Abstract
Background
Our goal is to study triggers of spontaneous preterm delivery using a case-crossover design.
Methods
In a pilot study, we enrolled 50 women with spontaneous preterm labor (PTL) and 50 with preterm premature rupture of membranes (PPROM) between 2011 and 2012. To assess non-transient risk factors, we also enrolled a control group of 158 pregnant women at their regular prenatal care visits matched to cases by gestational age and calendar time. The index time was defined as onset of PTL/ PPROM (for cases) or interview (for controls). Detailed data was collected through structured interviews regarding factors of interest during the 72 hours that preceded the index time. Within case subjects, we compared the frequency of transient factors from 0 to 24 hours before index time with that from 48 to 72 hours before index time and estimated matched odds ratios (OR) and 95% confidence intervals (CI).
Results
Previously hypothesized chronic risk factors for spontaneous preterm delivery, including mood disorders and stressful events, were more common among cases than among controls. Within cases, skipped meals (OR 4.3, 95% CI 1.2, 15.2), disturbed sleep (OR 4.5, 95% CI 1.5, 13.3), sexual activity (OR 6.0, 95% CI 0.7, 69.8) and intake of spicy foods (OR 7.0, 95% CI 1.6, 30.8) were associated with an increased risk for PTL/ PPROM within the subsequent 24 hours. For physical exertion and other potential risk factors evaluated the OR was close to the null.
Conclusion
Skipping meals and disturbed sleep may be associated with imminent PTL/ PPROM; sexual activity and spicy food may trigger PTL/ PPROM in susceptible women. Larger case-crossover studies will be able to evaluate the impact of modifiable risk factors and acute predictors of PTL/PPROM and might help guide obstetrical management.
Keywords: Preterm labor, spontaneous rupture of membranes, risk factors, case-crossover design
INTRODUCTION
Over 12% of infants in the U.S are born preterm (before the 37th week of gestation)1. Preterm delivery remains a major medical and public health concern; it is a leading cause of infant death and adverse long-term morbidities including cerebral palsy, vision and hearing impairment, and learning disabilities2,3. Spontaneous preterm delivery following premature labor (PTL) or preterm premature rupture of membranes (PPROM) accounts for approximately 50% of all preterm deliveries4. Although substantial research over the past 40 years has been conducted on risk factors related to this condition5,6 (e.g. race, twin pregnancy, maternal age, chronic illnesses), virtually nothing is known about its immediate triggers. While an assessment of chronic factors and fixed effects answers the question “why me?”, examination of immediate triggers seeks to answer the question “why now?”, i.e., “Why did the labor start this morning and not yesterday?”.
The effect of immediate determinants or triggers on health outcomes is difficult to assess. Previous observational studies suggest that coitus7, prolonged standing8,9, urinary tract and other infections10, physical exertion11,12, caffeine intake13, smoking14–16, cocaine use17, marijuana use18, alcohol use17, skipping meals19, and psychological stress20,21 may trigger preterm delivery. These triggers are biologically plausible as the activities may induce a cascade of events that result in an onset of labor through their effect on corticotropin-releasing hormone22. Nevertheless, the association of these factors with preterm delivery has been inconsistent and controversial23.
The dearth of information about acute causes and modifiable risk factors prevents clinicians from adequately advising women about behaviors that might be altered to reduce the risk of preterm delivery. Additionally, specifically defined prodromal symptoms may help guide obstetricians in the use of timely tocolytic or betamethasone therapy. Given the burden and cost associated with preterm delivery, the identification of potential interventions has the potential to be of great public health significance. Such work also can be useful in recognizing which factors are not triggers for preterm delivery: pregnant women might be unnecessarily worried about certain exposures based on untested popular beliefs.
The case-crossover study design was developed in the 1990s to study triggers of acute myocardial infarction and has subsequently been applied to several other acute conditions24,25. Although one previous study used a case-crossover design to evaluate the effects of high ambient temperature on preterm delivery, we are unaware of previous work that has used this design to study modifiable triggers of preterm delivery.26 Therefore, we conducted a pilot study to evaluate the feasibility of using an observational case-crossover design to evaluate the effect of intermittent exposures on the risk of acute onset of PTL or preterm premature rupture of membranes PPROM27–30.
