Abstract
Background: Triple negative breast carcinomas (TNBC) do not benefit from hormonal or Herceptin therapies. In search of novel therapeutic targets for TNBC, interest is escalating in a subset of these tumors that are androgen receptor (AR) positive with potential benefit from anti-androgen therapy. Against this background, the frequency of AR expression alone and in combination with other markers and morphologic features was assessed to identify TNBC subtypes for targeted therapy. Methods: 400 consecutive invasive mammary carcinomas with known estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR) and HER2 status were selected for study. The frequency of AR positivity alone or in combination with other markers was recorded with specific attention to the morphology of AR+ TNBCs. Ki67 was evaluated in selected group of cases. ASCO/CAP guidelines were used for interpretation of the various biomarkers. Results: Of the 400 tumors, 32 (8%) carcinomas were quadruple negative (ER-, PR-, AR-, Her2-), while 50 tumors (12.5%) were triple negative (ER-, PR-, Her2-); 18 (36%) of the triple negative tumors were AR positive and 10 (55%) of these were classic apocrine carcinomas. Fourteen cases, all apocrine carcinomas, were AR and Her2 positive. All 32 QN carcinomas were poorly differentiated and they had the highest Ki67 labeling index. Conclusion: The relatively high proportion of AR+ tumors (36%) among the 50 triple negative carcinomas is an important finding in support of routine assessment of AR in at least all TNBCs and apocrine carcinomas as a potential target for therapy.
Keywords: Androgen receptor (AR), estrogen receptor (ER), progesterone receptor (PR), HER2, breast cancer
Introduction
Breast cancer is the most common malignancy among women in the US, with 232,340 new cases accounting for 29% of all cancers estimated to develop in women in 2014 [1]. Furthermore, it is the second leading cause of death from cancer among women with 39,620 deaths, accounting for 14% of all deaths from cancer among women in 2014 [1]. Therefore, there is substantial interest in identification of novel markers that could be used as prognostic or predictive markers and therapeutic targets. Expression of estrogen receptor (ER), progesterone receptor (PR) and Human Epithelial Growth Factor Receptor 2 (Her2) as predictive and/or prognostic markers has been well established in multiple studies and has led to a major shift in treatment approach from nonspecific chemotherapy to more targeted treatments reducing the undesirable systemic side effects of chemotherapy [2-7]. These targeted approaches have improved prognosis and outcome among patients with ER, PR and/or HER2 positive breast carcinomas. Patients with triple negative breast cancer (TNBC) accounting for approximately 10% to 24% of all breast cancers [8-12], are excluded from the benefits of such targeted therapies, however [13].
The prevalence of TNBC depends on the threshold for positivity of biomarkers used in various studies. The latest CAP/ASCO guideline for ER and PR assessment [14] has recommended a threshold of 1% for positivity. The threshold for HER2 positivity has been reduced from 30% to 10% when using the immunohistochemical (IHC) approach and for in situ hybridization, the HER2/CEP17 ratio required for gene amplification is now ≥2 (reduced from 2.2) or a HER2 copy number of ≥6 signals per cell [15].
Novel therapeutic targets and options are particularly needed for the triple negative breast carcinoma (TNBC) due to the futility of conventional anti-hormonal therapies and HER2 blocking agents in this group [16]. Recently, there has been substantial interest in identifying novel therapeutic options for TNBC, the role of androgens and androgen receptor (AR) as a potential multifaceted biomarker. Available studies have provided divergent opinions on the role of androgens in TNBC and correlation of AR expression with prognosis, clinical outcome and chemosensitivity in various settings [13,16-27]. Frequently co-expressed with ER, PR and/or Her2, AR is the most commonly expressed (close to 90% in some studies) receptor among all types of breast cancer [25,28-30] with a frequency of 6.6-75% among TNBC cases [17,18,27,31-49,50]. Moinfar et al [25] found AR expression in 88% of grade 1 invasive breast cancers compared to 47% of grade 3 tumors and concluded that AR is the most frequently expressed marker even among high grade breast carcinomas. Although the reported expression rate for AR has varied widely, probably due to different cut off points used in various studies, it has been suggested that its expression in TNBC has prognostic value; the presence of an apocrine signature at the molecular level is also thought to have clinical relevance [17,28,31,32,50-52]. AR expression in combination with other markers has been linked to smaller tumor size, lower histological grade, lower clinical stage, lower mitotic rate, lower proliferation index (measured by Ki-67) and decreased aggressive behavior [18,19,34,40-42,46,47,50]; lower expression of AR correlates with earlier metastasis, shorter disease free intervals and lower survival rates [18,19,41,47]. There are variations in results of different studies, however, due to differences in the patient populations and/or due to variations in methodology.
One important consideration, not addressed in prior publications, however, is that AR is expressed in two types of mammary epithelial cells. It is most uniformly and diffusely expressed in metaplastic apocrine cells that are a common cell population in the breast particularly as a component of fibrocystic changes. Apocrine cells (benign or malignant) do not express either ER or PR in over 90% of cases even though they do have the messenger RNA for ER [53]. AR is also expressed in at least 5 to 30% of normal luminal epithelial cells that lack any evidence of apocrine differentiation where it is often, but not always co-expressed with ER and or PR; in a given duct, sometimes the AR + cells exceed the ER+ and/or PR+ cells (Figure 1A-C). It is important to note that expression of ER, PR or AR is not uniform in the normal epithelial cells lining mammary ducts and ductules; some ductules/ducts may show 100% positivity, while others may show 2% or no positivity. Tumors derived from these two different cell populations or showing apocrine vs non-apocrine differentiation may share expression of AR, but are morphologically quite distinct and they may also show different responses when AR is used as a therapeutic target since AR expression maybe variably controlled in the two cell types. Despite existence of some data supporting a prognostic value for AR positivity regardless of ER status [28], the lack of sufficient data in support of AR as a predictive marker and lack of consistency in various studies have prevented the routine assessment of AR expression even among patients with TNBC. Considering the availability of androgen inhibitors - i.e. Bicalutamide - currently under investigation in clinical trials (such as phase II trial of Bicalutamide in AR+/ER- metastatic breast cancer) and the possible prognostic value of AR status in breast cancer patients, investigation of AR as a potential target for therapy and clarification of the impact of AR positivity on tumor behavior and responsiveness to various chemotherapies is crucial [18,19,26,27,36,39-42,47].
Figure 1.
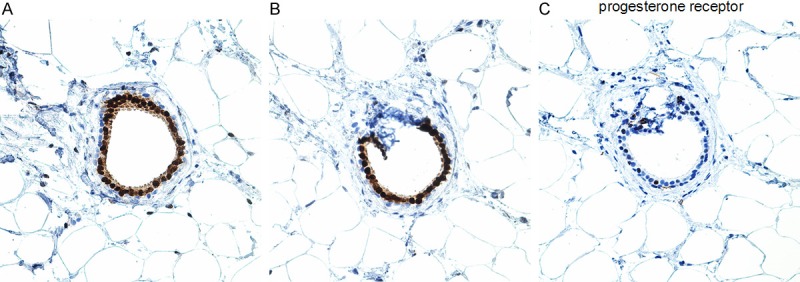
Normal luminal epithelium in the same duct showing diffuse (100%) positivity for AR (A) and 80% positivity ER (B), but substantially less positivity for PR (C) (Immunostains for AR and PR, Dako, Carpinteria, CA; immunostain for ER, Marque, Rocklin, CA).
