Abstract
Epidemiological evidence supports a protective effect of physical activity for breast cancer but pre-clinical studies are needed to help define the underlying mechanisms in an age-related manner. We utilized 18-month old BALB/c mice injected in the mammary fat pad with syngeneic 4T1 tumor cells as a model of invasive breast cancer. A negative correlation was observed between daily distance ran, prior to tumor injection, and absolute tumor mass measured at necropsy (Pearson’s r = -0.89, P = 0.0066, R2 = 0.80). A correlation was also observed between distance ran before tumor implant and the histological score for mitotic index (Pearson’s r = -0.85, P = 0.034, R2 = 0.72). Runners showed an increased respiratory exchange ratio during the light cycle (P = 0.029) suggesting that voluntary running shifted resting substrate metabolism toward glucose oxidation, relative to lipid oxidation. The shift in substrate metabolism was significantly different from baseline for both groups of animals, indicating that the tumor burden might have been responsible. The observations from this study indicate that running longer distances is associated with decreased breast tumor burden in old mice, suggesting that physiological factors generated by exercising before tumor onset are protective against tumor progression. The mechanisms for this protective effect are not known but the data show that older mice are useful models to address specific questions in cancer research and support further studies on the ability of exercise training to protect older women at risk for breast cancer.
Keywords: Breast cancer, exercise, protective effect
Introduction
Cancer is an age-associated disease, with advancing age a primary risk factor for developing the disease in humans. Supporting this view is the observation of an exponential increase in the age-specific incidence of breast cancer in women until menopause and then a slower rate of increase thereafter [1]. In addition, up to 80% of women diagnosed with breast cancer are over 50 years of age [2]. The explanations for the increased incidence of age-dependent breast cancer are multi-factorial, including prolonged lifetime exposure to carcinogens and reproductive hormones, and age-associated changes in tissue repair and immune function. Consequently, the biological characteristics of breast cancer are different between young and old individuals and can be distinguished by their histology and molecular markers. Histologically, breast tumors from older individuals are frequently papillary, lobular and mucinous whereas breast tumors from young individuals are usually inflammatory and medullary cancers [2]. As well, breast tumors from older individuals are commonly estrogen receptor positive and have low proliferation rates [2]. Finally, younger women tend to be diagnosed with higher grade breast cancers that are related to poor prognosis, compared with older women. This does not mean that the proportion of benign tumors is higher in older women, but a reflection of the complex nature of the disease, which may be modified by age, the host milieu and lifestyle behaviors.
Epidemiological evidence supports a protective effect of physical activity against breast cancer. For example, the risk of death from invasive breast carcinoma was 30% lower in American women aged 35-64 years that participated in recreational physical activity throughout their lifetime compared with women that were sedentary [3]. Women with stage I-III breast cancer who participated in more than 9 metabolic equivalent hours per week had decreased risk of breast cancer recurrence and mortality [4]. Moderate physical exercise, including brisk walking, reduced postmenopausal breast cancer risk suggesting that increases in activity after menopause are beneficial [3]. In a 6 year follow up of women diagnosed with local or regional breast cancer, any recreational physical activity and consumption of better quality diets was associated with a 91% reduced risk of death from breast cancer [5]. However, not all studies have shown positive associations. For example, a negative correlation between amount of physical activity and risk of breast cancer mortality was recently reported [6]. The limitation of physical activity-focused epidemiological studies is that they are observational. Therefore, in order to investigate and define the underlying mechanisms associated with a beneficial effect of physical activity on cancer biology, pre-clinical studies would be useful.
