Abstract
Background
H.P. Acthar® Gel is currently the only Food and Drug Administration therapy approved for the treatment of nephrotic syndrome. Active drug ingredients include structurally related melanocortin peptides that bind to cell surface G-protein-coupled receptors known as melanocortin receptors, which are expressed in glomerular podocytes. In animal models of membranous nephropathy, stimulation has been demonstrated to reduce podocyte injury and loss. We hypothesized that H.P. Acthar® Gel would improve symptoms of the nephrotic syndrome in patients with idiopathic membranous nephropathy.
Methods
Twenty patients received a subcutaneous dose of 40 or 80 IU twice weekly. Changes in proteinuria, albumin, cholesterol profile, estimated glomerular filtration rate and serum anti-PLA2R antibodies were assessed at baseline and in response to treatment along with tolerance and safety.
Results
Baseline characteristics included mean proteinuria (9.1 ± 3.4 g/day), albumin (2.7 ± 0.8 g/dL), estimated glomerular filtration rate (77 ± 30 mL/min) along with elevated total and low-density lipoprotein (LDL) cholesterol. By 12 months of follow-up, there was a significant improvement in proteinuria in the entire cohort, decreasing to 3.87 ± 4.24 g/day (P < 0.001) with significant improvements in serum albumin, total and LDL cholesterol. A >50% decrease in proteinuria was noted in 65% of the patients with a trend toward better outcomes among patients who received greater cumulative doses. No significant adverse effects were documented. Clearing of serum anti-PLA2R antibodies prior to or in parallel with proteinuria improvement was noted in some, but not all patients.
Conclusions
H.P. Acthar® Gel is a potential therapy for nephrotic syndrome secondary to idiopathic membranous nephropathy that deserves further study.
Keywords: ACTH (H.P. Acthar® Gel), membranous nephropathy, nephrotic syndrome
INTRODUCTION
Primary or idiopathic Membranous Nephropathy (iMN) is a common cause of nephrotic syndrome in adults representing ∼1/3 of biopsy-proven cases as noted in a 30-year study conducted in the USA [1]. It typically presents with nephrotic range proteinuria. The most relevant factors influencing the rate of progression are the severity and persistence of proteinuria with the 10-year renal survival ranging from 60 to 80% [2–6]. Further, complications related to the nephrotic syndrome including venous thromboembolism, edema and hyperlipidemia can result in significant morbidity and mortality [7, 8]. As such, immunosuppressive therapy is now recommended in addition to supportive measures that include restriction of dietary protein intake, blood pressure control and edema management in patients with persistent high-grade proteinuria, complications of the nephrotic syndrome or an early rise in serum creatinine [9]. Existing immunosuppressive treatment options, however, are associated with significant side effects (cytotoxic agents) as well as a significant potential for relapse after withdrawal of therapy (calcineurin inhibitors).
H.P. Acthar® Gel (ACTH) is obtained from processing of the porcine pituitary gland and is currently the only Food and Drug Administration (FDA) approved therapy for the treatment of nephrotic syndrome. The active ingredients in H.P. Acthar® Gel are part of the family of structurally related peptides known as melanocortin peptides. Melanocortin peptides, which include adrenocorticotrophic hormone (ACTH) and the α-, β- and γ-melanocyte stimulating hormones, are derived from the natural protein pro-opiomelanocortin (POMC) and bind to the cell surface G-protein-coupled receptors known as melanocortin receptors (MCRs) [10]. To date, five forms of MCRs have been cloned, each with different tissue distributions, affinities and physiological roles. Although the functional roles of all the peptides in H.P. Acthar® Gel have not been fully elucidated, ACTH is known to have activity at all five MCRs.
MCRs are expressed in glomerular podocytes and receptor stimulation has been demonstrated to reduce oxidative stress and improve glomerular morphology by diminishing podocyte apoptosis, injury and loss in the remnant kidney animal model [11]. Human data also support an anti-proteinuric effect in addition to a pronounced lipid-lowering effect in healthy individuals [12, 13], in steroid-treated patients with renal disease [14] and in hemodialysis patients [15], using a similar synthetic ACTH preparation that is not available in North America. As such, we hypothesized that ACTH therapy in the form of H.P. Acthar® Gel may prove an effective therapy in patients with severe nephrotic syndrome secondary to iMN.
In this manuscript, we report the findings from a phase Ib/II pilot study using H.P. Acthar® Gel in patients with iMN. We hypothesized that H.P. Acthar® Gel would be a safe alternative therapy that would improve proteinuria as well as symptoms of the nephrotic syndrome, including lipid profiles, in patients with severe nephrotic syndrome secondary to iMN. We also assessed the predictive value of the antibodies to the podocyte-expressed phospholipase A (2) receptor (anti-PLA2R), which have been previously reported in at least 70% of patients with iMN with titers shown to be associated with disease activity [16].
MATERIALS AND METHODS
Study subjects
Participating patients were adults (>18 years of age) with biopsy-proven iMN. The diagnostic biopsy was performed within 36 months of study initiation and did not demonstrate in excess 30% glomerulosclerosis and/or interstitial fibrosis or tubular atrophy. Potential study participants required at least 3 months of treatment with renin angiotensin system (RAS) blockade to lower blood pressure to <130/75 mmHg in >75% of the readings prior to the initiation of ACTH treatment. All study participants had nephrotic range proteinuria as defined by Uprot/Ucr ≥4.0 on a spot sample aliquot from a 24-h urine collection without significant renal insufficiency as defined by an estimated glomerular filtration rate (eGFR) ≥ 40 mL/min/1.73 m2 while taking blockade of the RAS. The choice of Uprot/UCr was in accord with recent National Kidney Foundation Chronic Kidney Disease (NKF-CKD) guidelines, and glomerular filtration rate (GFR) was estimated using the 4 variable Modification of Diet in Renal Disease (MDRD) equation as published in the NKF-CKD guidelines [17]. The rationale for the GFR criteria was that patients with severely reduced GFR might have significant interstitial and glomerular scarring that would not benefit from treatment.