MATERIAL AND METHODS
Study Design
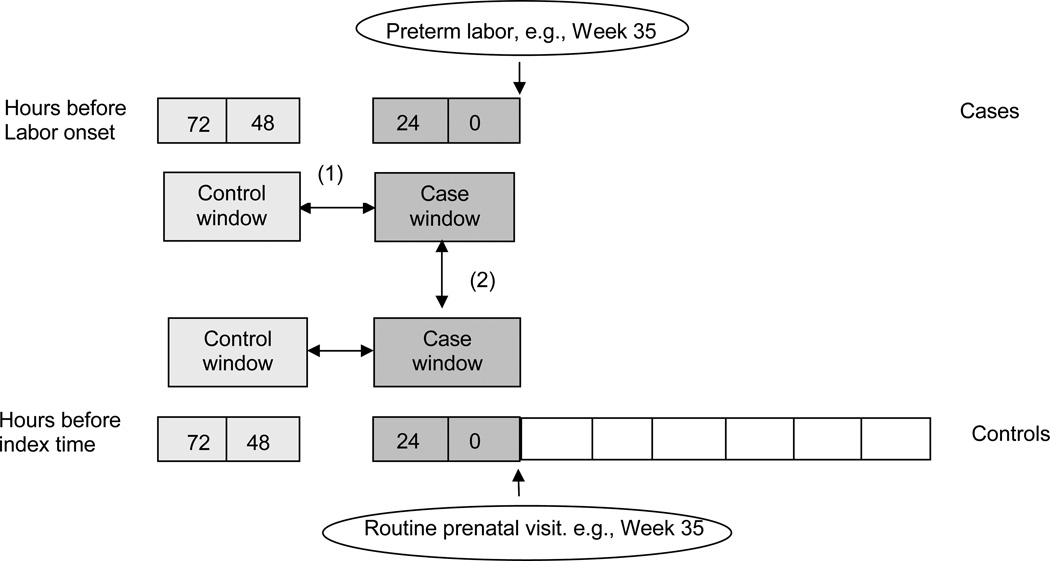
In this non-interventional case-crossover design cases serve as their own controls, but each case contributes one case window and one or more control windows. Figure 1. The case window was arbitrarily defined as the 24 hours preceding the onset of labor or rupture of membranes, which we hypothesised is the period when exposures might be most likely to affect the risk of PTL/ PPROM. The control window was defined as the 48 to 72 hours before the onset of labor/rupture of membranes, implicitly assuming any potential carry-over effect from exposures during this window lasted less than 24 hours. The 48–72 hour window provided an estimate of the expected prevalence of these exposures for each case. We left a gap (“washout period”) of 24 hours between the case and control windows. Exposure frequencies from 0–24 hour and 48–72 hour windows were then compared within each subject. Through stratification of the population to the extreme of one-person strata, it is warranted that controls will be representative of cases (i.e., of themselves). The case-crossover approach effectively eliminates potential confounding effects from both measured and unmeasured subject characteristics that are constant during the case and control period (e.g., socio-demographic and genetic factors). However, the case-crossover approach is more susceptible to reverse causality since early signs, symptoms, or premonitions of preterm delivery may lead to changes in behaviours herein hypothesized as triggers.
Figure 1.
Study design: Case-crossover (1) and case-control (2) designs.
We also compared the frequency of both persistent and transient factors at the time of PPROM or PTL onset among case subjects with that among a reference group of pregnant women without PPROM or PTL at similar gestational age. This analysis allows the identification of non-transient factors, or non-transient effects, which would be missed by the case-crossover design. For both those with PTL/PPROM and the comparison group, the expected date of confinement was determined based on the patient’s report of her last menstrual period (LMP) and a clinically confirming ultrasound scan during the first trimester. If the pregnancy was conceived through in vitro fertilization, those procedure dates established the date of confinement. Usual care was provided to both cases and controls.
Study Population
The source population comprised women age 18 and older that delivered, or planned to deliver, at the Massachusetts General Hospital (MGH) between September 2011 and June 2012. This research was approved by the Partners Institutional Review Board.
Case subjects
Our case group consisted of prospectively identified women admitted to the MGH Obstetric Service with spontaneous PTL or PPROM. Since PTL and PPROM are probably related conditions and share common risk factors,30 we combined them in our primary analyses. Specific results for PTL and PPROM are provided in the Appendix. All women who presented to the obstetric ward before 37 completed weeks of gestation after spontaneous onset of PTL or spontaneous rupture of membranes were eligible. Women were diagnosed with PTL or PPROM based on the attending obstetrician's clinical assessment. PPROM diagnosis was based on routine clinical practice, including amnicators, where applicable. PTL cases included women who delivered as a result of spontaneous preterm labor at less than 37 weeks, as well as women with preterm labor at less than 37 weeks receiving tocolysis. If a woman reported both PTL (i.e., painful contractions) and PPROM prior to admission, she was classified for analysis according to which occurred first. Indicated preterm births (maternal or fetal conditions resulting in planned early delivery) were excluded. The index time for cases was defined as the first painful contraction of labor or rupture of membranes, whatever came first. Inclusion of false positives would tend to dilute any potential association.
Control subjects
Our control group consisted of pregnant women between 24 weeks and 37 weeks gestation who were seen within the MGH obstetrical practice for routine prenatal care. Controls were frequency matched to cases so that they had a similar distribution of gestational ages (in completed weeks) and calendar month of visit as cases, to account for gestational and seasonal trends in the factors under study (e.g. avoidance of physical exertion as women advance in pregnancy or during the winter months). Controls were assigned an index time equal to the time of interview.
