL cholesterol concentrations >50 mg/dL were associated with a significant increase in CV and overall mortality. These findings are in contrast to the associations observed in the general population in whom increasing HDL cholesterol concentrations were associated with decreased CV mortality and improved survival [21–24]. Furthermore, in MHD patients increments in total cholesterol to HDL cholesterol concentration ratios were associated with improved survival contrasting the findings in the general population [36, 37]. This observation further supports the finding that very high plasma HDL cholesterol concentrations are not associated with improved survival and instead may be associated with increased adverse outcomes in patients with ESRD. It should be noted that a low total cholesterol/HDL cholesterol concentration ratio may be due to a low plasma cholesterol concentration as opposed to a high HDL concentration. In such cases increased risk of CV and overall mortality may be due to the high burden of systemic inflammation which results in a significant fall in plasma cholesterol concentration. In the subgroup analyses, these paradoxical associations persisted, although they tended to be stronger in diabetics, men, patients <65 years of age and those with higher ferritin and albumin concentrations.
In a series of earlier studies, we found that compared with the HDL from the healthy controls, the HDL from MHD patients exhibits markedly reduced antioxidant activity [25, 38]. This was associated with and, in part, due to the significant reduction of paraoxonase and glutathione peroxidase activity, the key HDL-associated antioxidant enzymes, which are crucial for its antioxidant–anti-inflammatory functions [25, 38]. The reduction of the antioxidant and anti-inflammatory properties of HDL can contribute to the atherogenic diathesis in the ESRD population. However, the association between high HDL cholesterol levels and increased CV and overall mortality in the subpopulation of ESRD patients shown here cannot be solely attributed to the impaired antioxidant and anti-inflammatory activities of HDL. This is because HDL cholesterol levels up to 50 mg/dL were associated with reduced CV and overall mortality in this population. Thus, despite its diminished antioxidant and anti-inflammatory functions, HDL appears to confer some protective action in the majority of ESRD patients. However, in the current study, we also found that the highest HDL cholesterol concentrations were associated with increased overall and CV mortality. This can be explained by a growing body of evidence which indicate that in the presence of oxidative stress and inflammation, HDL is transformed from an antioxidant and anti-inflammatory to a pro-oxidant, pro-inflammatory particle known as acute-phase HDL [39–41]. In fact, Honda et al. have recently shown that in a cohort of Japanese MHD patients, higher HDL cholesterol concentrations were associated with higher levels of oxidized HDL [32]. Furthermore, they found that elevated oxidized-HDL concentrations were associated with increased CV mortality. They concluded that excess oxidative stress may have yielded dysfunctional HDL in patients on MHD, and patients with high HDL cholesterol under these conditions may have enriched oxidized HDL which can then result in increased CV disease and CV mortality [32]. Yamamoto et al. also recently noted that HDL of patients with ESRD on MHD had reduced antichemotactic activity and increased macrophage inflammatory cytokine response (tumor necrosis factor alpha, interleukin 6 and interleukin 1 beta), when compared with HDL from healthy controls [42]. This is in agreement with the findings of Weichhart et al. who observed that while HDL isolated from healthy individuals inhibited the production of inflammatory cytokines by peripheral monocytes, HDL isolated from the majority of patients with ESRD did not show an anti-inflammatory property and many HDL samples even promoted production of inflammatory cytokines [43]. Therefore, in the setting of inflammation (i.e. patients with ESRD, diabetes and elevated ferritin levels), HDL may not only be dysfunctional but may also have a deleterious effect by promoting inflammation and adding to the CV disease burden [44].