Patients with documented resistance to immunosuppressive routines used in iMN (e.g. calcineurin inhibitors plus or minus steroids or cytotoxic agents plus or minus steroids) were ineligible to participate to exclude a patient population resistant to all forms of treatment. However, patients who had had only a partial response to other regimens or significant side effects were eligible to participate. These study patients were required to be off glucocorticoid therapy, calcineurin inhibitors (cyclosporin A, tacrolimus) or mycophenolic mofetil for >1 month, and alkylating agents for >6 months to exclude patients in whom concurrent immunosuppression or a delayed effect of recently discontinued immunosuppression may have a beneficial effect on the disease course as well as to reduce the risk of immunosuppressive complications. Other exclusion criteria included active infections or secondary causes of membranous nephropathy (e.g. hepatitis B, SLE, medications, malignancies), Type 1 or 2 diabetes mellitus to exclude proteinuria secondary to diabetic nephropathy, pregnancy or nursing women for safety reasons, as well as patients with documented acute thrombosis, requiring anticoagulation therapy.
The study was performed at the Mayo Clinic and the University of Toronto. Research ethics boards at both sites approved the study. All subjects provided informed consent as per the Declaration of Helsinki for Medical Research Involving Human Subjects.
Study protocol
This was a phase Ib/II, non-blinded, dose-finding study using ACTH in the form of H.P. Acthar® Gel provided by Questcor Pharmaceuticals, Inc. Patients were randomized to receive ACTH at the dose of either 40 or 80 units subcutaneously. Randomization occurred on a 1:1 ratio using a block randomization technique.
Prior to initiating active therapy, target blood pressure was achieved during a 3-month run-in period. In incident patients not on RAS blockade, angiotensin receptor blockers were used preferentially as better tolerated, with minimal cough or angioedema. The dose was increased at 2-week intervals until the maximum tolerated/FDA approved dose was achieved or until intolerable side effects occurred. Prevalent patients already stable on the maximum dose of RAS blockade, including dual blockade, were allowed to continue on their therapy as prescribed. Patients whose blood pressure control was not at target, additional medication was added as necessary at the discretion of the attending nephrologist, but the choice of a non-dihydropyridine calcium channel blockers was made because of concerns that dihydropyridine-type may have a delayed anti-proteinuric effect. As part of the standard of care for patients with nephrotic syndrome and severe hyperlipidemia, patients were started on atorvastatin 10 mg a day (or its equivalent) and if tolerated the dose was increased to the maximum recommended according to the recently published Kidney Disease Outcomes Quality Initiative-dyslipidemia guidelines [18]. Finally, all patients received dietary counseling to maintain a low salt diet (2–3 g/day) and a dietary protein target intake of 0.8 g/kg ideal body weight/day of high quality protein throughout the duration of the study. Once stabilized, further escalations to drugs that block the RAS or the dose of lipid-lowering agents was not permitted. Dose reductions, however, were guided by side effects (i.e. hyperkalemia, myalgia, etc.).
The dose of ACTH was increased from one injection every other week to two injections per week. It was then continued at full dose, either 40 or 80 units twice per week, for 12 weeks. The injections were given on Days 0, 14, 21, 28 (one injection per week), 31, 35, 38, 42, 45, 49, 52, 56, 59, 63, 66, 70, 73, 77, 80, 84, 87 and 91 (two injections per week). At the Mayo Clinic only, patients on 40 units that did not demonstrate a significant improvement in urine protein by Day 91 were offered a dose increase to 80 units given twice per week for an additional 12 weeks. These patients received an additional 90 days of therapy.
Blood samples and aliquots of 24-h urine were collected in the morning at baseline and then at 1, 3, 6 and 12 months. Samples were obtained immediately before the administration of ACTH, if scheduled for that day, and always in the fasting state. Measurements included serum concentrations of cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein cholesterol, creatinine, albumin, cortisol, blood glucose as well as anti-PLA2R antibody. The glomerular filtration rate was estimated by the MDRD equation. Proteinuria was monitored by 24-h collections. At each visit, patients were questioned about their symptoms and possible side effects of therapy. Physical examination included the measurement of blood pressure and body weight. Patients were given the option to participate in a sub-study designed to determine the pharmacological response to ACTH by measuring cortisol levels in their blood and urine. Fasting blood samples for serum cortisol measurements were collected before the injection of ACTH and after 0.5, 1, 2, 3, 4, 6, 8, 12 and 24 h, while urine cortisol was assessed at 24 and 48 h. Six patients in the 40 IU group and seven patients in the 80 IU group agreed to this extra testing.
The primary outcome measures included changes in the measures of nephrotic syndrome including improvements in proteinuria, serum albumin and cholesterol profile as well as documented side effects and toxicity. Secondary end points included the proportion of subjects that achieved a complete remission (CR = proteinuria <0.3 g/day), partial remission (PR =reduction in proteinuria by >50% with a final urine protein < 3.5 g/day, but >0.3 g/day) and no response (NR = reduction in proteinuria by <50% or worsening of proteinuria).
Laboratory determinations
Most values were determined by site-specific laboratory evaluations. Samples were tested for the presence of anti-PLA2R antibodies by western blot immunoassay against both native PLA2R (present in extracts from human glomeruli) and cell-expressed recombinant human PLA2R, as previously described [19]. The assessors of the assay were blinded to the clinical results. Subsequent sera from patients initially found to be positive in both assays were tested against native PLA2R only. Sera were routinely tested at a titer of 1:25, which has been shown to be both sensitive and specific.