This study was initiated to determine the prevalence of AR positivity when assessed uniformly among 400 invasive breast carcinomas and correlate AR positivity with other predictive markers (ER, PR, and HER2), patient age, and morphologic subtype to ultimately determine the proportion of carcinomas that would qualify as quadruple negative (ER-, PR-, AR- and HER-2), those that are only AR positive, those that are positive only for AR and HER2 as well as those that would qualify as quadruple positive. Our major interest was to determine the frequency of tumors that are solely AR+ as this could provide a new therapeutic option for the so-called “triple negative” carcinomas. We also compared the morphology and the Ki67 labeling index of “AR+ only” tumors with the quadruple negative tumors and those that are positive only for AR and HER2.
Materials and methods
After Institutional Review Board approval, a total of 400 consecutive mammary invasive carcinomas of any type (ductal, lobular, apocrine, micro-papillary, tubular, and special types) in women diagnosed either on core biopsies or more extensive surgery at Yale-New Haven Hospital between July 2012 and July 2013, with known status for four markers - estrogen receptor (ER), progesterone receptor (PR), androgen receptor (AR) and HER2 - were selected for study. For a minority of tumors that included the quadruple negative (QN), the Her2+/AR+ and the “AR+ only” tumors, Ki-67 was also used to determine the proliferative rate based on presence of nuclear reaction; Ki67 was available on a small proportion of the remaining cases. The frequency of positivity for each marker alone and in various combinations with any and all of the other markers was recorded.
The findings were analyzed using Microsoft Excel 2010. The relationship between expressions of different markers was analyzed using Chi- square test. A P-value of less than 0.05 was considered statistically significant. The data regarding patients’ demographics, histopathologic subtypes and receptor expressions were extracted from patients’ reports; the diagnostic slides of all quadruple negative (QN) carcinomas, cancers that were positive only for AR and those positive for AR and Her2 were re-examined for confirmation of the morphologic subtypes. Approximately 20% of the remaining cases were randomly selected for histologic review by one of the authors (F.A.T.).
Pure intraepithelial neoplasias (in situ carcinomas) were excluded. For immunohistochemical and FISH analysis, 4-micron thick serial tissue sections prepared from formalin-fixed, paraffin embedded blocks were used. In addition to immunohistochemical assessment of HER2, FISH was performed on all cases and information regarding amplification and ratio of HER2/CEP17 was available for all tumors.
The thresholds suggested by the 2011 ASCO/CAP guidelines [54] for ER/PR and the 2007 ASCO/CAP guidelines for HER2 interpretation [55] were used in all 400 cases. ER (estrogen receptor) and PR (progesterone receptor) assays were considered positive if at least 1% of tumor cells’ nuclei show positivity regardless of intensity (1+ to 3+). We applied the same approach for androgen receptor (AR) and considered at least one percent nuclear staining of any intensity (1+ to 3+) as a positive AR assay. The specific percentage of positive cells and their intensity of staining (1+ to 3+) with each of the 3 receptors were also recorded for all cases. For immunohistochemical (IHC) assessment of HER2, the results were semi-quantitatively scored on a scale of 0 (no staining or faint/weak membrane staining in ≤10% of tumor cells), 1+ (faint partial membrane staining detected in >10% of invasive tumor cells), 2+ (weak to moderate complete membrane staining in >10% of invasive tumor cells) and 3+ (uniform, intense membrane staining in >30% of invasive tumor cells). Scores of 0 and 1+ are considered negative, 2+ is indeterminate, and 3+ is positive. A fluorescent in situ hybridization (FISH) ratio (HER2 gene signals to chromosome 17 signals) of more than 2.2 was considered positive; a ratio of 1.8 to 2.2 was considered indeterminate and <1.8 was considered negative. At our institution, HER2 status of all breast carcinomas is assessed routinely by both immunohistochemistry (IHC) and fluorescent in situ hybridization (FISH).
For Ki67 labeling index (LI), the percent of cells with nuclear positivity and the intensity (1+ to 3+) of staining were recorded. The assessment was based on counting the number (ultimately percent) of positive nuclei in 500 (5 X100) cells from different areas of the tumor to include at least one hot spot; a percentage of ≥14% was considered elevated. The antibodies used for AR, ER, PR, HER 2 and Ki-67 in our laboratory, the vendors and the exact dilutions that were used are listed in Table 1. The frequency of positivity for each marker alone and in various combinations with any and all other markers was recorded.
Table 1.
Specifications of various antibodies used
| Antibody | Clone | Cat#/Vendor | Dilution | Retrieval |
|---|---|---|---|---|
| Androgen Receptor | AR441 | M3562/Dako, Carpinteria, CA | 1:150 | High pH 20 min |
| Estrogen Receptor | SP-1 | 249R-16/Cell Marque, Rocklin, CA | 1:50 | High pH 20 min |
| Progesterone Receptor | PgR636 | N1630 | 1:2 | Low pH 20 min |
| Ki-67 | MIB-1 | M7240/Dako, Carpinteria, CA | 1:300 | High pH 20 min |
| HER2 | Poly | K5207/Dako, Carpinteria, CA | Kit | Low pH 60 min |
Results
Frequency of AR, ER, PR and HER2 expression-overall, in various combinations or alone
Among the four markers evaluated, AR was the most frequently expressed with positivity in 351 cases (87.8%), followed by ER with 332 positive cases (83%), PR expressed in 295 cases (73.75%) and finally HER-2 that was positive (by IHC and/or FISH) in 41 cases (10.25%).
We compared all possible combinations of receptor positivity among the tumors. Some of these receptor-positive groups have clear overlap with others; the various combinations are shown in Supplementary Chart 1.
Of the 400 tumors evaluated, 32 (8%) carcinomas were quadruple negative (ER-, PR-, AR- and HER2-), while 15 tumors (3.75%) were positive for all 4 biomarkers (quadruple positive: ER+, PR+, HER2+, and AR+). Fifty tumors (12.5%) were triple negative (ER-, PR-, and HER2-), while 15 (3.75%) patients were triple positive (ER+, PR+, and HER2+) and 255 (63.8%) were positive for ER, PR and AR. Of the 50 triple negative carcinomas, 18 (36%) were positive for AR.
Tumors with AR as the only positive marker (18 of 400, 4.5%) were far more common than cases with PR as the only positive receptor (1 patients; 0.25%), those with only HER2 positivity (3 tumors, 0.75%) and even those with only estrogen receptor positivity (3 cases, 0.75%). Furthermore, the case that was positive for PR only showed a low level of positivity (reported as 3% of cells with faint intensity of positivity). The most common “two receptors only” positive combination was androgen and estrogen receptor positive tumors accounting for 9.5% of all patients (38/400), while 3.5% of tumors (14/400) were AR and HER2 positive and 9 patients (2.3%) were ER and PR positive only.
