Coastal cliff-tops are specific saline environments, where only highly specialized halophytes can thrive. Limonium spp. are commonly found in these ecological conditions, many of them being considered as threatened or with an unknown conservation status. The habitat requirements of Limonium multiflorum, an apomictic halophyte endemic to western Portugal, were investigated. Results showed the species narrow habitat specificity as well as its intolerance to competition with invasive alien plants. We conclude that in situ conservation of this rare and vulnerable species emerges as a priority in order to ensure that its biodiversity is not lost.
Keywords: Agamospermic species, cliff-dwelling species, conservation, habitat specificity, halophyte, Limonium.
Abstract
Coastal areas and other saline environments are major contributors to regional and global biodiversity patterns. In these environments, rapidly changing gradients require highly specialized plants like halophytes. In European coastal cliff-tops, rocky and sandy seashores, and saltmarshes, typical halophytes from the genus Limonium are commonly found. Among them, the aneuploid tetraploid (2n = 4x = 35, 36, 37) Limonium multiflorum, endemic to the west coast of Portugal, is an interesting case study for investigating the ecology and conservation of a halophyte agamospermic species. Although it is listed in the IUCN red list of threatened species, information on its population size or rarity, as well as its ecology, in some respects is still unknown. Field surveys in the largest known population were performed (Raso cape, Portugal) in order to determine habitat requirements and conservation status. A total of 88 quadrats were monitored, 43 of which contained at least one L. multiflorum individual. For each sampled quadrat, four abiotic and four biotic variables as well as two spatially derived variables were recorded. Principal component analysis and cluster analysis showed narrow habitat specificity for this species which appeared to be intolerant to competition with invasive alien plants. We conclude that in situ conservation in a local ‘hotspot’ of this rare and vulnerable species emerges as a priority in order to ensure that biodiversity is not lost.
Introduction
Habitat assessment is a fundamental requirement for species conservation. Attempts to set plant conservation priorities revealed the need to consider attributes such as species ecological specificity, geographical rarity and rate of threat (Domínguez Lozano et al. 2003; Pärtel et al. 2005). Furthermore, a study on plant life history traits in rare versus common taxa also showed that infrequent species exhibited a narrower geographical range and more habitat specialization than their common relatives (Farnsworth 2007). Thus, knowledge on habitat requirements of a rare species is crucial, in particular in the case of agamospermic species (defined by an asexual breeding system) (Domínguez Lozano et al. 2003), to help establish the best conservation methods.
In Europe, coastal vegetation presents lower diversity values when compared with other world regions (Mucina 2013) but European dry coastal terrestrial habitats, which include maritime rocks, sea-cliffs and coastal slopes, show higher values in terms of plant diversity (van der Maarel 1993; van der Maarel and van der Maarel-Versluys 1996). Among the best represented flowering plant families of these habitats are the Plumbaginaceae with nearly 90 % of coastal species, including most members from the genus Limonium (Kubitzki 1993; van der Maarel and van der Maarel-Versluys 1996). This cosmopolitan halophytic genus comprises annual and perennial species found on cliff-tops, rocky and sandy seashores, and saltmarshes (Erben 1993; Kubitzki 1993). These habitats are an important source of endemics (van der Maarel and van der Maarel-Versluys 1996), and nearly 37 % of all typical littoral species are considered threatened either because they have an extremely local distribution or because they are in decline as a result of negative human impacts in coastal areas (van der Maarel and van der Maarel-Versluys 1996).
Knowledge of habitat requirements of threatened populations from rare species selected for conservation is crucial for assuring their viability (Simberloff 1988; Brussard 1991; Schemske et al. 1994; Heywood and Iriondo 2003), since the establishment and expansion of a species is dependent on growth under favourable ecological conditions (e.g. Baumberger et al. 2012). Increasing a species' survival prospects through reintroduction or reinforcement (increase population size and diversity) of native species (Akeroyd and Wyse Jackson 1995), and knowing ecological processes in combination with demographic and genetic processes and breeding systems is therefore essential (Godefroid et al. 2011). However, objective data for documenting habitat preferences of rare plant species are relatively scarce, in particular those on ocean-exposed high cliff-tops subjected to salt spray transported inland by wind (Salisbury 1952; Maun 2004; Frederiksen et al. 2006).