To standardize the assay for comparison of anti-PLA2R levels between different subjects, baseline samples from all 20 subjects were run in a single assay (primary assay). Identical lanes containing 30 µL of a single large batch preparation of human glomerular extract were electrophoresed by SDS–PAGE and transferred to nitrocellulose membranes. Individual lanes were cut and incubated overnight at 4°C with serial serum samples; all samples from an individual patient were processed in parallel. IgG subclass-specific sheep anti-human IgG4 (The Binding Site) was used at 1:3000 and ultimately detected with peroxidase-conjugated donkey anti-sheep IgG (Jackson ImmunoResearch), followed by reaction in a chemiluminescent substrate and a 10-s exposure to autoradiography film. Bands were quantitated using a Hewlett Packard scanner, aligned in Adobe PhotoShop CS4, and densitometry performed using NIH ImageJ. Subsequently, and separately for each individual patient, available samples were assayed (secondary assay) and normalized according to the initial baseline value in the primary assay. In this manner, we were able to compare anti-PLA2R values between subjects and between samples from each subject at all time points.
Statistical analysis
Descriptive statistics were calculated for all variables of interest. Continuous measures were summarized using mean ± standard deviation, whereas categorical measures were summarized using counts and percentages. Paired testing was used to assess changes over time. Fisher's exact test was utilized to compare proportions. An analysis of variance was used to compare responses by dose with the Newman–Keuls test utilized for between group comparisons. Correlation was used to assess the relationship between response to treatment, i.e. proteinuria reduction, and serum anti-PLA2R antibody levels. A P-value of <0.05 was deemed statistically significant. All analyses were carried out using SAS Version 9.1 (SAS Institute, Cary, NC, USA)
RESULTS
Baseline characteristics
The cohort had an average age of 51 ± 15 years. The majority of the cohort was male (63%) and Caucasian (58%). All but one patient were stable on either monotherapy (14 patients) or dual blockade (5 patients) of the RAS prior to the run-in phase with no noted changes in urine protein. The single patient not on RAS blockade did not tolerate the therapy due to hypotension, and half of the patients could not tolerate the manufacture's maximum recommended dose for hypertension. The median disease latency was 14 months (IQR 8.5–47 months), and seven patients had previous exposure to immunosuppressive agents alone or in combination, including prednisone, calcineurin inhibitors, mycophenolate mofetil and cyclophosphamide.
Other population baseline characteristics are displayed in Table 1. Blood pressure was controlled. The cohort was nephrotic with a mean 24-h urine collection for protein of 9.1 ± 3.4 g. The patients had relatively well-preserved renal function with an eGFR of 77 ± 30 mL/min. They had evidence of the nephrotic syndrome with mean serum albumin levels of 2.72 ± 0.83 g/dL and abnormal cholesterol profiles, including elevated levels of total and LDL cholesterol. None of the patients had elevated blood glucose (93 ± 11 mg/dL) or cortisol levels (11 ± 3 μg/dL) at baseline. There were no statistically significant differences in any of the baseline variables between the two doses. At baseline, anti-PLA2R antibodies were detected in 15/20 (75%) of the patients.
Table 1.
Baseline and follow-up variables in the overall cohort
| Baseline | ACTH completion | 12 months | P-value | |
|---|---|---|---|---|
| Systolic BP (mmHg) | 121 ± 16 | 124 ± 18 | 119 ± 16 | NS |
| Diastolic BP (mmHg) | 72 ± 8 | 75 ± 7 | 74 ± 11 | NS |
| Proteinuria (g/day) | 9068 ± 3384 | 6155 ± 4754* | 3866 ± 4243*, ** | <0.001 |
| eGFR (mL/min) | 77 ± 30 | 78 ± 25 | 76 ± 30 | NS |
| Albumin (mg/dL) | 2.72 ± 0.83 | 3.25 ± 0.60* | 3.56 ± 0.76*, ** | 0.001 |
| Cholesterol (mg/dL) | 306 ± 133 | 230 ± 95* | 187 ± 49*, ** | <0.001 |
| LDL (mg/dL) | 182 ± 85 | 116 ± 52* | 93 ± 37*, ** | 0.001 |
| HDL (mg/dL) | 67 ± 29 | 66 ± 27 | 59 ± 23 | NS |
| Trigycerides (mg/dL) | 225 ± 190 | 247 ± 260 | 176 ± 103 | NS |
BP, blood pressure; NS is not significant
*P < 0.05 versus Baseline.
**P < 0.05 versus ACTH Completion.
Treatment response
There was a significant improvement in urine protein and other features of the nephrotic syndrome by the completion of ACTH treatment, which continued to improve over the subsequent months of follow-up even while off therapy (Table 1). Mean proteinuria improved significantly from 9.1 ± 3.4 to 6.2 ± 4.8 g/day at the point of completion of the ACTH treatment to 3.9 ± 4.2 g/day at 1 year of follow-up. This was paralleled by a rise in serum albumin from 2.72 ± 0.83 to 3.56 ± 0.76 mg/dL along with a decrease in both total and LDL cholesterol (306 ± 133 to 187 ± 49 mg/dL and 182 ± 85 to 93 ± 37 mg/dL, respectively) over the same time period. In the overall cohort, blood pressure remained controlled throughout the trial, and there was no significant loss of renal function during the period of treatment or follow-up.