We found that androgen receptor expression has a strong positive correlation with estrogen or progesterone receptor expression (p value <0.0001), but there was no specific correlation between AR and HER2 expression (Table 2).
Table 2.
Correlation between expression of different receptors (using Chi-Square test); AR expression is positively correlated to ER expression and PR expression, but it is not correlated to the expression of HER-2
| Observed | HER2+ | HER2- | ER+ | ER- | PR+ | PR- |
|---|---|---|---|---|---|---|
| AR+ | 36 | 318 | 322 | 32 | 286 | 68 |
| AR- | 5 | 41 | 11 | 35 | 9 | 37 |
| Predicted* | HER2+ | HER2- | ER+ | ER- | PR+ | PR- |
| AR+ | 36.3 | 317.7 | 294.7 | 59.3 | 261.075 | 92.925 |
| AR- | 4.72 | 41.28 | 38.3 | 7.7 | 33.93 | 12.075 |
| P-value | 0.95 | 0.89 | <0.0001 | <0.0001 | 0.003 | <0.0001 |
Predicted values are calculated values that would have been observed if each receptor expression was completely independent of all other receptors’ expression, and is calculated based on the observed ratio of each receptor’s positivity in total population.
To explore how changing the threshold for AR positivity would impact the total number of AR positive cases, the AR+ cases were divided into two groups: those with positivity in 1-9% of the cells and those with expression in 10% or more. From a total of 351 AR+ cases, 16 (4.6%) expressed AR in 1-9% of the invasive cells, while the remainder (335; 83.75%) had ≥10% positivity. Among the 18 cases only positive for AR, 4 (22%) had AR expression in 1-9% of the invasive cells (2 of these in 1% of the cells only), while 14 cases (78%) showed ≥10% staining. Likewise, application of 1% threshold for ER positivity compared to the higher percentage required in prior CAP/ASCO guidelines, transformed at least 8 (16%) triple negative tumors to hormone receptor positive cases.
Three tumors with AR positivity also had nodal metastases that were evaluated for AR and all three metastases also expressed AR. A fourth case with AR expression in the primary tumor also had retention of AR expression in a local recurrence. None of these 4 cases was AR+ only.
Age distribution
The patients - all female - ranged in age from 25 to 93 years with a median of 62 years and an average of 62.5 years. To determine the potential impact of age on type of receptor positivity, the patients were divided into six age groups (20-39, 40-49, 50-54, 55-59, and ≥60); the distribution of various positive markers relative to the age groups is illustrated in Supplementary Figure 1.
The 32 (8%) women with quadruple negative (ER-, PR-, AR-, Her2-) tumors ranged in age from 29 to 84 years with a median of 58 years. Of these, 14 (46.9%) were 60 years of age or older, 6 were in the 55-59 year group, 3 in the 50 to 54 year group, 7 (23.5%) in the 40 to 49 year group, and 2 (6.5%) in the 20 to 39 year group.
The 50 patients with triple negative (ER-, PR-, HER2-) tumors ranged in age from 29 to 90 years with a median of 62.5 years. Of these, 28 (56%) were 60 years or older, while 12 (23.5%) were in the 40-49 year group. The 18 women with TNBC who were AR+ (“AR+ only”) ranged in age from 41 to 90 years of age with a median of 65 years; 12 (66%) patients were over 60 years of age, 2 (11%) were in the 55-59 year group (both 58 years of age), 0 in the 50 to 54 year group, 4 (22%) in the 40 to 49 year group, and 0 in the 20 to 39 year group.
The 15 patients with quadruple positive tumors (ER+, PR+, AR+, HER2+) ranged in age from 45 to 85 years with a median of 62 years. Of these, 9 were 60 years or older, 2 were in the 55-59 year group, 1 in the 50 to 54 year group, 3 in the 40 to 49 year group, and 0 in the 20 to 39 year group.
The 14 AR+ and HER2+ tumors occurred in 13 women who ranged in age from 45 to 85 years with a median of 59 years. Of these, 7 (50%) were 60 years or older, 3 (20%) were in the 55-59 year group, 2 (15%) in the 50 to 54 year group, 2 (15%) in the 40 to 49 year group, and 0 in the 20 to 39 year group.
The number of patients with only ER, PR, or Her2 (3, 1 and 3 respectively) was too small to find a possible relationship with age; the ER+ and PR+ patients (total of 4) were all 60 years of age or older, while the 3 with tumors positive only for HER2 were 33 and 39 and 84 years of age. Interestingly, the highest percentage of patients ≥60 years of age was among the AR+ only cases.
Age distributions for patients with only one positive receptor, those with QP (TP and AR+) or TN (and AR+) tumors are illustrated in Supplementary Figure 1.
Morphology
Of the 18 triple negative, but AR positive tumors (AR+ only), 10 (55%) had classic apocrine morphology (Figure 2A and 2B), while the 8 remaining carcinomas were poorly differentiated carcinomas with 2 of the 8 showing some apocrine features. Those with apocrine features were composed of cells that had prominent nucleoli, but limited eosinophilic to basophilic cytoplasm without the granularity of classic apocrine cells.
Figure 2.
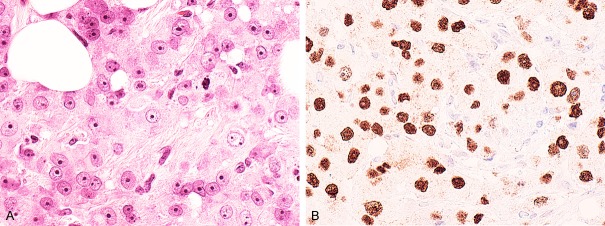
A. The tumor cells in this (AR+, Triple negative breast carcinoma) apocrine carcinoma have granular, pink cytoplasm and prominent nucleoli (Hematoxylin and eosin stain). B. There is diffuse nuclear AR positivity in the tumor cells (Immunostain for AR, Dako, Carpinteria, CA).
We also reviewed all 32 quadruple negative (ER-, PR-, HER2-, AR-) cases. Morphologically, 4 of the 32 tumors were metaplastic/special type carcinomas with squamous (n=3) or chondroid (n=1) differentiation, 3 were moderately differentiated ductal carcinomas, one was a poorly differentiated apocrine carcinoma, while three of the remaining 24 poorly differentiated carcinomas had apocrine features. The poorly differentiated infiltrating carcinomas had high mitotic rates, variable lympho-plasmacytic infiltrate and/or tumor cell necrosis. Furthermore, 4 of the 24 poorly differentiated carcinomas had BRCA germ line mutations (3 tumors had BRCA1 mutation and 1 had BRCA2 mutation); there may have been additional BRCA+ cases, but the information was not available in the pathology files.
Of the 400 tumors, 14 (3.5%) were AR and HER2 positive only; all had apocrine differentiation. Thirteen of the 14 cases were apocrine ductal carcinomas, 4 of which were poorly differentiated; the 14th case was a pleomorphic lobular carcinoma with apocrine differentiation.