Another possible mechanism responsible for increased mortality observed with elevated HDL cholesterol levels in our patient population was recently described by Huang et al. in an in vitro study using early endothelial progenitor cells (EPCs), which served as a prognostic indicator of clinical atherosclerosis. In this study, it was demonstrated that while protective at low concentrations, moderate-to-high concentrations of HDL from healthy subjects paradoxically impaired EPCs and related angiogenesis in the absence of oxidized LDL hence providing in vitro mechanistic evidence of potential toxic effects of elevated HDL concentrations under some specific circumstances [45]. In addition, defective unloading of the HDL's cholesterol cargo in the liver which is the final step in RCT may be another possible mechanism which has not been studied to date. Such a defect can lead to a highly atherogenic state despite marked elevation of plasma HDL cholesterol. Another possible mechanism that can explain the association between an elevated serum HDL cholesterol concentration and increased CV mortality is a deficiency of the enzyme cholesteryl ester transfer protein (CETP). CETP deficiency may possibly lead to the development of atherosclerosis despite high HDL cholesterol levels as reported by Nagano et al. [46]. Also, there are studies which show that serum HDL cholesterol elevation caused by CETP-deficient mutations can be associated with a high prevalence of CV disease [47, 48]. Furthermore, Kimura et al. demonstrated that CETP may serve as a protective factor against vascular disease in MHD patients with elevated serum HDL cholesterol levels [49]. Therefore, deficiency or inhibition of the CETP pathway can explain the association of an elevated serum HDL cholesterol concentration with an increase in CV mortality. Overall, the reason for the adverse outcomes seen with elevated HDL cholesterol concentrations in a subgroup of ESRD patients is presently unknown and requires further investigation. However, it is important to note that similar paradoxical associations between elevated HDL cholesterol level and mortality have also been reported in other patient populations such as those with non-ST-elevation myocardial infarction and coronary artery disease [50–52]. Therefore, it is imperative that future studies focus on determining the mechanisms responsible for these observations.
Several limitations of our study should be mentioned. First, the current findings should be qualified given the observational nature of our study design. In addition, a mixed incident/prevalent MHD population was examined in this study. However, we did adjust for dialysis vintage in all case–mix models and also performed separate analysis for incident (vintage ≤6 months) dialysis patients as shown in Figures 3 and 4. Another limitation is the potential confounding of therapy with lipid-altering agents which was not examined because home medication data were not available systematically in this national cohort. However, given the results of the 4D, AURORA and SHARP studies [53–55], it is unlikely that the inclusion of such data would have resulted in significantly different associations. It should be mentioned that our studied cohort represented only 20% of the entire national DaVita database and even smaller fractions for HDL subgroup analyses. However, we do not believe confounding by indication was present given that in 2006, the decision to measure serum HDL levels was made uniformly at the clinic level and was not individualized. In addition, we believe the risk of selection bias was not high given that all DaVita dialysis facilities are under uniform administrative care, and all laboratory tests are performed in one single laboratory with optimal quality-assurance monitoring. Furthermore, we performed a sensitivity analysis to compare DaVita patients included and excluded in this study and did not find any major differences (data not shown).
CONCLUSIONS
Serum HDL cholesterol levels were below the normal limits in the majority of ESRD patients, but were markedly elevated in a minority of these patients. Increments in HDL cholesterol concentration up to 50 mg/dL were associated with reduced CV and overall mortality but increments >50 mg/dL were paradoxically associated with increased mortality. The underlying mechanism(s) responsible for the observed elevation of HDL cholesterol and its adverse consequences in the subpopulation of ESRD patients is unknown and awaits investigation.
FUNDING
K.K.-Z. is supported in part by National Institute of Health (NIH) grants K24-DK091419 and R01-DK078106. H.M. is supported by NIH DK082130.
CONFLICT OF INTEREST STATEMENT
K.K.-Z. was a medical director of DaVita dialysis clinic in California 2008–2012.
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research for providing access to their database.