Upon completion of the course of ACTH therapy, 10/20 patients had a >50% reduction in urine protein, improving to 13/20 of the patients (65%) by 1 year of follow-up. Among these 13 patients, 2 patients ultimately achieved a complete remission (10%) and 10 patients achieved a partial remission (50%), while one had a significant improvement in proteinuria (18.2–8.1 g/day), but did not meet the criteria for a partial remission. The remaining seven patients had either no improvement or progressed with respect to their proteinuria. Two of these seven patients were given alternate immunosuppression prior to completion of the trial and were therefore excluded from the 1-year follow-up data.
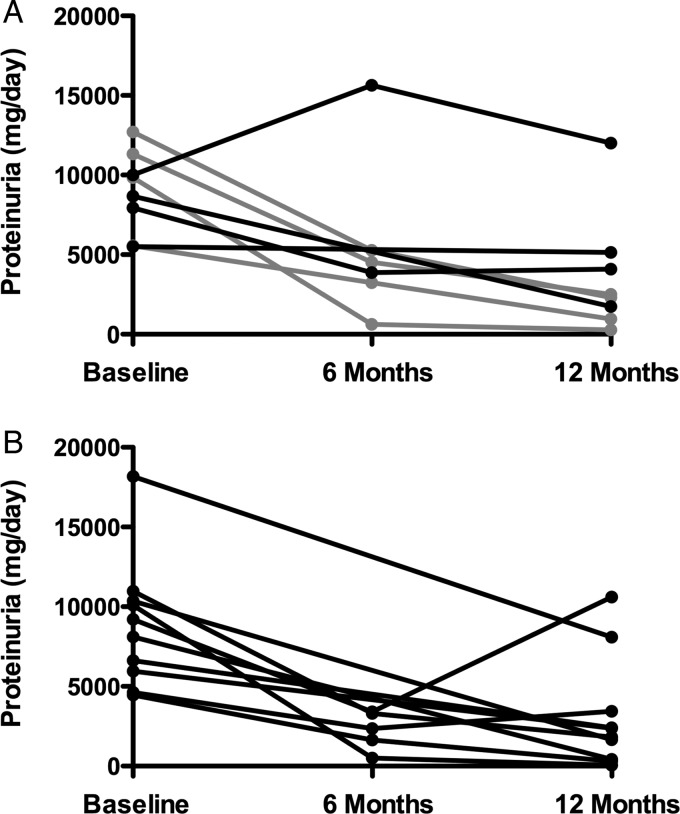
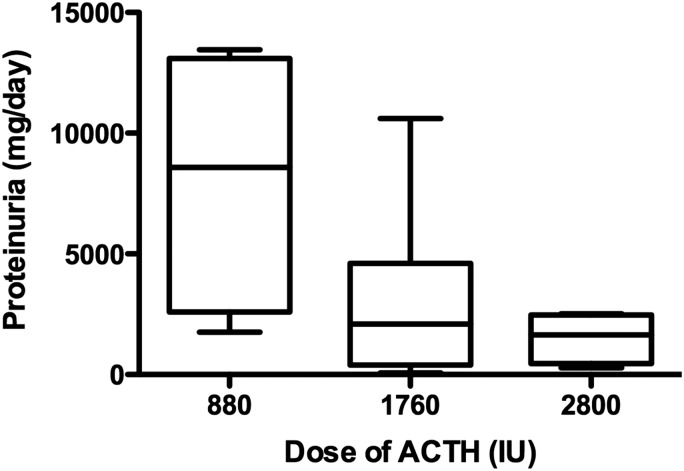
With respect to the drug dose and response, there was no meaningful change in proteinuria after 12 weeks of therapy noted in any of the patients who received 40 IU, whereas 5/11 patients receiving the higher dose demonstrated at least a 30% reduction in their level of urine protein (P = 0.038) with further improvements in urine protein noted over time (Figure 1). Given that the dose was increased to 80 IU in 5/9 patients originally randomized to receive 40 IU, comparison of follow-up data at 1 year was limited by the small numbers of patients who received only 40 units throughout the duration of the trial. Of the four patients who were maintained on the lower dose, three had no response to treatment, while one achieved a partial remission. Of the 16 patients who received a full course of 80 units of H.P. Acthar® Gel (11 who were initiated on 80 IU and the 5 whose dose was increased from 40 to 80 IU), 11/16 patients achieved remission with a complete remission occurring in 2/16 and a partial remission in 9/16, while 5 did not respond. The assessment of patients based on the cumulative dose received (Table 2) did reveal trends with respect to improved proteinuria (Figure 2, R = 0.53, P < 0.05) as well as the symptoms of the nephrotic syndrome with the larger cumulative doses.
FIGURE 1:
Proteinuria response to treatment. The proteinuria response over time is graphed with (A) depicting the patients initiated at 40 IU and (B), those initiated at 80 IU. The patients wherein the dose was escalated from 40 to 80 IU are shown in gray.
Table 2.
Outcomes at 12 months of follow-up by cumulative dose
| 880 IU | 1760 IU | 2800 IU | |
|---|---|---|---|
| Systolic BP (mmHg) | 128 ± 7 | 115 ± 18 | 119 ± 18 |
| Diastolic BP (mmHg) | 78 ± 11 | 70 ± 7 | 77 ± 17 |
| Proteinuria (g/day) | 8090 ± 5571 | 3120 ± 3501 | 1505 ± 1072* |
| eGFR (mL/min) | 64 ± 16 | 78 ± 35 | 83 ± 28 |
| Albumin (mg/dL) | 2.75 ± 1.05 | 3.74 ± 0.40 | 3.95 ± 0.60** |
| Cholesterol (mg/dL) | 238 ± 17 | 163 ± 37** | 194 ± 60 |
| LDL (mg/dL) | 125 ± 31 | 80 ± 31 | 91 ± 43 |
| HDL (mg/dL) | 75 ± 14 | 53 ± 24 | 56 ± 25 |
| TG (mg/dL) | 190 ± 92 | 147 ± 79 | 234 ± 159 |
*P < 0.05 for trend across groups.