Ki67 labeling index
The Ki67 labeling index was not available on all cases, but was available for nearly all (17 of 18) tumors that were quadruple negative, the “AR+ only” group, and the 14 that were AR+ and Her2+. Among the 17 QN tumors (Figure 3A and 3B), the Ki67 labeling index ranged from 18% to over 90%, with a median of 64%. Three of the four cases with values of less than 60% were apocrine carcinomas with Ki67 LI of 18%, 22% and 22%. A carcinoma with chondroid differentiation also had a Ki67 LI of 18. Of the 11 “AR+ only” tumors with available Ki67 assessment, the labeling index ranged from 8% to 96%, with a median of 54%. For the 10 of 14 AR+ and Her2+ tumors with Ki67 assessment, the Ki67 LI ranged from 16% to 86% with a median of 48%. Of the remaining cases, 36 had Ki67 LI available and these ranged from 8% to 24% with only 9 having LI values of ≥14%.
Figure 3.
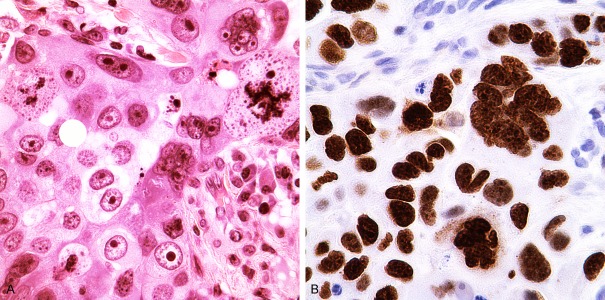
A. Quadruple Negative poorly differentiated apocrine carcinoma with bizarre nuclei and numerous abnormal mitotic figures (Hematoxylin and eosin stain). B. Ki67 proliferation index was 86% in this case; the negative nuclei are mostly stromal cells and lymphoplasmacytic infiltrate (Immunostain for Ki67, Dako, Carpinteria, CA).
The highest Ki67 labeling index was noted among the QN and AR+ only tumors. A majority of the QN carcinomas were poorly differentiated infiltrating duct carcinomas of no special type and had a very high Ki67 LI of >60%.
Discussion
For patients with TNBC (ER-, PR-, Her2-), systemic therapy is limited predominantly to chemotherapeutic options. While some of the TNBC cases are of special types (squamous, chondroid, etc) and a few have medullary features (with or without BRCA germ-line mutations), a substantial proportion is poorly differentiated ductal carcinomas, not otherwise specified (NOS). Androgen receptor is the most commonly expressed receptor [25] in breast carcinoma, but it is generally co-expressed with one or more of the other biomarkers (ER, PR, HER2). Our major interests included determination of the proportion of TNBCs that would have expression of AR and might potentially benefit from AR-related targeted therapy and how the expression of AR only correlates with morphology. Expressed in 351 (87.8%) of our 400 cases, AR was the sole receptor expressed in 18 cases reflecting 4.5% of the 400 cases and 36% of the 50 TNBC, and it was the only steroid receptor expressed in combination with HER2 in another 14 (3.5%) of the tumors. This implies that about 8% of all tumors and 36% of TNBCs could potentially benefit from using AR as a target for therapy in the neo-adjuvant setting. Also, it seems logical to consider AR as an alternate target when ER+ and/or HER2+ carcinomas become resistant to targeted therapies against ER and/or HER2. At our institution, we currently evaluate all invasive carcinomas for expression of AR in addition to ER, PR and HER2 (the latter by both IHC and FISH) in the hope of ultimately using this information for prognostic and therapeutic purposes.
Given the strong correlation of apocrine differentiation with expression of either “AR only” (10 of 18 with classic apocrine morphology and another 2 with apocrine features) or AR and Her2 (14/14 cases), it is only reasonable to routinely assess for expression of AR if not in all breast carcinomas, at least in all apocrine and triple negative carcinomas to avail the patients of the potential benefits of AR as a therapeutic target.
An important consideration for future studies would be to separate apocrine carcinomas that are AR positive from non-apocrine, AR+ tumors to determine the possible impact of morphologic apocrine differentiation on the ultimate response and also level of response to therapies against AR. Also, it would be important to determine if AR expression is retained in metastatic and recurrent tumors.
In our study, a classic apocrine carcinoma was defined as a tumor in which 90% of the tumor was composed of cells with abundant pink cytoplasm with variable granularity and nuclei with prominent nucleoli. Tumors with only one of the two features were interpreted as having apocrine features. Interestingly, many of the apocrine carcinomas in our study were not only AR+, but also had a high Ki67 labeling index, but most of them were moderately to poorly differentiated. The Ki67 labeling index was also high for tumors that were AR+ and Her2+ only, but was highest for the quadruple negative carcinomas (median of 48%; range 16% to 86%). Again, a majority of these cases were poorly differentiated carcinomas. Nonetheless, it appears that at least in the setting of apocrine differentiation, either AR and/or Her2 expression develop even in relatively primitive cells that are undergoing rapid proliferation, whereas substantial cellular differentiation may be required for expression of ER and PR.
Furthermore, 12 of the 18 (66%) AR+ only tumors occurred in women ≥60 years of age; while 7 (50%) of those with Her+ and AR+ tumors were ≥60 years. Apocrine cells (whether benign or malignant) are generally AR positive, but negative for ER and PR in over 90% of cases; a small proportion are occasionally ER and/or PR positive particularly with the current low threshold of 1%. Interestingly, it has been shown that apocrine carcinomas have the mRNA for ER, but do not generally produce the protein [53].
Prevalence of AR in TNBC and other biomarker combinations-literature review
Among the 22 studies summarized in Table 3, the percentage of patients with TNBCs ranged from 7.1% to 60% and the proportion of tumors with positive AR among TNBCs ranged from 6.6% to 75% (Table 3). In our study, 12.5% of all breast cancers (50/400) were triple negative, and 36% (18/50) of these expressed AR. It is noteworthy that only 6 of the 22 studies in Table 3 had used the most recent ASCO/CAP guidelines (1% and more) for ER, PR, and AR positivity, while most had used the ≥10% as the minimum staining required for a positive interpretation. In some studies, even older scoring systems were used - such as Remmele Score published in 1987 [56]. In fact, if a threshold of ≥1% were used, many of these could be AR positive and at least some of the triple negative carcinomas would be positive for one or more of the markers. We used 1% as the minimum requirement for AR positivity similar to the most recent ASCO/CAP guidelines [54] for ER and PR. In our study, changing the minimum threshold for AR positivity from ≥1% to ≥10% could result in losing 4.6% of all AR+ cases, and 22% of AR+ TNBC cases. The College of American Pathologists (CAP) along with the American Society of Clinical Oncology (ASCO) has recommended the routine assessment of HER2, ER and PR status for all invasive breast cancers. However, no organization has recommended AR assessment for all invasive breast cancers or even for the triple negative breast cancers. Given the absence of specific guidelines for interpretation of AR, various approaches or requirements for positivity have been used accounting for the substantial variation in the reported frequency of AR positivity in breast carcinoma (Table 3).
Table 3.