Ascertainment and enrollment
Study subjects were identified through review of admissions and through regular contact with the Labor and Delivery and postpartum censuses (cases) and outpatient clinics (controls). Women were approached at the hospital room (cases) or at the waiting room for their regular outpatient prenatal visit (controls), and asked to sign a consent form. For cases, interviews were conducted within 48 hours of admission to the hospital to minimize the potential for recall bias. For controls, interviews were conducted right before or right after their prenatal visit. All personal or potentially identifiable data was retained within the MGH; analytic files and communications among investigators refer to study numbers only.
Data collection
Data were collected through in-person interviews with direct entry of responses and instantaneous coding. The interviewer followed a standardized protocol and script for each question. The questionnaire contained close-ended questions on self-declared racial, educational and economic background, personal and family history of reproductive diseases, reproductive events during the current pregnancy including multiple gestation, gestational hypertension or diabetes, vaginal bleeding, clinically relevant infections, and hospitalizations. Detailed information was collected regarding the transient factors of interest (i.e., heavy physical exertion, sexual activity, skipping meals, eating spicy food, caffeine and alcohol intake, acute infections, use of licit and illicit drugs, traumas and stressful events) during the 72 hours before the index time. Stressful events were defined as life events or significant life changes (e.g., moving, travel, learn of the death of a close person, suffer any injury, have a serious quarrel, have any serious financial problems, appear in court or be imprisoned, lose a job, be left by a husband). Exercise was coded using Metabolic Equivalent of Task (MET) charts. Time between each transient exposure of interest and the index time was collected in hours. Pertinent prior obstetrical and medical history was collected directly from the patient’s electronic chart. At the end of the interview, we asked women open ended questions about their beliefs regarding potential triggers of preterm labor: “Were there any events or things that happened to you that you think might have caused you to go into labor (or cause your water to break)? “
Statistical Analysis
We first described the distribution of socio-demographic and clinical characteristics among case and control subjects and compared them through odds ratios (ORs). To evaluate potential circadian patterns we explored the timing of PTL or PPROM onset among cases. For the main analysis, we compared the frequency of transient factors from 0–24 and 48–72 hours windows within case subjects through a matched (on subject) OR, which is an unbiased estimate of the relative risk. The case-crossover OR is estimated by the ratio of the number of cases exposed only during the 0–24 hour window to the number of cases exposed only during the 48–72 hour window (i.e., ratio of discordant pairs). Therefore only discordant pairs contribute to the estimation. We used conditional logistic regression to estimate matched ORs and 95 percent confidence intervals (CI). Given the small sample size, only crude estimates are presented. We implicitly assumed that the underlying subject-specific probability of being exposed to each potential trigger was constant within the 72 hours interval between the control and the case period. We tested this assumption comparing 0–24 and 48–72 hours windows within control subjects of similar gestational age. Study data were collected and managed using REDCap electronic data capture tools hosted at MGH.31 Statistical analyses were conducted using SAS (at least version 9.1; SAS Institute, Cary, NC).
RESULTS
We enrolled and interviewed 50 women with PTL, 50 with PPROM and 158 controls. By the time of the interview, 18 women with PTL (3 of them despite tocolysis) and 22 women with PPROM (also 3 with tocolysis) had delivered. Other women with PTL/PPROM delivered during that hospitalization or later, but after participating in the study. The mean number of hours from onset (index time) to admission were 4 for PPROM and 11 for PTL cases.
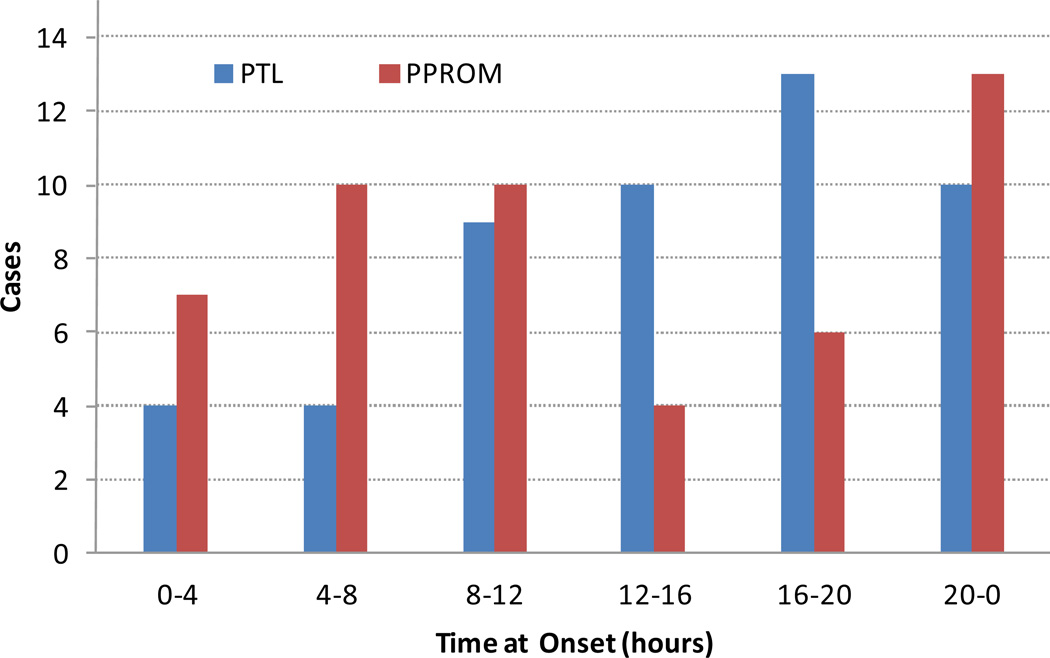
Circadian distributions of labor onset are presented in Figure 2: For PTL, onset of contractions was more common during the day (8:00 to 20:00) and dropped during the night (0:00 to 8:00). Premature rupture of membranes seemed to occur more often during the night and morning (20:00 to 12:00).