**P < 0.05 versus 880 IU.
FIGURE 2:
Proteinuria and cumulative ACTH dose. Proteinuria was inversely related to cumulative ACTH dose and the trend of this relationship was statistically significant (R = 0.53, P < 0.05).
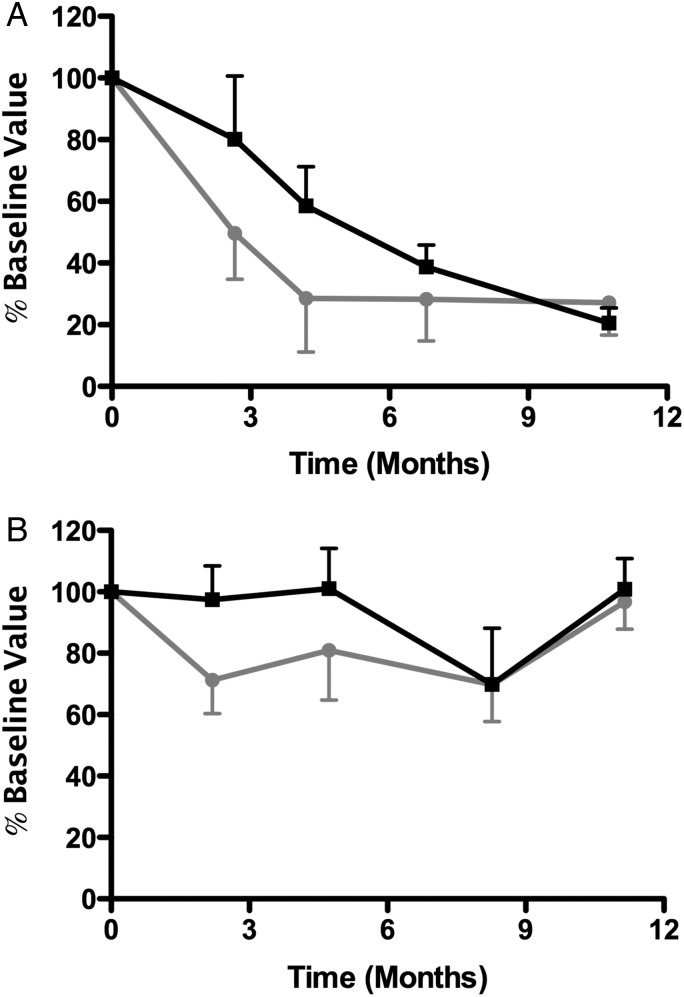
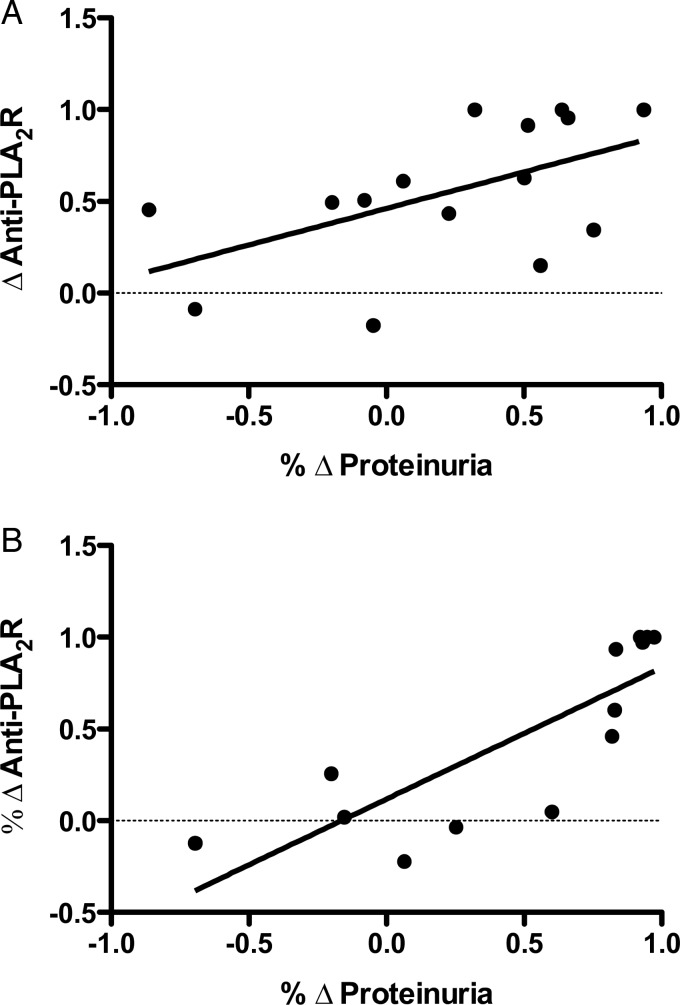
In the 15 patients with anti-PLA2R antibodies detected during screening, the antibody was cleared completely in 3 patients and was reduced in another 4 patients by the completion of the ACTH treatment. Antibody clearing typically preceded improvement in the level proteinuria (five patients) or paralleled improvement in proteinuria (two patients). In patients who did not respond, there was also typically no change in the antibody (Figure 3). As such, there was a statistically significant correlation between the percentage change anti-PLA2R antibodies and improvement in proteinuria after completion of the ACTH therapy (R2 = 0.29; P = 0.04, Figure 4A) that improved after 1 year (R2 = 0.67; P < 0.001, Figure 4B). In the five patients wherein no anti-PLA2R antibodies were detected at baseline, one entered a complete remission, three patients entered a partial remission and one had a significant improvement in proteinuria (18 to 8 g/day), but did not meet the criteria for a partial remission.