The dichotomy in tumor pathology between young and old women suggests that breast cancer is a heterogeneous disease stratified by age. This stratification of an age-dependent nature of breast cancer necessitates an individualized interventional approach in cancer prevention and treatment tailored to women of different ages. Pre-clinical animal models are useful and necessary to address the molecular and cellular complexities of age-dependent breast cancer and response to physical activity and chemotherapy. However, the majority of cancer researchers utilizing model organisms to elucidate mechanisms of disease progression or develop therapeutics, commonly use young mice or rats. This experimental approach does not recapitulate the physiological changes due to aging and thus misses critical molecular and cellular targets that are expressed differently between the young and old. For instance, Gravekamp and colleagues vaccinated young and old BALB/c mice with Mage-b, a breast cancer vaccine, and found a lack of an immune response in the old mice, corresponding with their inability to inhibit primary tumor growth and metastatic outgrowth [7]. In a different study, depletion of immunosuppressive T-regulatory cells that contribute to tumor growth elicited different responses in young and old C57BL/6 mice [8]. Hence, pre-clinical studies designed to address the effects of drugs or other intervention strategies in cancer need to consider the age-associated biological responses.
We utilized old BALB/c mice orthotopically implanted with 4T1 breast cancer cells as a model of breast cancer and aging. The 4T1 breast cancer cell line was originally isolated from a spontaneous breast tumor of a BALB/c mouse that expressed the mouse mammary tumor virus [9]. This cancer cell line is injectable in the mouse mammary fat pad and is highly invasive, with the ability to metastasize to lungs, brain, liver, bone, lymph nodes and blood. The disease in mice has some characteristics similar to triple negative invasive breast cancer in women [9]. We report here that voluntary wheel running before tumor challenge attenuated primary tumor growth in old mice in a distance-dependent manner.
Materials and methods
Animals and wheel running activity
Studies were conducted using BALB/cBy (National Institute on Aging) females 18 months of age. Mice were randomly selected, with 15 mice in the running group and 15 mice in the non-running group. Runners were given access to free running wheels in individual cages and non-runners were also given access to running wheels that were locked. We monitored the running activity continuously for 60 days before 4T1 tumor injection and another 30 days following tumor cell injection, at which time the mice were terminated. Running wheels (measuring 15.5 cm by diameter) transmitted electronic signals wirelessly to a monitoring hub (Med Associates ENV-044, Vermont) and raw data was exported to Microsoft Excel for processing. The distance ran by the mice was calculated as (3.14 x 15.5 cm x number of revolutions)/(100 cm per m x 1000 m per km). Mice were housed in a standard rodent room within a specific pathogen free (SPF) barrier facility with 12-hour light and dark cycles. Ambient temperature in the room was kept at 70-74 degrees F. Mice were fed standard rodent chow (5053; Picolab, Richmond, IN) and water ad libitum. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Washington, Seattle.
Tumor model
4T1 cells (ATCC, Manassas, VA) were thawed from liquid nitrogen and cultured in 100 mm plates, using Dulbecco’s modified Eagle Media (DMEM, Life Technologies), supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were maintained in an incubator at 37°C, and 10% CO2. Cells were passaged routinely at 80-90% confluence and the total number of passages did not exceed 10. At passage 3, DMEM was removed and cells were washed with phosphate buffered saline (PBS), trypsinized (EDTA 0.05%) and counted with using trypan blue exclusion and a hemocytometer. The appropriate cell numbers were resuspended in 100 uL of PBS for each injection site. Each mouse was injected with 1 x 104 cells in the 4th mammary fat pad. Mice were palpated for tumor growth 1 week after tumor implantation. Once palpable, tumors were measured in two dimensions (length, width) with digital calipers to calculate surface area. At 30 days post-tumor cell injection, mice were euthanized via CO2 asphyxiation. Carcasses and mammary tumors were weighed to obtain tumor burden, defined as tumor weight normalized to carcass weight. The breast tumor from each mouse was cut in half, with half fixed in 10% neutral buffered formalin and the other half flash-frozen in liquid nitrogen and kept at -80°C. Lungs were removed and individual lobes perfused with 10% neutral buffered formalin. Hearts were removed, weighed and flash-frozen in liquid nitrogen.