Figure 2.
Circadian patterns for reported onset of premature labor (PTL) and premature rupture of membranes (PPROM).
Case Control Design
Controls had a mean age of 32 years at interview; 72% were White and 9% Hispanic, 41% had graduate education, 70% had family income >$70,000 per year and 87% were married. Table 1. Compared with controls, cases were more likely to be Hispanic, had slightly lower family income and fewer years of education, and were less likely to be married. Cases were also more likely to have been diagnosed with depression/anxiety, have a history of preterm labor, have required fertility treatments such as IVF, carry twins, and have had vaginal bleeding during pregnancy.
Table 1.
Characteristics of case and control subjects.
| Cases (n=100) | Controls (n=158) | OR (95% CI) | |
|---|---|---|---|
| n (%) | n (%) | ||
| Maternal age, years. Mean (SD) | 31.5 (5.6) | 32.3 (4.8) | 1.0 (0.9, 1.0) |
| Gestational age, complete weeks. Mean (SD) | 33.0 (3.3) | 32.7 (3.3) | N/A* |
| Prepregnancy BMI. Mean (SD) | 25.6 (5.4) | 25.1 (5.2) | 1.0 (1.0, 1.1) |
| Race | |||
| White | 69 (69.0%) | 114 (72.2%) | 1.0 (Reference) |
| Black | 9 (9.0%) | 9 (5.7%) | 1.7 (0.6, 4.4) |
| Asian | 11 (11.0%) | 15 (9.5%) | 1.2 (0.5, 2.8) |
| Other | 11 (11.0%) | 20 (12.7%) | 0.9 (0.4, 2.0) |
| Hispanic descent | 17 (17.0%) | 14 (8.9%) | 2.1 (1.0, 4.5) |
| Annual income ($/year) | |||
| >70 K | 54 (54.0%) | 111 (70.3%) | 1.0 (Reference) |
| 50–70 K | 7 (7.0%) | 12 (7.6%) | 1.2 (0.4, 3.2) |
| 30–50 K | 9 (9.0%) | 9 (5.7%) | 2.1 (0.8, 5.5) |
| <30 K | 13 (13.0%) | 8 (5.1%) | 3.3 (1.3, 8.5) |
| Missing | 17 (17.0%) | 18 (11.4%) | 1.9 (0.9, 4.1) |
| Marital status | |||
| Married, living together | 68 (68.0%) | 137 (86.7%) | 1.0 (Reference) |
| Not married, living together | 15 (15.0%) | 15 (9.5%) | 2.0 (0.9, 4.4) |
| Not married, not living together | 10 (10.0%) | 5 (3.2%) | 4.0 (1.3, 12.3) |
| Divorced, separated or widow | 7 (7.0%) | 1 (0.6%) | 14.1 (1.7, 116.8) |
| Education | |||
| Graduate/professional school | 39 (39.0%) | 64 (40.5%) | 1.5 (0.8, 2.7) |
| College | 27 (27.0%) | 66 (41.8%) | 1.0 (Reference) |
| Some college | 16 (16.0%) | 17 (10.8%) | 2.3 (1.0, 5.2) |
| High school | 14 (14.0%) | 9 (5.7%) | 3.8 (1.5, 9.8) |
| Some high school | 4 (4.0%) | 2 (1.3%) | 4.9 (0.8, 28.3) |
| Insurance type | |||
| Private/HMO | 84 (84.0%) | 133 (84.2%) | 1.0 (Reference) |
| Medicaid/Mass Health | 16 (16.0%) | 24 (15.3%) | 1.1 (0.5, 2.1) |
| Employed | 86 (86.0%) | 126 (79.8%) | 1.6 (0.8, 3.1) |
| Anxiety disorder | 17 (17.0 %) | 11 (7.0%) | 2.7 (1.2, 6.1) |
| Depression | 18 (18.0%) | 9 (5.7%) | 3.6 (1.6, 8.4) |
| Uterine fibroids | 7 (7.0%) | 6 (3.8%) | 1.9 (0.6, 5.8) |
| Asthma | 20 (20.0%) | 19 (12.0%) | 1.8 (0.9, 3.6) |
| Primiparous | 63 (63.0%) | 94 (59.9%) | 1.1 (0.7, 1.9) |
| Previous therapeutic abortions | 19 (19.0%) | 17 (10.8%) | 1.9 (1.0, 3.9) |
| Previous miscarriages | 24 (24.0%) | 37 (23.8%) | 1.0 (0.6, 1.8) |
| Prior preterm gestation (<37 weeks) | 9 (9.0%) | 2 (1.3%) | 7.7 (1.6, 36.3) |
| Difficulties becoming pregnant (>6 months) | 31 (31.3%) | 35 (22.2%) | 1.6 (0.9, 2.8) |
| Planned pregnancy | 72 (72.0%) | 113 (71.5%) | 1.0 (0.6, 1.8) |