The Portuguese coast is known for the richness of its flora due to a singular biogeographic position (Braun-Blanquet et al. 1972; Asensi et al. 1993; van der Maarel and van der Maarel-Versluys 1996). About 35 % of all Portuguese Natura 2000 habitats consist of coastal habitats, including Atlantic ocean-exposed cliff-tops (Costa et al. 2007; Martins et al. 2013). These locations present the original flora and vegetation on limestone, sandstone and marly clay areas, and include the rare and endemic cliff-dwelling Limonium multiflorum (Erben 1978, 1993; Costa et al. 2000; Espírito-Santo et al. 2012). This species is listed in Annex II of Habitats Directive (Council Directive 92/43/EEC 1992) and in the IUCN red list of threatened species (IUCN 2013), and most of its known populations are located in Portuguese NATURA 2000 Sites of Community Importance (EC 2013). Although L. multiflorum is considered a Portuguese crop wild relative (Magos-Brehm et al. 2008) and has an assessed conservation status of ‘Least Concern’ in the IUCN red list (IUCN 2013), information on its ecological preferences, essential for recovery plans, especially population restoration, population augmentation or population reintroduction is lacking.
In the present study we assessed habitat requirements for L. multiflorum. Two main questions were addressed: (i) What are the main abiotic and biotic variables favourable for its persistence? (ii) What is the vegetation cover and respective species composition associated with its presence?
Methods
Study species
Limonium multiflorum (2n = 4x = 35, 36, 37) (Erben 1993; Róis et al. 2012) is a perennial species endemic to a 120-km-long shoreline stretch in western Portugal (ICNF 2013) (Fig. 1) (Espírito-Santo et al. 2012). It is mainly found in NATURA 2000 habitat 1240 Vegetated sea cliffs of the Mediterranean coasts with endemic Limonium spp. and habitat 1330 Atlantic salt meadows (APA 2011). Along the western coast, populations are found on different cliffs separated by unsuitable habitats such as acid rock cliffs or steep slopes (mostly granite and syenite), sandy beaches or pinewoods. It occurs in small populations from <10 individuals to 1000 flowering plants on average (Caperta et al. 2013; Róis et al. 2013). Seedling emergence is mainly observed in autumn, following dispersal of seeds in summer. However, a persistent seed bank is not formed in such a thin soil and only a small amount of seeds originate seedlings (A.D.C. and A.S.R., unpubl. res.). Plants then grow as vegetative rosettes for several years on cliff-tops and in nearby rocky areas with a high degree of exposure to salt spray and salt-laden winds from the Atlantic Ocean (Caperta et al. 2013). Flowering mainly occurs in spring and summer (April to July), although in some years, it is also observed in November and February (A.D.C. and A.S.R., unpubl. res.).
Figure 1.