Indirect calorimetry
A subset of mice was randomly chosen from each group and placed in metabolic cages. Metabolic rates for a 24-hour period were assessed in these mice using indirect calorimetry (Oxymax; Columbus Instruments, Columbus, OH). The system consisted of metabolic cages, each fitted with water bottles. The rates of oxygen consumption (VO2; mL/kg/hour) and carbon dioxide production (VCO2; mL/kg/hour) was measured at intervals of 20 minutes over 24 hours. Mice were given free access to regular chow (5053; Picolab, Richmond, IN) and water. The amount of food given and remaining were weighed. Metabolic experiments were conducted prior to group randomization (baseline, N = 7 runners, N = 6 non-runners) and one week prior to termination (end-point, N = 8 runners, N = 8 non-runners). The respiratory exchange ratio (RER) was calculated from the ratio of VCO2/VO2.
Histopathology
Formalin-fixed tumors and lungs were processed routinely and stained with Hematoxylin and Eosin. Stained sections of primary tumors were evaluated for mitosis, necrosis and inflammation at various magnifications (20X to 400X) with a light microscope. If multiple tumor masses were present on the primary tumor slide, scores were performed on the largest tumor. Normal and aberrant mitotic figures were characterized by tri-radial or circular metaphase plates and counted in three random fields at 400X magnification. Necrosis was estimated as the percentage of necrotic area within the largest tumor section, that is, regions that were hypocellular and hypo-or hypereosinophilic with basophilic cellular and nuclear debris. Inflammation was graded as 0 (minimal), 1 (mild), 2 (moderate), or 3 (marked) at a 40X or 100X magnification. Invasiveness was characterized by neoplastic epithelial cells within skeletal muscle, outside of the confines of the basement membrane. Lung sections were examined for metastatic lesions.
Statistics
Student’s t-test was used to detect significant differences between groups for the following dependent variables: running distance, body weights, lean mass, fat mass, tumor outcomes, immunostaining intensity and immuno-labeling index, tibia length, cardiac weights and food intake. Pearson’s correlation analysis was used to assess relationships between distance ran by runners and the following dependent variables: tumor burden (absolute, normalized), mitotic index, necrosis index. Prism (Graph Pad, Version 5) was used to perform the statistical analyses. All results are presented as means ± standard deviation unless stated otherwise.
Results and discussion
Old mice with cancer run less that old mice without cancer
Mice randomized to the running group ran an average daily distance of 4.89 ± 1.73 km over 60 days prior to 4T1 tumor cell injection, and 2.38 ± 1.51 km over 30 days after tumor cell injection (Figure 1A, P = 0.0006). The fact that old female BALB/c mice reduced their running distance after cancer onset suggests that they are sensitive to the debilitating effects of invasive tumor growth. The decrease in physical activity closely mimics the debilitating effects of invasive cancer in older people, and provides compelling evidence that old mice are useful models to address specific questions in cancer research.
Figure 1.