FIGURE 3:
Association between proteinuria and anti-PLA2R antibody. In antibody-positive patients, the association between proteinuria (black squares) and the anti-PLA2R antibody (gray circles) versus time is plotted. (A) Patients wherein a response was noted. (B) Patients who did not respond either clinically or immunologically to ACTH therapy.
FIGURE 4:
Correlation between proteinuria and anti-PLA2R antibody. There was a statistically significant correlation between the percentage change in anti-PLA2R antibodies (% Δ anti-PLA2R antibody) and improvement in proteinuria (% Δ proteinuria) after completion of the ACTH therapy (R2 = 0.29; P = 0.04, A) and after 1 year (R2 = 0.67; P < 0.001, B).
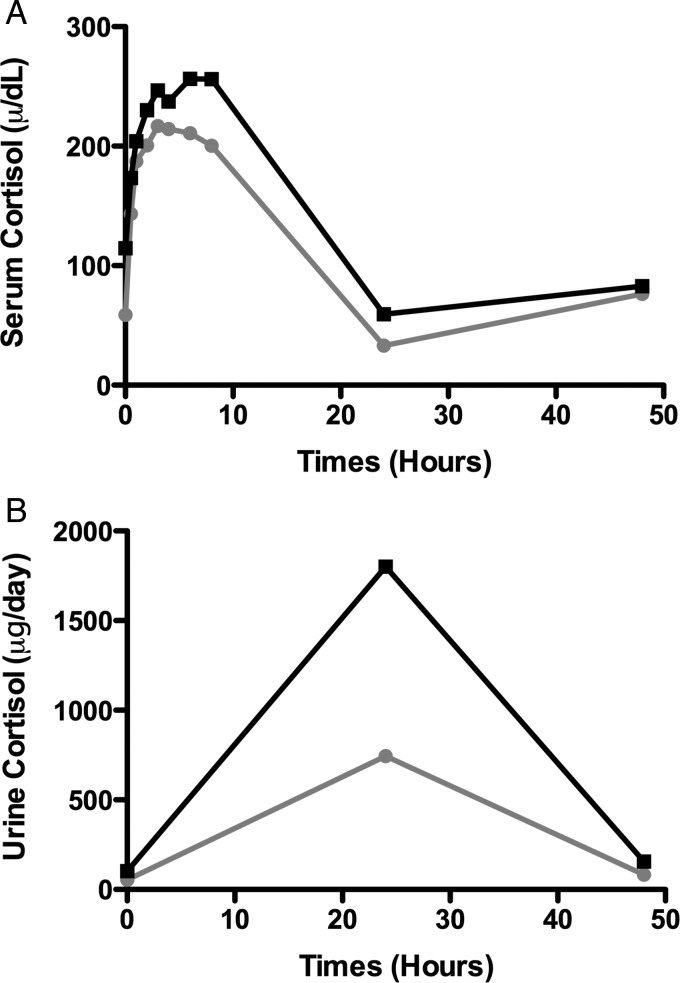
Overall, the treatment was well tolerated with no patients requiring discontinuation of therapy for treatment-related side effects irrespective of the total dose received. Serum ACTH and cortisol as well as urine cortisol levels increased acutely after both the 40 IU and the 80 IU doses. Although there were no dose-related differences in serum ACTH levels, there was a larger increase in both serum and urine cortisol after the 80 IU dose that decreased toward baseline within 24 h (Figure 5). With respect to steroid-related side effects, only three patients were noted to have a cushingoid appearance. Weight gain was noted in five patients (≤7 kg) and transient worsening of edema or bloating was noted in three patients. Skin changes included acne (n = 2), flushing (n = 3) and bronzing (n = 2). With respect to potential psychological effects of the treatment, increased irritability, depression and improved mood were all noted and fluctuated throughout the follow-up period (six patients), while six patients noted transient insomnia. Tremulousness (n = 3), hoarseness (n = 2), dizziness (n = 5), muscle aches or pain (n = 5), headaches (n = 5), gastrointestinal symptoms (n = 7), blurred vision (n = 2) as well as generalized weakness or fatigue (n = 9) were also described throughout the year of follow-up, but were not in all cases clearly related to the therapy. Bruising at the injection site was noted in five patients. The serum blood glucose increased in 13/20 patients following ACTH administration, but there were no significant differences between the two doses. Transient increases to a blood glucose level ≥130 mg/dL occurred in five patients, but only one patient had a clinically important increase in glucose that was sustained, that patient required only dietary treatment, and subsequently improved with weight loss.
FIGURE 5:
The pharmacological response to ACTH. The serum (A) and urine cortisol (B) response to therapy is plotted against time where black represents the patients treated with the 80 IU dose and gray the patients treated with 40 IU.
DISCUSSION
In summary, H.P. Acthar® Gel (ACTH) in this pilot study appears to be safe and may be an effective therapy in patients with nephrotic syndrome secondary to iMN, as significant and progressive reductions in proteinuria were seen along with improvements in other features of the nephrotic syndrome in the majority of patients. Although the poor response in patients who received the 40 IU dose suggests a dose–response relationship, the dose was escalated in the majority of non-responding patients. As such, we cannot be sure that longer courses at lower doses would not have yielded the same benefits. Trends emerged with respect to improved levels of urine protein and measures of the nephrotic syndrome at higher cumulative doses. Toxicity was minimal and side effects were manageable even with the increased exposure with no patients requiring treatment discontinuation, but longer trials will be necessary to determine the safety after more prolonged administration. An association with anti-PLA2R antibodies and response was evident in some, but not all patients. However, both antibody positive and negative patients followed a similar trajectory with respect to time to remission, again suggesting a treatment effect.