REFERENCES
- 1.United States Renal Data System. USRDS 2006 annual data report: atlas of end-stage renal disease in the United States. 2008. USRDS Annual Report. National Institutes of Health NIoDaDaKD, Bethesda, MD: NIH/NIDDK http://www.usrds.org/atlas_2006.htm. (6 August, 2010, date last accessed)
- 2.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Trivedi BK, Anderson JE. Associations of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis. 2006;13:183–188. doi: 10.1053/j.ackd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 5.Noori N, Caulfield MP, Salameh WA, et al. Novel lipoprotein subfraction and size measurements in prediction of mortality in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2861–2870. doi: 10.2215/CJN.03650411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aminzadeh MA, Nicholas SB, Norris KC, et al. Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrol Dial Transplant. 2013;28:2038–2045. doi: 10.1093/ndt/gft022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: reconciling low protein intake with nutritional therapy. Am J Clin Nutr. 2013;97:1163–1177. doi: 10.3945/ajcn.112.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoccali C, Mallamaci F, Tripepi G. Inflammatory proteins as predictors of cardiovascular disease in patients with end-stage renal disease. Nephrol Dial Transplant. 2004;Suppl 5:V67–V72. doi: 10.1093/ndt/gfh1059. [DOI] [PubMed] [Google Scholar]
- 9.Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol. 2013;38:136–148. doi: 10.1159/000351758. [DOI] [PubMed] [Google Scholar]
- 10.Wilensky RL, Hamamdzic D. The molecular basis of vulnerable plaque: potential therapeutic role for immunomodulation. Curr Opin Cardiol. 2007;22:545–551. doi: 10.1097/HCO.0b013e3282f028fe. [DOI] [PubMed] [Google Scholar]
- 11.Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des. 2005;11:3061–3072. doi: 10.2174/1381612054865064. [DOI] [PubMed] [Google Scholar]
- 12.Obryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208–222. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Botham KM, Moore EH, De Pascale C, et al. The induction of macrophage foam cell formation by chylomicron remnants. Biochem Soc Trans. 2007;35:454–458. doi: 10.1042/BST0350454. [DOI] [PubMed] [Google Scholar]
- 14.Gleissner CA, Leitinger N, Ley K. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension. 2007;50:276–283. doi: 10.1161/HYPERTENSIONAHA.107.089854. [DOI] [PubMed] [Google Scholar]
- 15.Davidson MH, Toth PP. High-density lipoprotein metabolism: potential therapeutic targets. Am J Cardio. 2007;100:n32–n40. doi: 10.1016/j.amjcard.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi L, Gomaraschi M, Franceschini G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler Thromb Vasc Biol. 2003;23:1724–1731. doi: 10.1161/01.ATV.0000094961.74697.54. [DOI] [PubMed] [Google Scholar]
- 17.Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson AD, Berliner JA, Hama SY, et al. Protective effect of high density lipoprotein associated paraxonase: inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest. 1995;96:2882–2891. doi: 10.1172/JCI118359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansell BJ, Navab M, Hama S, et al. Inflammatory/anti-inflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-denisty lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 2003;108:2751–2756. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 20.Feig JE, Shamir R, Fisher EA. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr Drug Targets. 2008;9:196–203. doi: 10.2174/138945008783755557. [DOI] [PubMed] [Google Scholar]
- 21.Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels: the Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 22.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104:1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 23.Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 24.Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 25.Moradi H, Pahl MV, Elahimehr R, et al. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl Res. 2009;153:77–85. doi: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Pahl MV, Ni Z, Sepassi L, et al. Plasma phospholipid transfer protein, cholesteryl ester transfer protein and lecithin:cholesterol acyltransferase in end-stage renal disease (ESRD) Nephrol Dial Transplant. 2009;24:2541–2546. doi: 10.1093/ndt/gfp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moradi H, Said HM, Vaziri ND. Post-transcriptional nature of uremia-induced downregulation of hepatic apolipoprotein A-I production. Transl Res. 2013;161:477–485. doi: 10.1016/j.trsl.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaziri ND, Deng G, Liang K. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol Dial Transplant. 