| Fertility treatment to become pregnant | 24 (33.3%) | 16 (14.2%) | 3.1 (1.5, 6.4) |
| In vitro fertilization (IVF) | 22 (22.0%) | 12 (7.6%) | 3.4 (1.6, 7.3) |
| Twin pregnancy | 19 (19.0%) | 4 (2.5%) | 9.0 (3.0, 27.4) |
| Vaginal bleeding/spotting in 1st trimester | 37 (37.4%) | 29 (18.4%) | 2.7 (1.5, 4.7) |
| Vaginal bleeding/spotting in 2nd trimester | 15 (15.0%) | 7 (4.4%) | 3.8 (1.5, 9.7) |
| Prescribed progesterone | 9 (9.0%) | 2 (1.3%) | 7.8 (1.6, 36.9) |
There was no difference between groups because participants were matched on gestational age in weeks.
Stressful events were more frequent among cases than among controls; during the 24 hours preceding the index time, 10% of cases compared with 5% of controls reported a very stressful event (Table 2). Physical exertion overall was similar among cases and controls during the 24 hours before the index time, although exercise of more than 5 METs (vigorous exertion) was less frequent among cases. Similarly, cases were less likely to go to work and more likely to skip meals and have sleep interruptions during the 24h preceding onset of spontaneous preterm labor.
Table 2.
Distribution of selected exposures in 0–24 hour and 48–72 hour windows in cases with spontaneous preterm delivery and control subjects.
| Cases (n=100) | Controls (n=158) | |||
|---|---|---|---|---|
| 0–24 h window | 48–72 h window | 0–24 h window | 48–72 h window | |
| Any stress | 18 (18.0%) | 21 (21.0%) | 13 (8.2%) | 11 (7.0%) |
| At least very stressful event | 10 (10.0%) | 13 (13.0%) | 8 (5.1%) | 8 (5.1%) |
| Any work | 11 (11.0%) | 39 (39.0%) | 47 (29.8%) | 72 (45.6%) |
| Any sexual activity | 6 (6.0%) | 1 (1.0%) | 8 (5.1%) | 12 (7.6%) |
| Any exercise | 32 (32.0%) | 28 (28.0%) | 45 (28.5%) | 52 (32.9%) |
| 5+ METs | 12 (12.0%) | 15 (15.0%) | 33 (20.9%) | 32 (20.3%) |
| Ate spicy food | 15 (15.0%) | 3 (3.0%) | 25 (15.8%) | 20 (12.7%) |
| Skipped one or more meals | 17 (17.0%) | 7 (7.0%) | 17 (10.8%) | 11 (7.0%) |
| Disturbed sleep | 26 (26.0%) | 12 (12.0%) | 28 (17.7%) | 20 (12.7%) |
About half of the women (54% in cases, 58% in controls) had beliefs regarding what can trigger PTL or PPROM. The most commonly reported theories were stress (n=46), over-exertion (dancing, lifting weight), increased abdominal pressure (vomiting, sneezing, trauma, curling up for long time), spicy food intake, sexual activity, dehydration, and baby kicks.
Case crossover analysis
Within cases, the frequency of stressful events did not change comparing the 0–24 hours to the 48–72hours preceding PTL or PPROM. Table 2. Similarly, as shown in Table 3, neither exertion nor stressors had a significant association while going to work was inversely associated with PTL and PPROM. The factors associated with onset of spontaneous preterm delivery were intake of spicy food, sleep interruptions and skipping meals. Disturbed sleep and skipping meals were more common during the 0–24 hour window than during the 48–72 hour window among cases; but a similar pattern was also seen among controls. After adjustment for trends in controls,32 the ORs for skipped meals and sleep interruptions changed to 2.3 and 2.6, respectively. Sexual activity was also more common during the 0–24 hour window, although the 95%CI included the null.
Table 3.