Synthetic formulations of ACTH have previously proved effective in patients with nephrotic syndrome, and the use of ACTH for the treatment of elevated cholesterol and proteinuria dates back many decades [20, 21]. In Europe, the synthetic formulation of ACTH, tetracosactide (Synacthen), which is not available in North America, has proved similarly effective in both uncontrolled series and a small randomized controlled trial wherein it was compared with cytotoxic therapy [22–24]. The largest case series includes 23 patients with a variety of causes of nephrotic syndrome of which 10 had membranous nephropathy [22]. These patients received on average 25 μg/kg/week for ∼6 months with a range of 2–11 months, depending on response and side effects. All the patients achieved a complete or partial remission, and documented side effects were minimal. The only controlled trial to date randomized patients to receive either a combination therapy, which included methylprednisolone alternating with a cytotoxic agent, or a year of Synacthen [23]. They found no significant differences in treatment response between the two groups with 15/16 patients who received cytotoxic therapy and 14/16 patients who received Synacthen entering a complete or partial remission. In those patients treated with synthetic ACTH, one patient withdrew at 3 months due to lack of effect and one withdrew due to treatment-related side effects.
However to date, there are only limited, uncontrolled data describing the response to natural ACTH (H.P. Acthar® Gel), which is currently the only available ACTH preparation in North America. Bomback and colleagues summarized the available data wherein ACTH (H.P. Acthar® Gel) was dispensed to treat idiopathic, non-diabetic nephrotic syndrome in the USA (outside of a research setting) [25]. Twenty-one subjects were identified as having received H.P. Acthar® Gel for the treatment-resistant idiopathic nephrotic syndrome of which 11 patients had iMN. The most common treatment regimen was 80 units subcutaneous twice weekly for 6 months. Of the 11 subjects with iMN who failed a mean of 2.4 previous therapies, the complete and partial remission rates approached 80%. Those who did not respond received lower doses.
Similar to our trial, synthetic forms of ACTH have proven effective in reducing proteinuria and treating nephrotic syndrome in patients with iMN. It would appear, however, that doses of at least 80 IU at least twice weekly are necessary to achieve an effect, but the precise duration of therapy remains to be determined, as we did note ongoing further improvement in urine protein in most patients after completion of the treatment regimen. However, a longer course of therapy may have resulted in an increased rate of complete as opposed to partial remissions. Given the reasonable side effect profile noted in our study and in previous studies, utilizing synthetic forms of ACTH, longer treatment courses should be feasible, but ongoing close follow-up for side effects in larger, longer trials is warranted.
Recent understanding with respect to the mechanism of renal disease in iMN includes the notion of antibody reactivity to primary antigenic targets on the podocyte. Subjects with the CC genotype in rs35771982 in the phospholipase A (2) receptor (PLA2R) have been noted to have a higher susceptibility to idiopathic MN compared with subjects with other genotypes [26], and circulating autoantibodies against the M-type phospholipase A (2) receptor (anti-PLA2R) were recently identified in patients with iMN. This IgG4 subclass autoantibody has been noted to be present in the majority of patients with idiopathic disease (>70%) and has been noted to be associated with disease activity and treatment response [16]. In a recent study, treatment with rituximab resulted in clearing or improvement of the antibody titer in 68% of patients, and these patients faired clinically better than those who did not clear the antibody [27]. The timing of the response with decreasing proteinuria following the decrease in the antibody titer supports a cause and effect mechanism secondary to immunomodulation. Still, similar to our experience with ACTH, responses were also noted in patients without positive baseline anti-PLA2R autoantibodies suggesting perhaps the presence of other important autoantibodies or alternate mechanisms in addition to an immunomodulatory response. Although it has been previously suggested that patients without autoantibodies may be destined to achieve a spontaneous remission, and therefore, not require immunosuppressive treatment, the timeline for response in our antibody negative group was virtually identical to the antibody-positive patients, supporting a treatment effect.
How ACTH might work to modify the pathobiology of nephropathies like iMN is currently unknown. Its potential therapeutic mechanisms are numerous and complex [28]. Potential renoprotective mechanisms include corticosteroid-mediated systemic immunosuppression and anti-inflammatory actions subsequent to ACTH-induced steroidogenesis through MCR 2 interaction as well as direct MCR-mediated immunomodulation and anti-inflammatory effects (MCR 1, 3 and 5). Correction of dyslipidemia mediated by MCR 1 and 5 on hepatic cells and neurogenic anti-inflammatory effects mediated by MCR 3 and 4 expressed in the central nervous system are likely also beneficial to the kidneys. Finally, a direct MCR-mediated protective effect on kidney cells, particularly the podocytes, has been described. It has been hypothesized that by modulating apolipoprotein metabolism, ACTH might restore glomerular expression of clusterin, known to be reduced in glomerular diseases such as iMN [29]. In experimental models of iMN, clusterin has been shown to compete with the C5b-9 membrane attack complex, the terminal components of the complement cascade [30]. Clusterin, therefore, may play a critical role in the protection of the glomerular lesions caused by complement, whereas deficient expression could enhance these lesions. In humans, glomerular clusterin has been associated with better outcomes in iMN [31]. It is, therefore, possible that ACTH may act at least in part through a direct renal mechanism. This is supported by a recent publication in the rat model of passive Heyman nephritis that demonstrated improvement after treatment with a melanocortin receptor agonists [11]. Through synaptopodin staining, the authors were able to identify MCR 1 in the renal podocytes. Subsequent treatment with a MCR 1 agonist improved proteinuria as well as foot process effacement along with normalization of the glomerular basement membrane.