Various studies on the frequency of AR positivity among triple negative breast carcinomas (TNBC)
| Study | Number of all patients | Number of TN | Number of AR+ in TNs | AR positivity definition |
|---|---|---|---|---|
| Sutton et al, 2012 [17] | NA | 121 | 38 (31.4%) | ≥1% |
| Mrklic, et al, 2013 [31] | 1849 | 124 (6.7%)* | 27 (32.5%) | ≥1% |
| Alshenawy, 2012 [32] | 150 | 48 (32%) | 36 (75%) | At least >10% |
| McNamara et al, 2013 [18] | NA | 203 | 51 (25%) | >10% LI |
| Koo et al, 2009 [27] | NA | 47 | 5 (10.6%) | >10% |
| Qi e al, 2012 [33] | 980 | 158 (16%) | 84 (53.1%) | ≥10% |
| Tsutsumi, 2012 [34] | 325 | 51 (15.6%) | 21 (41.1%) | ≥1% |
| Gonzalez-Angulo et al, 2009 [35] | 347 | 97 (27.9%) | 17-81 (17.5-83.5%) | NA |
| Niemeier at al, 2010 [36] | 189 | 30 (15.8%) | 2 (6.6%) | >10% |
| He et al, 2012 [37] | NA | 287 | 74 (25.8%) | ≥5% |
| Luo et al, 2010 [38] | NA | 137 | 38 (27.7%) | 1-25%** |
| Park et al, 2010 [39] | 931 | 156 (16.7%) | 21 (13.4%) | ≥10% |
| Chae et al, 2011 [40] | 169 | 12 (7.1%) | 1 (8.3%) | ≥10% |
| Pristauz et al, 2010, [41] | 135 | 44 (32.5%) | 7 (15.9%) | ≥1% |
| Ogawa et al, 2008 [42] | 227 | 42 (18.5%) | 18 (42.9%) | ≥10% |
| Tang et al, 2012 [43] | NA | 127 | 16 (12.6%) | >10% |
| Rakha et al, 2006 [44] | 1726 | 282 (16.3%) | 36 (12.7%) | ≥1% |
| Hu et al, 2011 [45] | 1467 | 211 (14.3%) | 78 (37%) | ≥1% |
| Loibl et al, 2011 [46] | 673 | 111 (17%) | 24 (21.6%) | AR+*** |
| Micello et al, 2010 [47] | 226 | 135 (60%) | 41 (30.3%) | >10% |
| Masuda et al, 2010 [48] | 163 | 31 (20%) | 6 (18%) | Any positive staining |
| Hanley et al, 2008 [49] | 94 | 20 (21%) | 5 (25%) | >10% |
| Thike et al, 2014 [50] | 699 | 699 | 265 (38%) | ≥1% |
| Current study, 2014 | 400 | 509 (12.5%) | 18 (36%) | ≥1% |
83 selected after exclusion of patients who had received chemotherapy;
Minimum of 1-25% and at least 2+ intensity;
Remmele Score.
For AR, we used the same threshold (≥1%) proposed by the 2011 ASCO/CAP guidelines [53] for ER and PR, but more evidence would be valuable to determine the optimal approach for interpretation of positivity for all these markers rather than arbitrarily switching from 10% to 1% in cases of ER/PR and to 30% for HER-2 in the 2011 guidelines; the latter has been changed back to 10% in the 2013 ASCO/CAP, HER2 guidelines [15]. More studies are needed to determine the optimal/valid percentages required for a positive marker; this percentage could impact the level of predictability of a marker. Ultimately and optimally, a single standard criterion would be necessary to compare results of various studies. At least some of the Her2 negative cases by the 2007 ASCO/CAP standards [55] would qualify as positive by the current 2013 guidelines [15]. By current standards of positivity for ER and PR, a proportion (potentially significant) of triple negative carcinomas would be ER and/or PR positive.
Considering the percentage of AR positivity in triple negative patients in previously published studies and among our cases, routine determination of AR status could be critical at least for triple negative and apocrine carcinomas as well as the HER2 positive only cases. The favorable impact of AR positivity on outcome among TNBC and basal like breast cancer (BLBC) noted in the recent literature [50] is another incentive to determine AR even for prognostic purposes.
The co-expression of AR and HER-2 in ER+ and ER- groups has been examined previously. At least one study [39] concluded that the AR expression significantly correlated with HER2 overexpression in ER negative tumors (P<0.001) but not in ER positive tumors (P=0.297) and that HER-2 occurs in ER- carcinomas but not among ER+ tumors. The fact that we did have quadruple positive (15 of 400 cases; 3.75%) as well as ER+, AR+ and Her2+ (11 of 400 cases; 2.75%) among our 400 cases indicates that any combination may occur and exclusivity of combinations is rare.
The frequency of AR positive cases has been reported to be high among ER and/or PR positive patients in prior studies [25]. Our data also showed a statistically significant relationship between androgen receptor expression and estrogen and/or progesterone receptor expression (Table 2) but no correlation between AR expression and HER-2 expression when all cases were considered. However, such calculations are strongly dependent on sample size. As a result, due to the small size of the HER-2 positive group compared to AR positive group in our study, a statistically significant difference may not be detectable. This issue should be explored in larger scale studies.
Among our study cases, the AR+, HER2+, ER- and PR- immunoprofile was noted only among apocrine carcinomas. In association with other markers, however, AR and HER2 were expressed in both apocrine and non-apocrine carcinomas. Also, these tumors generally had a high proliferation index as determined by Ki67 immunostaining. The frequency of expression of ER and PR was substantially lower in poorly differentiated carcinomas; ER and PR expression with or without co-expression of AR was generally noted in well to moderately differentiated carcinomas that had lower Ki67 labeling index among the limited number of cases with these characteristics evaluated.
Based on the high frequency of expression of AR and/or Her2 among highly proliferative tumors with apocrine differentiation or poorly differentiated NOS type tumors, one could conclude that once apocrine metaplasia takes place, it determines AR positivity regardless of the level of cellular differentiation; it also appears to increase the chances of HER2 expression. The fact that sometimes either AR and/or HER2 may be the only marker(s) expressed among poorly differentiated carcinomas with a high proliferation rate suggests that the expression of AR and Her2 is independent of proliferative activity, while a higher level of cellular differentiation and lower proliferative activity is necessary for the expression of ER and PR.
As noted in our study and previously by Tsusumi [34], triple negative breast carcinomas can be subdivided into AR positive tumors (36% in our study) that predominantly show an apocrine morphology and quadruple negative (QN) carcinomas that are predominantly poorly differentiated carcinomas of no special type (64% in our study).
Apocrine carcinomas may be positive or negative for HER-2. One study noted HER2 positivity in 23 (52%) of 44 apocrine carcinomas [34]. The role of HER-2 receptor in apocrine breast carcinomas is not clearly understood. The interactions of AR and HER2 has been studied in greater detail in prostate carcinomas; in this setting, HER-2 kinase-dependent modulation of AR function through effects on DNA binding and stability has been suggested [57]. While many believe that HER-2 expression rate in the apocrine subtype (ER-, PR-, AR+) is higher compared to all breast cancers, not all apocrine breast cancers are HER2 positive as shown in our study, however. Tsutsumi and his colleagues found that while 18% of 440 breast cancer cases are HER2 positive, substantially more (52%) of the “apocrine” (ER-, PR-, AR+) subtype is HER2+ [34].