Selected results from case-crossover analysis: association between acute events and the risk of spontaneous preterm delivery.
| Discordant pair ratio |
Odds ratio (95% CI) | |
|---|---|---|
| Any stress | 5/8 | 0.6 (0.2, 1.9) |
| At least very stressful event | 3/6 | 0.5 (0.1, 2.0) |
| Any work | 4/32 | 0.1 (0.04, 0.4) |
| Any sexual activity | 6/1 | 6.0 (0.7, 49.8) |
| Any exercise | 21/17 | 1.2 (0.7, 2.3) |
| 5+ METs | 9/12 | 0.8 (0.3, 1.8) |
| Ate spicy food | 14/2 | 7.0 (1.6, 30.8) |
| Skipped one or more meals | 13/3 | 4.3 (1.2, 15.2) |
| Disturbed sleep | 18/4 | 4.5 (1.5, 13.3) |
Sub-analyses restricting the population to singleton pregnancies yielded similar results. Numbers were too small to evaluate triggers among twin pregnancies. Other potential triggers could not be evaluated because of lack of discordance (e.g. smoking, caffeine) or just too few exposed cases overall (e.g. illicit drugs, alcohol, heat exposure). Results were similar when PTL and PPROM were considered separately (Appendix).
COMMENT
Using a case-crossover design, we found that skipping meals, disturbed sleep, and intake of spicy food were associated with an increased risk of both PTL and PPROM within the following 24 hours. We did not find the previously suggested associations with physical exertion11,12 and psychological stress,20,21 although stress was associated with PTL in the case-control analysis.
In our study, going to work was inversely associated with the risk of labor onset or rupture of membranes during the following 24 hours. It is possible that early symptoms such as cramps or spotting had an effect on a woman’s ability or willingness to go to work and that such account for our finding. Although women clearly reported the onset of the first painful contraction or rupture of membranes, when prompted, they also mentioned prodromal symptoms, or just "feeling funny" during hours preceding onset. Not surprisingly, defining the exact onset of preterm labour, and therefore timing case and control windows, is challenging. If the onset of labour is gradual, factors reported right before the first painful contraction or rupture of membranes can be influenced by initial symptoms. Such reverse causation might have affected the estimates not only for working, but also for interrupted sleep and skipped meals since cramping or spotting can affect appetite and sleeping.
If the associations we found are the result of reverse causation, they would not identify possible interventions to prevent preterm delivery, i.e., avoiding skipping meals or improving sleep quality would not prevent onset of labour. However, associations would still be informative---suggesting that these behaviours may predict an imminent premature labour. The exact prediction of PTL and PPROM remains challenging even in those patients at high risk. Well-defined predictors may be useful to obstetricians who are trying to manage a woman at risk for preterm delivery, particularly when timing time-sensitive treatments such as tocolytic therapy or steroid administration for fetal lung maturity.
On the other hand, the non-statistically significant association with sexual activity would unlikely be explained by an increased frequency of sexual intercourse after early symptoms of labor. Actually, if women who had a sensation of imminent preterm labor were more likely to stop having intercourse, our estimates for sexual activity would be underestimating a potential triggering effect. Interestingly, the frequency of sexual intercourse during the 24 hours before the index time was similar for case and control subjects; however, it was lower during the 48–72 hour window among cases. These findings are consistent with the theory that sexual intercourse is an acute risk factor for PTL and PPROM, but only in a selective group of susceptible individuals (e.g., women with shortening cervical length). The existing literature suggests that sex does not increase the risk of preterm delivery in low-risk pregnancies; evidence is inconsistent for high-risk gestations.33 Yet, restriction of sexual activity is sometimes recommended for the prevention of preterm delivery in the presence of specific risk factors, e.g., previous spontaneous preterm labor, a short cervix, multiple gestation and cervical incompetence.
In general, it is worth noting that case-only designs are restricted, by definition, to subjects with a predisposition to develop the outcome. Therefore, relative risks estimated from this case-crossover design would not be comparable with those from a traditional cohort or case-control design, which would include subjects at low risk, or even immune, to the outcome. The higher prevalence of twin pregnancy, prior preterm, IVF/fertility treatment, asthma, and previous therapeutic abortion among cases compared to controls (Table 1) highlight that this is a susceptible population. These differences remind us of the confounding that would have biased a traditional case control (or cohort) design.
In the setting of relatively few discordant pairs, all confidence intervals were wide, including that for sexual activity. To replicate this potential association with sufficient power, future studies should include larger sample sizes. If the RR for sexual activity were really 6, we would need around 150 cases to reach statistical significance. However, if the RR for sexual activity were actually close to 2, the sample size would need to be one order of magnitude larger. With larger sample sizes, future studies should also consider potential effect modification by gestational age (e.g. divide sample into late preterm and very preterm).
The case-crossover design is best suited to evaluate transient exposures with immediate and transient effects in relation to abrupt outcomes. Stressful experiences (e.g. unemployment) or their impact (e.g. separation) likely last more than 24 hours and would extend over both 0–24 and 48–72 hours windows. As a result, they were not identified in our study as triggers. However, stressful events, as well as anxiety and depression, were associated with spontaneous preterm delivery in the case-control analysis. This argues that stress may be a non-transient risk factor; its effect may last longer than 72 hours. The discordance between the findings regarding stress in the case-control and case-crossover designs point to the care investigators must exercise in selecting exposures for study using the case-crossover approach, as well as in defining case and control windows. Although study protocols should specify the intended windows of analysis, it would have been informative to explore alternative windows. For example, asking “When was the last time of you had a stressful event? and “What was your usual frequency in the past month?” would permit sensitivity analyses for case window definition.