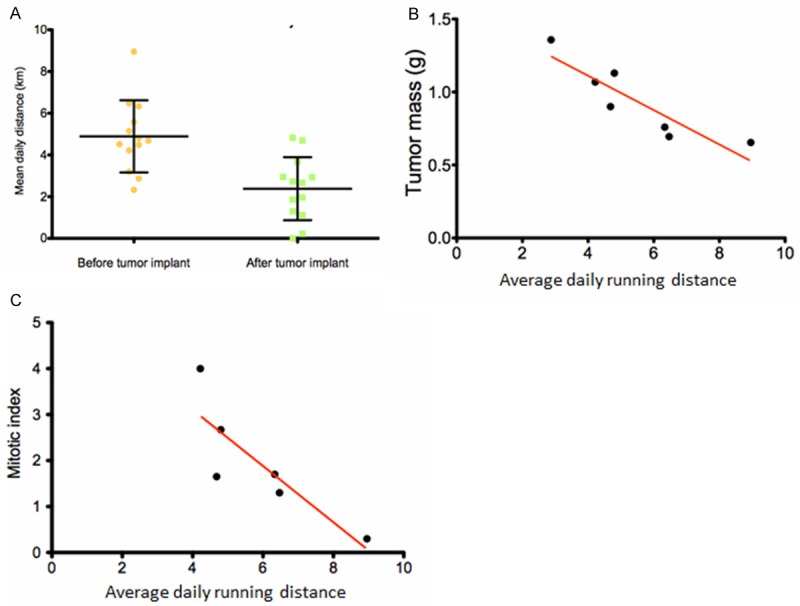
Singly housed, 18 months old BALB/c mice readily and consistently use slanted running wheels placed inside the cage in a tumor dependent manner. The mice were allowed to run 60 days before injection with 4T1 breast tumor cells, and an additional 30 days after tumor cell injection, at which time they were euthanized and tumors measured. A. Mice ran longer distances before tumor cell injection compared to after tumor cell injection, p = 0.0006. B. Mice that ran longer distances before tumor cell injection had decreased tumor mass measured at the time of euthanasia, Pearson’s r = -0.89, P = 0.0066, R2 = 0.80. C. There was also a negative correlation between pre-tumor running distance and primary tumor mitotic index, Pearson’s r = -0.85, P = 0.034, R2 = 0.72.
Old mice that run longer distances before tumor challenge have smaller tumors
Because many older women are at risk for breast cancer, but have not yet been diagnosed, we wanted to see if exercise before tumor onset would affect cancer risk in old mice. Therefore, we placed 18 month old BALB/c females on voluntary running wheels 60 days before orthotopic challenge with 4T1 breast tumor cells. There was a negative correlation between daily distance ran, prior to tumor cell injection, and absolute tumor mass measured 30 days after tumor cell injection when mice were euthanized (Figure 1B, Pearson’s r = -0.89, P = 0.0066, R2 = 0.80). The correlation was still significant when tumor mass was normalized to body weight at the termination of the experiment (Pearson’s r = -0.87, P = 0.011, R2 = 0.75, data not shown). A negative correlation was also observed between distance ran before tumor cell injection and the histological score for mitotic index (Figure 1C, Pearson’s r = -0.85, P = 0.034, R2 = 0.72), suggesting that pre-tumor running may release factors that prevent tumor cell multiplication. Runners and non-runners showed no differences in tumor growth either one week or two weeks after tumor cell injection. Tumor burden was similar between the two groups after 30 days of running when the mice were euthanized.
Few research groups have investigated the effects of exercise training or voluntary physical activity in old mouse cancer models. Our study is one of the first to demonstrate a significant correlation between tumor progression and average distance run in aged mice using the voluntary wheel running paradigm. This observation is based on distance run before mice were challenged with tumor suggesting that clinical studies may be of interest to see if older women without cancer could decrease their risk of developing breast cancer by engaging in some type of exercise program.
Runners develop a metabolic response to exercise training
A positive correlation in runners was observed between distance ran prior to tumor cell injection and average night VO2 at the termination of the study (Figure 2A, Pearson’s r = 0.83, P = 0.041, R2 = 0.69). The average 24-hour oxygen consumption during tumor progression was greater in non-runners compared to runners, 3552 ± 130.4 mL/kg/hr vs 3451 ± 100.9 mL/kg/hr, respectively, but did not reach statistical significance. Mice from both groups presented with changes in substrate oxidation over the course of the study, as evidenced by changes in respiratory exchange ratio (RER) between baseline and prior to euthanasia. Both runners and non-runners experienced significant increases in average RER, respectively (Figure 2B, P = 0.001; Figure 2C, P = 0.0024). Because nocturnal physical activities may shift the RER closer to 1.0, RER values were calculated for both light and dark cycles, which corresponded with day time and night time activity values respectively. Runners showed increased RER during the inactive light cycle (Figure 2D, P = 0.029) suggesting that voluntary running shifted resting substrate metabolism toward glucose oxidation, relative to lipid oxidation. This shift in substrate metabolism was significantly different from baseline for both groups of animals, indicating that the tumor burden might have been responsible. However, runners demonstrated an even greater increase in RER values suggesting that the ability to oxidize glucose was enhanced possibly due to the imposition of physical activity. Food intake measurements showed that runners had greater food intake than non-runners, 6 weeks after being randomized into running or non-running conditions (P = 0.043, data not shown). Runners did not demonstrate significant differences in heart weight at sacrifice, compared with non-runners. This was somewhat surprising since we have seen increased heart weights in younger mice after several weeks of voluntary wheel running [11]. Perhaps the hearts in older mice take longer to adapt to the physiological stress of running.