1999;14:1462–1466. doi: 10.1093/ndt/14.6.1462. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri ND, Moradi H. Mechanisms of dyslipidemia of chronic renal failure. Hemodial Int. 2006;10:1–7. doi: 10.1111/j.1542-4758.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 32.Honda H, Ueda M, Kojima S, et al. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis. 2012;220:493–501. doi: 10.1016/j.atherosclerosis.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 33.Hatamizadeh P, Ravel V, Lukowsky LR, et al. Iron indices and survival in maintenance hemodialysis patients with and without polycystic kidney disease. Nephrol Dial Transplant. 2013;28:2889–2898. doi: 10.1093/ndt/gft411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lertdumrongluk P, Lau WL, Park J, et al. Impact of age on survival predictability of bone turnover markers in hemodialysis patients. Nephrol Dial Transplant. 2013;28:2535–2545. doi: 10.1093/ndt/gft290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention NHANES 2009–2010: laboratory files, “HDL-Cholesterol ”. http://www.cdc.gov/nchs/nhanes/nhanes2009–2010/lab09_10.htm. (January, 2013, date last accessed)
- 36.Kinosian B, Glick H, Garland G. Cholesterol and coronary artery disease: predicting risks by levels and ratios. Ann Intern Med. 1994;121:641–647. doi: 10.7326/0003-4819-121-9-199411010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Ray K, Wainwright NW, Visser L, et al. Changes in HDL cholesterol and cardiovascular outcomes after lipid modification therapy. Heart. 2012;98:780–785. doi: 10.1136/heartjnl-2011-301405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moradi H, Ganji S, Kamanna V, et al. Increased monocyte adhesion-promoting capacity of plasma in end-stage renal disease - response to antioxidant therapy. Clin Nephrol. 2010;74:273–281. doi: 10.5414/cnp74273. [DOI] [PubMed] [Google Scholar]
- 39.Navab M, Reddy ST, Van Lenten BJ, et al. Role of dysfunctional HDL in atherosclerosis. J Lipid Res. 2009;50:S145–S149. doi: 10.1194/jlr.R800036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng C, Aikawa M. High-Density Lipoproteins: From Function to Therapy. J Am Coll Cardiol. 2012;60:2380–2383. doi: 10.1016/j.jacc.2012.08.999. [DOI] [PubMed] [Google Scholar]
- 41.Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S, Yancey PG, Ikizler TA, et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–2379. doi: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weichhart T, Kopecky C, Kubicek M, et al. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23:934–947. doi: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moradi H, Vaziri ND, Kashyap ML, et al. Role of HDL dysfunction in end-stage renal disease: a double-edged sword. J Ren Nutr. 2013;23:203–206. doi: 10.1053/j.jrn.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang CY, Lin FY, Shih CM, et al. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating rho-associated kinase pathways. Arterioscler Thromb Vasc Biol. 2012;32:2405–2417. doi: 10.1161/ATVBAHA.112.248617. [DOI] [PubMed] [Google Scholar]
- 46.Nagano M, Yamashita S, Hirano K, et al. Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese. J Atheroscler Thromb. 2004;11:110–121. doi: 10.5551/jat.11.110. [DOI] [PubMed] [Google Scholar]
- 47.Zhong S, Sharp DS, Grove JS, et al. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agerholm-Larsen B, Nordestgaard BG, Steffensen R, et al. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation. 2000;101:1907–1912. doi: 10.1161/01.cir.101.16.1907. [DOI] [PubMed] [Google Scholar]
- 49.Kimura H, Miyazaki R, Suzuki S, et al. Cholesteryl ester transfer protein as a protective factor against vascular disease in hemodialysis patients. Am J Kidney Dis. 2001;38:70–76. doi: 10.1053/ajkd.2001.25196. [DOI] [PubMed] [Google Scholar]
- 50.Duffy D, Holmes DN, Roe MT, et al. The impact of high-density lipoprotein cholesterol levels on long-term outcomes after non-ST-elevation myocardial infarction. Am Heart J. 2012;163:705–713. doi: 10.1016/j.ahj.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 51.Corsetti JP, Zareba W, Moss AJ, et al. Elevated HDL is a risk factor for recurrent coronary events in a subgroup of non-diabetic postinfarction patients with hypercholesterolemia and inflammation. Atherosclerosis. 2006;187:191–197. doi: 10.1016/j.atherosclerosis.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Corsetti JP, Salzman P, Ryan D, et al. Plasminogen activator inhibitor-2 polymorphism associates with recurrent coronary event risk in patients with high HDL and C-reactive protein levels. PLoS ONE. 2013;8:e68920. doi: 10.1371/journal.pone.0068920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 55.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]