It is worth noticing that we found certain differences within controls comparing the 24 hours before interview to the 48–72 hours before interview (i.e., going to work, skipping meals or disturbed sleep). Trends among controls might be due to gestational trends in exposure or, more likely, to the ascertainment methods. For example, our study identified controls at one of their prenatal visits, which could have affected their work, sleep or eating patterns. In a future study, the controls could be telephoned on a subsequent random day to assess whether days that they visit the clinic were representative.
It is also possible that some of the associations might be partially explained by differential recall within person across study periods. Women may be more likely to remember factors in the 24 hours before compared to 48 to 72 hours before labour onset. For example, given the popular belief that eating spicy food is associated with preterm labour, cases may preferentially report as "spicy" the meals preceding their onset of labour. (In particular if they had upset stomach as a result of prodromal symptoms.) We tried to reduce differential misclassification of exposure by narrowing the gap between case and control windows and not informing subjects of the hypotheses of interest and the corresponding windows. Ideally, recall bias would be avoided with prospective collection of information. On a practical level, however, neither existing ad hoc pregnancy cohorts nor automated health care databases typically include information on transient exposures occurring immediately prior to the onset of labour.
We found intriguing concentrations of PTL during the day hours. This finding, together with the associations found with not-sleeping and not-eating, raises the hypothesis that alterations of circadian rhythms may be associated with preterm labour. Prior studies on circadian patterns on the time of the onset of preterm labour provide conflicting results.34–36 Both normal labour onset and term premature rupture of membranes are more common at night; some authors have suggested an altered diurnal circadian pattern for PTL34, or very preterm spontaneous onset of labour,36 but not for PPROM.37
In conclusion, findings suggest that sexual intercourse may trigger preterm delivery in susceptible pregnancies; and that impaired appetite or sleep may be predictors of imminent onset of labor. By conducting this pilot study we demonstrated the feasibility of identifying, approaching, enrolling and interviewing women admitted for PTL or PPROM. Case-crossover designs can be applied to the study of transient factors associated with spontaneous preterm delivery. However, in the course of the study we also uncovered certain challenges associated with the application of this design to abrupt adverse pregnancy outcomes. Attention needs to be given to the potential bias produced by prodromal symptoms that precede defined onset of PTL and PPROM and influence behaviors/exposures. If control subjects are enrolled, they should be matched to cases not only on gestational age and season but also on day of the week. Future studies employing this approach to study spontaneous preterm delivery will necessarily have to enroll larger numbers of patients to confirm or refute our results and evaluate other triggers, or predictors, of interest.
Acknowledgments
Sources of funding: Supported by Harvard School of Public Health Initiative. Caroline Boeke was funded by the T32 CA 09001, Brian Bateman was funded by T32 GM007592
REFERENCES
- 1.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 2.Allen MC. Neurodevelopmental outcomes of preterm infants. Current Opinion in Neurology. 2008;21:123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- 3.Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Washington (DC): National Academies Press (US); 2007. The National Academies Collection: Reports funded by National Institutes of Health. [PubMed] [Google Scholar]
- 4.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steer P. The epidemiology of preterm labour. BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112(Suppl 1):1–3. doi: 10.1111/j.1471-0528.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 7.Ekwo EE, Gosselink CA, Woolson R, Moawad A, Long CR. Coitus late in pregnancy: risk of preterm rupture of amniotic sac membranes. American Journal of Obstetrics & Gynecology. 1993;168:22–31. doi: 10.1016/s0002-9378(12)90879-0. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen TB, Hedegaard M, Secher NJ, Wilcox AJ. Standing at work and preterm delivery. British Journal of Obstetrics and Gynaecology. 1995;102:198–206. doi: 10.1111/j.1471-0528.1995.tb09094.x. [DOI] [PubMed] [Google Scholar]
- 9.Launer LJ, Villar J, Kestler E, de Onis M. The effect of maternal work on fetal growth and duration of pregnancy: a prospective study. British Journal of Obstetrics and Gynaecology. 1990;97:62–70. doi: 10.1111/j.1471-0528.1990.tb01718.x. [DOI] [PubMed] [Google Scholar]
- 10.Mazor-Dray E, Levy A, Schlaeffer F, Sheiner E. Maternal urinary tract infection: is it independently associated with adverse pregnancy outcome? Journal of Maternal-Fetal and Neonatal Medicine. 2009;22:124–128. doi: 10.1080/14767050802488246. [DOI] [PubMed] [Google Scholar]