Figure 2.
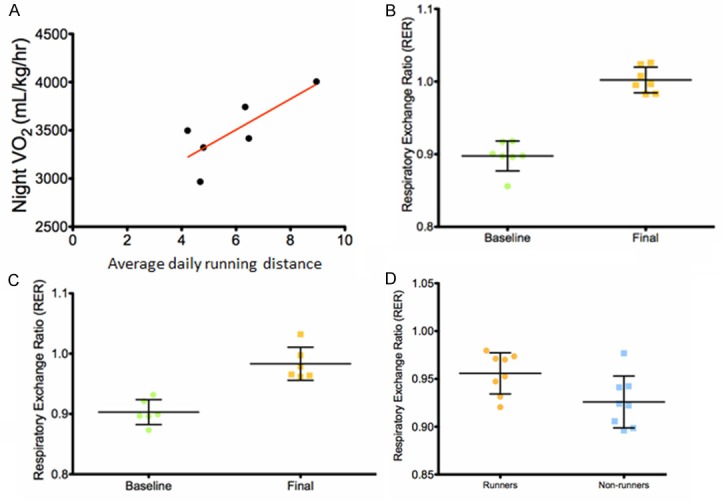
Voluntary wheel running induces a metabolic response in 18 months old BALB/c mice. The rates of oxygen consumption (VO2; mL/kg/hour) and carbon dioxide production (VCO2; mL/kg/hour) were measured at intervals of 20 minutes over 24 hours using the Oxymax system (Columbus Instruments, Columbus, OH). The respiratory exchange ratio (RER) was calculated from the ratio of VCO2/VO2. Assessments were conducted prior to group randomization (baseline) with 7 runners and 6 non-runners, and one week prior to euthanasia (final) with 8 runners and 8 non-runners. A. An increase in VO2 during the active night cycle measured one week before euthanasia was positively correlated with increased running distance measured in mice before 4T1 tumor cell injection, Pearson’s r = 0.83, P = 0.041, R2 = 0.69. B and C. The average RER increased in both runners (P = 0.001) and non-runners (P = 0.0024, respectively. D. The average RER measured during the inactive (daytime) cycle one week before euthanasia was significantly higher in runners compared to non-runners (P = 0.029).
Voluntary wheel running in mice is a translational model of exercise training
We would like to point out that while the voluntary wheel running paradigm has a number of strengths as an exercise training model, there is an issue of whether the intensity and duration of wheel running done by the mice compares to a reasonable exercise program for women. It has been our experience that all mice will run voluntarily, with older mice running less compared with younger mice. Running distance and rate are strain dependent, with BALB/c mice considered average distance runners [12]. Our old BALB/c females ran an average daily distance of close to 5 km before tumor onset during their 12 hour active night cycle period. This is less than one half a km per hour, which is very attainable by many older women in various types of exercise training. The question is whether one hour of training would provide the same protective anti-tumor benefits as 12 hours of voluntary wheel running. Mice run in spurts with periods of intense running and periods of light running or no running. We have observed that mice run most intensely the first several hours of the beginning of the active dark cycle and have shown that the first hour of running generates physiological responses similar to 12 hours of running [13]. It would be of interest to compare the anti-tumor effects of these two time frames.