In summary, this pilot study suggests that natural ACTH in the form of H.P. Acthar® Gel is a potential treatment for iMN that warrants a large-scale, randomized controlled trial. Although a cumulative dose of at least 80 IU twice weekly for 3–6 months appears to be necessary for a response, the precise dose and duration of therapy required to produce a sustained response remains unknown. In the short term, the drug is well tolerated and appears safe, but the long-term safety profile requires further study. Currently, the high cost of the drug is an additional significant barrier to widespread use of this therapy.
CONFLICT OF INTEREST STATEMENT
The study was supported by an unrestricted research grant from Questcor Pharmaceuticals. Questcor Pharmaceuticals had no role in the design of the study, evaluation of the results or writing of the manuscript.
REFERENCES
- 1.Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483–487. doi: 10.2215/CJN.00710805. [DOI] [PubMed] [Google Scholar]
- 2.MacTier R, Boulton Jones JM, Payton CD, et al. The natural history of membranous nephropathy in the West of Scotland. Q J Med. 1986;60:793–802. [PubMed] [Google Scholar]
- 3.Noel LH, Zanetti M, Droz D, et al. Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med. 1979;66:82–90. doi: 10.1016/0002-9343(79)90486-8. [DOI] [PubMed] [Google Scholar]
- 4.Zucchelli P, Ponticelli C, Cagnoli L, et al. Long-term outcome of idiopathic membranous nephropathy with nephrotic syndrome. Nephrol Dial Transplant. 1987;2:73–78. [PubMed] [Google Scholar]
- 5.Davison AM, Cameron JS, Kerr DN, et al. The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol. 1984;22:61–67. [PubMed] [Google Scholar]
- 6.Cattran DC, Pei Y, Greenwood CM, et al. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51:901–907. doi: 10.1038/ki.1997.127. [DOI] [PubMed] [Google Scholar]
- 7.Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 8.Polanco N, Gutierrez E, Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2012;2:186–197. KDIGO Clinical Practice Guideline for Glomerulonephritis Kidney Int Suppl. [Google Scholar]
- 10.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2012;8:122–128. doi: 10.1038/nrneph.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindskog A, Ebefors K, Johansson ME, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg AL, Hansson P, Nilsson-Ehle P. ACTH 1–24 decreases hepatic lipase activities and low density lipoprotein concentrations in healthy men. J Intern Med. 1991;229:201–203. doi: 10.1111/j.1365-2796.1991.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 13.Berg AL, Nilsson-Ehle P. Direct effects of corticotropin on plasma lipoprotein metabolism in man—studies in vivo and in vitro. Metabolism. 1994;43:90–97. doi: 10.1016/0026-0495(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 14.Berg AL, Nilsson-Ehle P. ACTH lowers serum lipids in steroid-treated hyperlipemic patients with kidney disease. Kidney Int. 1996;50:538–542. doi: 10.1038/ki.1996.346. [DOI] [PubMed] [Google Scholar]
- 15.Arnadottir M, Berg AL, Dallongeville J, et al. Adrenocorticotrophic hormone lowers serum Lp(a) and LDL cholesterol concentrations in hemodialysis patients. Kidney Int. 1997;52:1651–1655. doi: 10.1038/ki.1997.498. [DOI] [PubMed] [Google Scholar]
- 16.Hofstra JM, Beck LH, Jr, Beck DM, et al. Anti-phospholipase A(2) receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 18.Clinical Practice Guidelines for Treatment of Dyslipidemia. Am J Kidney Dis. 2003;41(4 Suppl 3):I–IV, S1–91. [PubMed] [Google Scholar]
- 19.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauson HD, Forman CW, Mc NH, et al. The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J Clin Invest. 1954;33:657–664. doi: 10.1172/JCI102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soshea JW, Farnsworth EB. Serum lipid analysis in the nephrotic syndrome under ACTH administration. J Lab Clin Med. 1951;38:414–419. [PubMed] [Google Scholar]
- 22.Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004;19:1305–1307. doi: 10.1093/ndt/gfh110. [DOI] [PubMed] [Google Scholar]
- 23.Ponticelli C, Passerini P, Salvadori M, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Rauen T, Michaelis A, Floege J, et al. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1–24. Clin Nephrol. 2009;71:637–642. doi: 10.5414/cnp71637. [DOI] [PubMed] [Google Scholar]
- 25.Bomback AS, Canetta PA, Beck LH, Jr, et al. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36:58–67. doi: 10.1159/000339287. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Chin HJ, Na KY, et al. Single nucleotide polymorphisms in the phospholipase A2 receptor gene are associated with genetic susceptibility to idiopathic membranous nephropathy. Nephron Clin Pract. 2011;117:c253–c258. doi: 10.1159/000320194. [DOI] [PubMed] [Google Scholar]
- 27.Beck LH, Jr, Fervenza FC, Beck DM, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catania A, Lonati C, Sordi A, et al. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghiggeri GM, Bruschi M, Candiano G, et al. Depletion of clusterin in renal diseases causing nephrotic syndrome. Kidney Int. 2002;62:2184–2194. doi: 10.1046/j.1523-1755.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Hori Y, Hanafusa N, et al. Clusterin is up-regulated in glomerular mesangial cells in complement-mediated injury. Kidney Int. 2001;59:137–146. doi: 10.1046/j.1523-1755.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- 31.Rastaldi MP, Candiano G, Musante L, et al. Glomerular clusterin is associated with PKC-alpha/beta regulation and good outcome of membranous glomerulonephritis in humans. Kidney Int. 2006;70:477–485. doi: 10.1038/sj.ki.5001563. [DOI] [PubMed] [Google Scholar]