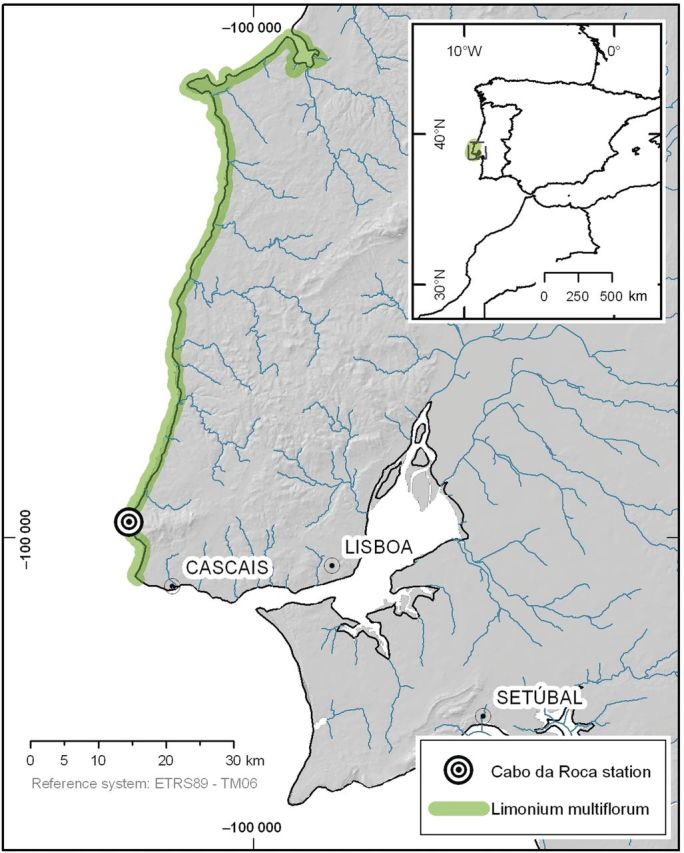
Map of L. multiflorum distribution in the western coast of Portugal.
Study site
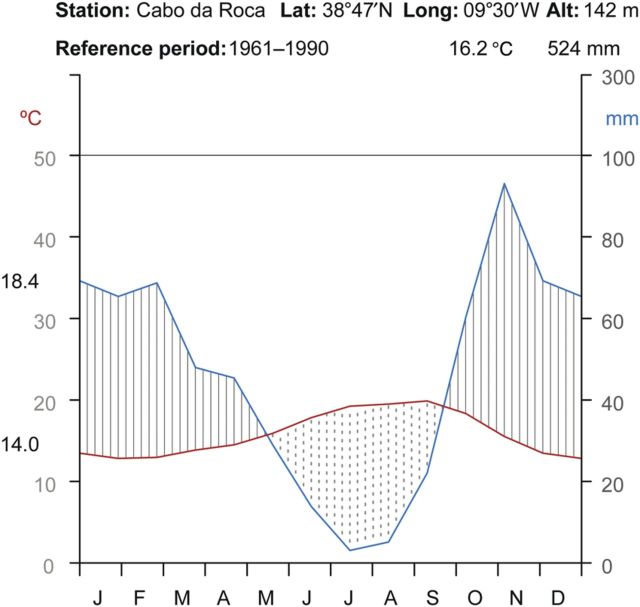
Our study was conducted in Raso cape, a broad promontory found in the west of Lisbon (municipality of Cascais, district of Lisbon, Portugal; mean location coordinates are 38°42′34″N and 9°29′12″W) (Fig. 2). Presently, the largest known population of L. multiflorum (about 1000 individuals) is found at this site, within an area of ∼0.6 km2, inside a Site of Community Importance (SCI Sintra/Cascais PTCON0008) for the Mediterranean biogeographical region. This cape consists of gently folded limestone, forming a rocky shoreline with low cliffs and numerous deep incisions along fault lines or abraded mylonitic rocks (Scheffers and Kelletat 2005). In terms of biogeographical typology this site is included in the Olissiponean District, Dividing Portuguese Sector, Sadensean-Dividing Portuguese Subprovince and Coastal Lusitan-Andalusian Province (Costa et al. 1998a; Rivas-Martínez 2007). The region's climate is well represented by the thermopluviometric diagram of Cabo da Roca station (Fig. 3), which presents a typical hyperoceanic Mediterranean climate pattern, as indicated by the low variation of average monthly temperatures and the existence of more than two consecutive dry months in the summer period. Another noteworthy feature of the region's climate is the high frequency of relatively strong winds coming from the northern and northwestern sectors (Fig. 4). Bioclimatically speaking (sensu Rivas-Martínez 2007), most of this region falls in the upper thermomediterranean thermotype and in the lower sub-humid to upper dry ombrotypes (Mesquita and Sousa 2009; Monteiro-Henriques 2010). Here, the predominant plant community is Limonietum multiflori-virgati (Costa et al. 1998b, 2012) which integrates L. multiflorum, L. virgatum, Dactylis marina, Plantago coronopus and Crithmum maritimum, among other species. This area encompasses different human occupations, namely buildings and other built structures like seafood tanks, off-road motorized driving and tourism which contribute to population fragmentation.