Another question is whether energy expenditure needs to be comparable. If we consider that the average stride of a mouse is 5 cm and the average stride of an older woman is 135 cm, then a comparable one hour running distance would be about 10 km. A 10 km run over one hour is probably doable by many older women but most likely not a reasonable expectation. However, if the same energy expenditure needed to complete the 10 km run were compressed into a shorter time frame with more enticing but comparable energy demanding types of exercise training, then older women might be more enthused about participating. The metabolic parameters of energy expenditure can easily be measured in tumor and non-tumor bearing mice engaged in exercise training experiments as we have demonstrated in the present study. These same parameters can also be easily measured in women so that relative energy expenditures can be compared to help further validate voluntary wheel running as a translational model for exercise training.
In summary, our results demonstrate that running longer distances is associated with decreased tumor burden in 18 month old BALB/c mice injected with 4T1 mouse breast cancer cells, suggesting that physiological factors generated by exercising before tumor onset are protective against tumor progression. The mechanisms for this protective effect are not known but the data support further studies on the ability of exercise training to protect older women at risk for breast cancer.
Acknowledgements
This work was supported by R21 CA 140916, NIH/NCI and P01 AG01751 and P30 AG013280, NIH/NIA. We thank Dr. Piper Treuting for the histological evaluation of tumor sections and Dr. Ruby Mangalindan for mouse health support.
Disclosure of conflict of interest
None.
References
- 1.Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol. 2008;66:65–74. doi: 10.1016/j.critrevonc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas GA, Leonard RCF. How age affects the biology of breast cancer. Clin Oncol. 2009;21:81–85. doi: 10.1016/j.clon.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Patel AV, Press MF, Meeske K, Callel EE, Bernstein L. Lifetime recreational exercise activity and risk of breast carcinoma in situ. Cancer. 2003;98:2161–2169. doi: 10.1002/cncr.11768. [DOI] [PubMed] [Google Scholar]
- 4.Holmes M, Chen WDF, Kroenke C, Colditz G. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.George SM, Irwin ML, Smith AW, Neuhouser ML, Reedy J, McTiernan A, Alfano CM, Bernstein L, Ulrich CM, Baumgartner KB, Moore SC, Albanes D, Mayne ST, Gail MH, Ballard-Barbash R. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control. 2011;22:589–598. doi: 10.1007/s10552-011-9732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 7.Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C. Vaccination with Mage-b DNA induces CD8 T-cell responses at young, but not old age in mice with metastatic breast cancer. Br J Cancer. 2009;101:1329–1337. doi: 10.1038/sj.bjc.6605329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurez V, Daniel BJ, Sun L, Liu AJ, Ludwig SM, Kious MJ, Thibodeaux SR, Pandeswara S, Murthy K, Livi CB, Wall S, Brumlik MJ, Shin T, Zhang B, Curiel TJ. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res. 2012;72:2089–2099. doi: 10.1158/0008-5472.CAN-11-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001;Chapter 20:Unit 20.2. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 10.Montel V, Mose ES, Tarin D. Tumor-stromal interactions reciprocally modulate gene expression patterns during carcinogenesis and metastasis. Int J Cancer. 2006;119:251–263. doi: 10.1002/ijc.21757. [DOI] [PubMed] [Google Scholar]
- 11.Goh J, Tsai JM, Bammler TK, Farin FM, Endicott E, Ladiges WC. Exercise training in transgenic mice is associated with an early attenuation of mammary tumor growth in a dose-dependent manner. PLoS One. 2013;8:e80123. doi: 10.1371/journal.pone.0080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 13.Goh J, Ladiges W. A novel long-term, short interval physical activity regime improves body composition in mice. BMC Res Notes. 2013;19:6–66. doi: 10.1186/1756-0500-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]