Distinction of apocrine type TNBC from non-apocrine type
The term apocrine carcinoma should be used only for breast cancers with apocrine type morphology. While the expression of AR as the only biomarker correlated with apocrine morphology in our study, it was not exclusive to apocrine morphology. Eight of the 18 tumors with only AR positivity were poorly differentiated ductal carcinomas, not otherwise specifiable (NOS) that lacked classic apocrine morphology; two of the 8 poorly differentiated carcinomas had at least some apocrine features.
Due to involvement of different pathways in the pathogenesis of apocrine TNBC and non-apocrine TNBC and also prognostic and predictive variations, it is very important to distinguish these types of TNBC. This will be an important step toward potential AR directed targeted therapy in each subtype. Recently, Farmer et al [58] suggested using the term “molecular apocrine” for tumors that are ER- and AR+. Since they also noted that all of these tumors had strong apocrine features on histologic examination [58], it would be best to confine the “apocrine subtype” for tumors that display apocrine morphology.
When defined based on immunoprofile (ER-; PR-; AR+), 44 cases in Tsutsumi et al’s study [34] qualified as “apocrine subtype”, though only 22 had classic apocrine morphology. In this study, p53 was detected among 86% of the IHC defined “apocrine subtype” TNBC compared to 46% among the non-apocrine type. Also, though EGFR was highly expressed in 90% of all TNBCs, its expression was even higher (100%) among the “apocrine” TNBC [34]. On the other hand, the rate of CK5/6 expression and Ki67 labeling index were higher among non-apocrine TNBC cases compared to the “apocrine” TNBC [34].
AR-negative TNBCs essentially reflect quadruple negative carcinomas (QN) as noted by Tsu-tsumi et al [34]. In our study, of the 18 quadruple negative tumors, only 4 were of special types with a vast majority reflecting poorly differentiated carcinoma; these tumors also had high Ki67 labeling index and appeared more aggressive morphologically. Currently, there is no targeted therapy available for quadruple negative cancers and further evaluation and exploration of this group is needed to search for therapeutic targets.
Predictive and prognostic value of androgen receptor in TNBC
Several reports have addressed the prognostic and predictive role of androgen receptor in ER+ and ER- breast cancer [36,37,59-61], but there is limited and conflicting data about the role of AR in TNBCs [37,61-63]. He et al [37] compared AR+ and AR- cases among 287 TNBC patients (AR-: 213, AR+: 74) and correlated the AR status with age, tumor size, stage, grade of disease, nodal status, and type of treatment (surgery, chemotherapy and radiotherapy). They concluded that patients with AR negative tumors have a higher frequency of positive lymph nodes compared to those with AR positive tumors [37]. After multivariate analyses, the disease free survival (DFS) and overall survival (OS) were statistically shorter in AR negative group [37], which is in agreement with the results of other studies [38,39]. Luo et al [38] also found that among TNBCs, AR+ tumors have statistically higher 5-year disease fee survival (DFS) compared to AR- TNBCs [38]. However, Gonzalez-Angulo et al [35] did not find any association between AR status in TNBC women, and 5-year OS or relapse-free survival [35]. In a large scale study with long follow-up period, Hu et al [45] also found that among TNBC, women with AR positive tumors had a higher mortality rate compared with those with AR- tumors (p value=0.02) [45]; all of the patients in the study were in menopausal age group.
While higher grade (III) tumors were more common among AR- group compared to the AR+ group, Tang et al [43] found no correlation between lymph node metastasis and AR status among 127 TNBC [43]. They also found that 75% (12/16) of AR+ patients are postmenopausal women compared to 43% (48/111) of patients in the AR- group [43].
Androgen therapy in “AR+ only” breast carcinomas
In pre-clinical models of breast cancer, androgens induce divergent proliferative responses being able to either stimulate or inhibit cell proliferation [64]. Many breast cancers co-express AR along with ER and or PR that can affect AR signaling, basically interfering with the effects of androgens on the breast cancer cells. The role of androgens in breast cancer can be different depending on their estrogen and progesterone receptor status. Therefore, using models that utilize cancer cells that lack expression of ER and PR is important in better understanding the potential value of androgenic therapy in breast cancer [65]. Androgens such as testosterone and Dihydrotestosterone (DHT) can have either an inhibitory or a stimulatory effect on breast cancer cell lines depending on their positivity for other steroid hormone receptors and presence or absence of breast adipose tissue fibroblasts (BAF) in the cell culture [66]. Testosterone induced cell proliferation in ER+ MCF-7 and T47D cell lines, but not in the ER-, MDA-MB-231 tumor cells [66] in the presence of BAF; this has been explained by the high expression of aromatase which converts androgens to estrogens in BAF followed by ER-mediated cell proliferation [66]. In contrast, DHT caused a suppression of cell proliferation in both ER+ MCF-7 and T47D as well as in ER-, MDA-MB-231 cell lines since DHT is not a substrate for aromatase [66]. Both the stimulatory and inhibitory effects of DHT on AR+ cell lines disappeared after adding androgen receptor antagonist, hydroxy flutamide, to the cell culture [64]. Clearly, some androgens may stimulate growth in ER+, AR+ cell lines through estrogen receptor [64,66], while other androgens can prevent the growth of cancer cells and increase cancer cell death in ER-, AR+ cell lines [64,66]. Among tumors that are only positive for AR, current studies have shown conflicting results regarding application of androgen or anti-androgen therapies. As mentioned earlier, many studies advocate the efficacy [26] of androgen agonists in the treatment of ER-, PR-, AR+ (apocrine subtype) TNBC, while other studies [66] have shown the efficacy of anti-androgens in growth restriction of apocrine TNBC cell line (MDA-MB-453). This cell line was one of the four cell lines in the group of Luminal AR (LAR) TNBC in the study of Lehmann and his colleagues [51]. All of these LAR cell lines showed reversal of growth in response to AR antagonist Bicalutamide [51]. The other common characteristic of LAR cell lines in the study of Lehmann et al. was the presence of PIK3CA mutation in all of them which could explain the possible efficacy of AR antagonist and PI3K/TOR targeting in LAR TNBC patients [51]. The efficacy of anti-androgen therapy alone (daily oral Bicalutamide) has been studied in 26 patients with AR+, ER-, PR- breast cancers in a recent Phase II study [26]. Although this study met its pre-specified end point (five patients with clinical benefits), larger scale studies are needed to test the efficacy of anti-androgens alone or in combination with other drugs in AR+ TNBC group and any potential differences in response related to the presence or absence of cytologic apocrine differentiation. Preclinical data also suggest that some agents (enzalutamide) may be useful in treatment of AR+ tumors regardless of ER status since enzalutamide blocks both androgen-mediated and estrogen-mediated cell growth [67].
The exact mechanism of the effect of therapies directed at AR is yet to be determined and may be related to whether classic apocrine differentiation is present or absent and may be influenced by whether the tumor is positive for one or more of other biomarkers (ER, PR, HER2) and the amount of adipose tissue in the body.