- 11.Homer CJ, Beresford SA, James SA, Siegel E, Wilcox S. Work-related physical exertion and risk of preterm, low birthweight delivery. Paediatric and Perinatal Epidemiology. 1990;4:161–174. doi: 10.1111/j.1365-3016.1990.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez G, Grimes RM, Annegers JF, Davis BR, Slater CH. Occupational physical activity and other risk factors for preterm birth among US Army primigravidas. American Journal of Public Health. 1990;80:728–730. doi: 10.2105/ajph.80.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams MA, Mittendorf R, Stubblefield PG, Lieberman E, Schoenbaum SC, Monson RR. Cigarettes, coffee, and preterm premature rupture of the membranes. American Journal of Epidemiology. 1992;135:895–903. doi: 10.1093/oxfordjournals.aje.a116385. [DOI] [PubMed] [Google Scholar]
- 14.Cnattingius S, Forman MR, Berendes HW, Graubard BI, Isotalo L. Effect of age, parity, and smoking on pregnancy outcome: a population-based study. American Journal of Obstetrics & Gynecology. 1993;168:16–21. doi: 10.1016/s0002-9378(12)90878-9. [DOI] [PubMed] [Google Scholar]
- 15.Guinn DA, Wigton TR, Owen J, Socol ML, Frederiksen MC. Prediction of preterm birth in nulliparous patients. American Journal of Obstetrics & Gynecology. 1994;171:1111–1115. doi: 10.1016/s0002-9378(13)90046-6. [DOI] [PubMed] [Google Scholar]
- 16.Wright SP, Mitchell EA, Thompson JM, Clements MS, Ford RP, Stewart AW. Risk factors for preterm birth: a New Zealand study. New Zealand Medical Journal. 1998;111:14–16. [PubMed] [Google Scholar]
- 17.Sokol RJ, Janisse JJ, Louis JM, et al. Extreme prematurity: an alcohol-related birth effect. Alcoholism: Clinical and Experimental Research. 2007;31:1031–1037. doi: 10.1111/j.1530-0277.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 18.Burns L, Mattick RP, Cooke M. Use of record linkage to examine alcohol use in pregnancy. Alcoholism: Clinical and Experimental Research. 2006;30:642–648. doi: 10.1111/j.1530-0277.2006.00075.x. [DOI] [PubMed] [Google Scholar]
- 19.Siega-Riz AM, Herrmann TS, Savitz DA, Thorp JM. Frequency of eating during pregnancy and its effect on preterm delivery. American Journal of Epidemiology. 2001;153:647–652. doi: 10.1093/aje/153.7.647. [DOI] [PubMed] [Google Scholar]
- 20.Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. British Medical Journal. 1993;307:234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 22.Lockwood CJ. Stress-associated preterm delivery: the role of corticotropin-releasing hormone. American Journal of Obstetrics & Gynecology. 1999;180:S264–S266. doi: 10.1016/s0002-9378(99)70713-1. [DOI] [PubMed] [Google Scholar]
- 23.Savitz DA, Murnane P. Behavioral influences on preterm birth: a review. Epidemiology. 2010;21:291–299. doi: 10.1097/EDE.0b013e3181d3ca63. [DOI] [PubMed] [Google Scholar]
- 24.Maclure M. The Case-Crossover Design: A Method for Studying Transient Effects on the Risk of Acute Events. American Journal of Epidemiology. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 25.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. New England Journal of Medicine. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 26.Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. American Journal of Epidemiology. 2010;172:1108–1117. doi: 10.1093/aje/kwq170. [DOI] [PubMed] [Google Scholar]
- 27.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. American Journal of Epidemiology. 1995;142:91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 28.Maclure M, Mittleman MA. Should we use a case-crossover design? Annual Review of Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 29.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American Journal of Epidemiology. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 30.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. American Journal of Epidemiology. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suissa S. The Case-Time-Control Design. Epidemiology. 1995;6:248–253. doi: 10.1097/00001648-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Sayle AE, Savitz DA, Thorp JM, Jr, Hertz-Picciotto I, Wilcox AJ. Sexual activity during late pregnancy and risk of preterm delivery. Obstetrics and Gynecology. 2001;97:283–289. doi: 10.1016/s0029-7844(00)01147-9. [DOI] [PubMed] [Google Scholar]
- 34.Cooperstock M, England JE, Wolfe RA. Circadian incidence of labor onset hour in preterm birth and chorioamnionitis. Obstetrics and Gynecology. 1987;70:852–855. [PubMed] [Google Scholar]
- 35.Vatish M, Steer PJ, Blanks AM, Hon M, Thornton S. Diurnal variation is lost in preterm deliveries before 28 weeks of gestation. BJOG: An International Journal of Obstetrics & Gynaecology. 2010;117:765–767. doi: 10.1111/j.1471-0528.2010.02526.x. [DOI] [PubMed] [Google Scholar]
- 36.Lindow SW, Jha RR, Thompson JW. 24 hour rhythm to the onset of preterm labour. BJOG: An International Journal of Obstetrics & Gynaecology. 2000;107:1145–1148. doi: 10.1111/j.1471-0528.2000.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 37.Cooperstock M, England JE, Wolfe RA. Circadian incidence of premature rupture of the membranes in term and preterm births. Obstetrics and Gynecology. 1987;69:936–941. [PubMed] [Google Scholar]