The results of studies on androgen-targeted therapies in prostate cancer should be considered as we search for optimal therapies for AR+ TNBCs. Recent studies regarding the efficacy of the FDA approved Abiraterone acetate (68-73) in castration-resistant prostate carcinoma (CRPC) has shown promising results [70-73]. Abiraterone acetate is a selective inhibitor of CYP17A1 (an important enzyme in production of DHEA) [69], currently used in combination with prednisone in CRPC [68,69], and it is well tolerated. This could be a candidate for AR+ TNBC. Besides Abiraterone acetate, other new drugs with similar mechanism of action (androgen synthesis inhibition and androgen receptor antagonism) such as TOK-001, MDV3100 (Enzalutamide), and TAK-700 are currently under investigation for prostate cancer [68].
The efficacy and safety of Bicalutamide in ER-, PR-, AR+, and metastatic breast cancer is being investigated in a phase II trial; also, disease free survival (DFS) and other outcome indices are being evaluated [26]. It is particularly important to also record the development and severity of any side effects of the various AR related therapeutic agents used in management of women with breast cancer.
Conclusion
A substantial proportion of triple negative breast carcinomas are AR+; often, but not always, these show apocrine morphology. It is important to include assessment of AR in our guidelines as part of routine evaluations of at least TNBC and preferably all breast carcinomas and explore AR as a target for therapy. The expression of AR among TNBC has also been shown to be associated with a better survival and its assessment would have prognostic value as well. Any future studies should specify whether the triple negative AR positive tumors have classic apocrine morphology or are of the poorly differentiated, NOS subtype. The morphology of tumors co-expressing Her2 and AR should also be specified to determine whether either AR positivity or apocrine morphology in Her2 positive tumors influences response to Her2 targeted therapies. This information may help explain some of the differences noted in response to AR targeted therapies in prior studies and would simplify future selection of specific therapies (agonist vs antagonist) for patients with AR+ breast carcinomas. It is also important to correlate the threshold of positivity with the level of response in order to optimally select the threshold that provides a certain level of response to a given therapy; one may have to use different thresholds for treatment of primary versus recurrent or metastatic tumors. Finally, the side effects of these novel therapies in AR+ breast cancers should be documented to weigh the benefits vs disadvantages of any regimen.
Supporting Information
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effects of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Tolcher AW, Rowinsky EK. The future of cytotoxic therapy: selective cytotoxicity based on biology is the key. Breast Cancer Res. 2003;5:154–159. doi: 10.1186/bcr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrest AP. Beatson: Hormones and the management of breast cancer. J R Coll Surg Edin. 1982;27:253–263. [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8:135–155. doi: 10.2174/1574884711308020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J Breast Cancer International Research Group. Adjuvant Trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, Veronesi P, Intra M, Torrisi R, Cardillo A, Campagnoli E, Goldhirsch A, Colleoni M. Invasive ductal carcinoma of the breast with “triple negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 9.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J Danish Breast Cancer Cooperative Group. Estrogen receptor, progesterone receptor, HER-2 and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J. Clin. Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KB. Triple negative breast cancers: an updated review on treatment options. Current Oncol. 2011;18:e173–e179. doi: 10.3747/co.v18i4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carey LA. Directed therapy of subtypes of triple- negative breast cancer. Oncologist. 2011;16(Suppl 1):71–78. doi: 10.1634/theoncologist.2011-S1-71. [DOI] [PubMed] [Google Scholar]
- 14.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:e48–72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gucalp A, Traina TA. Triple-negative breast cancer: Role of the androgen receptor. Cancer J. 2010;16:62–65. doi: 10.1097/PPO.0b013e3181ce4ae1. [DOI] [PubMed] [Google Scholar]
- 17.Sutton LM, Cao D, Sarode V, Molberg KH, Torgbe K, Haley B, Peng Y. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinoma. Am J Clin Pathol. 2012;138:511–516. doi: 10.1309/AJCP8AVF8FDPTZLH. [DOI] [PubMed] [Google Scholar]
- 18.McNamara KM, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, Kongdan Y, Wang L, Takagi K, Mayu T, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Ohuchi N, Sasano H. Androgenic pathway in triple negative invasive ductal tumors: its correlation with tumor cell proliferation. Cancer Sci. 2013;104:639–346. doi: 10.1111/cas.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witzel I, Graeser M, Karn T, Schmidt M, Wirtz R, Schütze D, Rausch A, Jänicke F, Milde-Langosch K, Müller V. Androgen receptor expression is a predictive marker in chemotherapy treated patients with endocrine receptor-positive primary breast cancers. J Cancer Res Clin Oncol. 2013;139:809–816. doi: 10.1007/s00432-013-1382-8. [DOI] [PubMed] [Google Scholar]
- 20.Razzak AR, Lin NU, Winer EP. Heterogeneity of breast cancer and implications of adjuvant chemotherapy. Breast Cancer. 2008;15:31–4. doi: 10.1007/s12282-007-0007-y. [DOI] [PubMed] [Google Scholar]
- 21.Baird RD, Caldas C. Genetic heterogeneity in breast cancer: the road to personalized medicine? BMC Med. 2013;11:151. doi: 10.1186/1741-7015-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–8. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 23.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The trile negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 24.Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev. 2009;36:206–15. doi: 10.1016/j.ctrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Moinfar F, Okcu M, Tsybrovsky O, Regitnig P, Lax SF, Weybora W, Ratschek M, Tavassoli FA, Denk H. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–711. doi: 10.1002/cncr.11532. [DOI] [PubMed] [Google Scholar]
- 26.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA Translational Breast Cancer Research Consortium (TBCRC 011) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo JS, Jung W, Jeong J. The predictive role of E-cadherin and androgen receptor on in vitro chemosensitivity in triple-negative breast Cancer. Jpn J Clin Oncol. 2009;39:560–8. doi: 10.1093/jjco/hyp065. [DOI] [PubMed] [Google Scholar]
- 28.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E. Androgen receptor expression and outcome in early breast cancer: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:djt319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 29.Hall RE, Aspinall JO, Horsfall DJ, Birrell SN, Bental JM, Sutherland RL, Tilley WD. Expression of the androgen receptor and an androgen-responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer. 1996;74:1175–1180. doi: 10.1038/bjc.1996.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, Henzen-Logmans SC. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996;32A:1560–1565. doi: 10.1016/0959-8049(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 31.Mrklic I, Pogorelic Z, Capkun V, Tomic S. Expression of androgen receptors in triple-negative breast carcinomas. Acta Histochemica. 2013;115:344–348. doi: 10.1016/j.acthis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Alshenawy HA. Prevalence of androgen receptors in invasive breast carcinoma and its relation with estrogen receptor, progesterone receptor and Her2/neu expression. J Egypt Natl Canc Inst. 2012;24:77–83. doi: 10.1016/j.jnci.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Qi JP, Yang YL, Zhu H, Wang J, Jia Y, Liu N, Song YJ, Zan LK, Zhang X, Zhou M, Gu YH, Liu T, Hicks DG, Tang P. Expression of the androgen receptor and its correlation with molecular subtypes in 980 Chinese breast cancer patients. Breast Cancer (Auckl) 2012;6:1–8. doi: 10.4137/BCBCR.S8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsutsumi Y. Apocrine Carcinoma as Triple-negative Breast Cancer: Novel Definition of Apocrine-type Carcinoma as Estrogen/Progesterone Receptor-negative and Androgen Receptor-positive Invasive Ductal Carcinoma. Jpn J Clin Oncol. 2012;42:375–386. doi: 10.1093/jjco/hys034. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, Traina TA, Hudis C, Hortobagyi GN, Gerald WL, Mills GB, Hennessy BT. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–8. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 36.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–12. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 37.He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, Jiang X, Qin T. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol. 2012;29:406–10. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
- 38.Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer. 2010;29:585–590. doi: 10.5732/cjc.009.10673. [DOI] [PubMed] [Google Scholar]
- 39.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 40.Chae BJ, Lee A, Bae JS, Song BJ, Jung SS. Exression of nuclear receptor DAX-1 and androgen receptor in human breast cancer. J Surg Oncol. 2013;103:768–772. doi: 10.1002/jso.21861. [DOI] [PubMed] [Google Scholar]
- 41.Pristauz G, Petru E, Stacher E, Geigl JB, Schwarzbraun T, Tsybrovskyy O, Winter R, Moinfar F. Androgen receptor expression in breast cancer patients tested for BRCA1 and BRCA2 mutations. Histopathology. 2010;57:877–84. doi: 10.1111/j.1365-2559.2010.03724.x. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, Sakurai K, Inoue T, Nishiguchi Y. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13:431–5. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 43.Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012;29:526–533. doi: 10.1007/s12032-011-9948-2. [DOI] [PubMed] [Google Scholar]
- 44.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 45.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, Marotti JD, Hankinson SE, Colditz GA, Tamimi RM. Androgen Receptor Expression and Breast Cancer Survival in Postmenopausal Women. Clin Cancer Res. 2011;17:1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loibl S, Müller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, Ataseven B, du Bois A, Fissler-Eckhoff A, Gerber B, Kulmer U, Alles JU, Mehta K, Denkert C. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130:477–87. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 47.Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457:467–76. doi: 10.1007/s00428-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 48.Masuda H, Masuda N, Kodama Y, Ogawa M, Karita M, Yamamura J, Tsukuda K, Doihara H, Miyoshi S, Mano M, Nakamori S, Tsujinaka T. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother Pharmacol. 2011;67:911–7. doi: 10.1007/s00280-010-1371-4. [DOI] [PubMed] [Google Scholar]
- 49.Hanley K, Wang J, Bourne P, Yang Q, Gao AC, Lyman G, Tang P. Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high-grade ductal carcinoma of the breast. Hum Pathol. 2008;39:386–92. doi: 10.1016/j.humpath.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Hyat Bay B, Tse GM, Iqbal J, Tan PH. Loss of androgen receptor expression predicts early recurrence in triple negative and basal-like breast cancer. Mod Pathol. 2014;27:352–360. doi: 10.1038/modpathol.2013.145. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campagnoli C, Pasanisi P, Castellano I, Abbà C, Brucato T, Berrino F. Postmenopausal breast cancer, androgens, and aromatase inhibitors. Breast Cancer Res Treat. 2013;139:1–11. doi: 10.1007/s10549-013-2505-2. [DOI] [PubMed] [Google Scholar]
- 53.Bratthauer GL, Lininger RA, Man YG, Tavassoli FA. Androgen and estrogen receptor mRNA status in apocrine carcinomas. Diagn Mol Pathol. 2001;11:113–8. doi: 10.1097/00019606-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Hammond ME, Hayes DF, Wolff AC. Clinical notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J. Clin. Oncol. 2011;29:e458. doi: 10.1200/JCO.2011.35.2245. [DOI] [PubMed] [Google Scholar]
- 55.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology; College of American Pathologists. American Society of Clinical Oncology/College of American Pathologist guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 56.Remmelle W, Stegner HE. Recimmendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 57.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–71. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 59.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120:725–731. doi: 10.1309/42F0-0D0D-JD0J-5EDT. [DOI] [PubMed] [Google Scholar]
- 60.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW, Lee KS. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22:1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 61.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD. Androgen receptor inhibits estrogen receptor alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69:6131–40. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 62.Shah PD, Gucalp A, Traina TA. The role of the androgen receptor in triple-negative breast cancer. Womens Health (Lond Engl) 2013;9:351–60. doi: 10.2217/whe.13.33. [DOI] [PubMed] [Google Scholar]
- 63.Peng Y. Potential prognostic tumor biomarkers in triple-negative breast carcinoma. Beijing Da Xue Xue Bao. 2012;44:666–672. [PubMed] [Google Scholar]
- 64.Birrell SN, Bentel JM, Hickey TE, Ricciardelli C, Weger MA, Horsfall DJ, Tilley WD. Androgens Induce Divergent Proliferative Responses in Human Breast Cancer Cell Lines. J Steroid Biochem Mol Biol. 1995;52:459–67. doi: 10.1016/0960-0760(95)00005-k. [DOI] [PubMed] [Google Scholar]
- 65.Garay JP, Park BH. Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res. 2012;2:434–445. [PMC free article] [PubMed] [Google Scholar]
- 66.Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. Effect of androgens on different breast cancer cells co-cultured with or without breast adipose fibroblasts. J Steroid Biochem Mol Biol. 2013;138:54–62. doi: 10.1016/j.jsbmb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gómez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK. Role of androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16:R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohlmann CH, Kamradt J, Stöckle M. Third generation anti-androgen therapy of advanced prostate cancer. Urologe A. 2012;51:522–6. doi: 10.1007/s00120-011-2760-y. [DOI] [PubMed] [Google Scholar]
- 69.Kluetz PG, Ning YM, Maher VE, Zhang L, Tang S, Ghosh D, Aziz R, Palmby T, Pfuma E, Zirkelbach JF, Mehrotra N, Tilley A, Sridhara R, Ibrahim A, Justice R, Pazdur R. Abiraterone Acetate in Combination with Prednisone for the Treatment of Patients with Metastatic Castration-Resistant Prostate Cancer: U. S. Food and Drug Administration Drug Approval Summary. Clin Cancer Res. 2013;19:6650–6. doi: 10.1158/1078-0432.CCR-13-2134. [DOI] [PubMed] [Google Scholar]
- 70.Nandha R. Abiraterone acetate: a novel drug for castration-resistant prostate carcinoma. J Postgrad Med. 2012;58:203–6. doi: 10.4103/0022-3859.101400. [DOI] [PubMed] [Google Scholar]
- 71.Ryan CJ, Cheng ML. Abiraterone acetate for the treatment of prostate cancer. Expert Opin Pharmacotherapy. 2013;14:91–6. doi: 10.1517/14656566.2013.745852. [DOI] [PubMed] [Google Scholar]
- 72.Dupuy L, Long J, Ranchoup Y. Metastatic castration-resistant prostate cancer: two case reports of dramatic response with abiraterone acetate in patients heavily pretreated with chemotherapy. Case Rep Oncol. 2011;6:325–30. doi: 10.1159/000353131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Logothetis CJ, Efstathiou E, Manuguid F, Kirkpatrick P. Abiraterone acetate. Nat Rev Drug Discov. 2011;10:573–4. doi: 10.1038/nrd3516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