Figure 2.
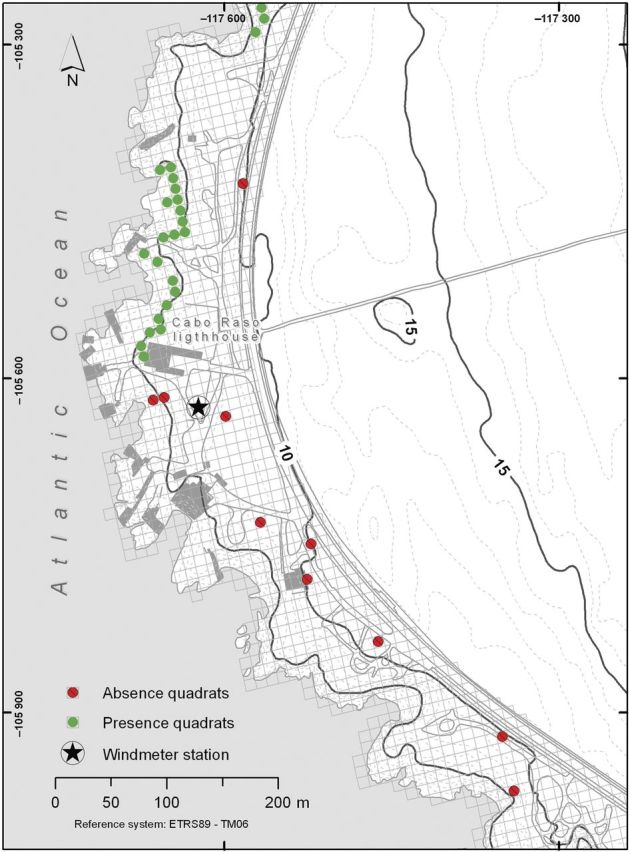
Schematic drawing of the quadrat sampling of L. multiflorum in the coast of Raso cape.
Figure 3.
Thermopluviometric diagram based on the weather station in the coast of Raso cape. The red line corresponds to mean monthly temperature (°C) and the blue line corresponds to mean monthly precipitation (mm).
Figure 4.
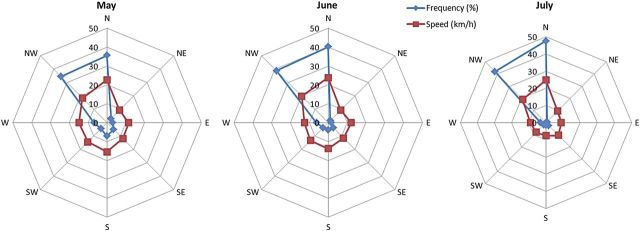
Wind regime diagrams based on the weather station in the coast of Raso cape during L. multiflorum flowering peak (May–July).
Sampling design
Field surveys used a grid of 10 × 10 m quadrats which was overlaid over the aerial photographs of the site and later transferred to the site using global positioning system (GPS) receivers. A total of 88 quadrats were surveyed, 43 of which contained at least one L. multiflorum individual (presence quadrat) and the remaining 45 were randomly selected quadrats containing no individuals from this species (absence quadrat) (Fig. 2). The random selection procedure was performed using R software's ‘sample’ routine (R Core Team 2013). In quadrats where L. multiflorum was present censuses were carried out from April to June 2013. For each sampled quadrat four abiotic variables, namely rock formation (RockForm), cobble (Cobble), coarse sand (CoarseSd) and fine sand (FineSand) (all expressed in %), were recorded. Four biotic variables related to vegetation cover (Coverage) as well as invasive non-native species (INNS) cover, dead organic matter (lignified or not) (DOM) cover and litter (Residues) cover were also estimated (expressed in %) in each quadrat. To facilitate data collection, each quadrat was then sub-divided into sub-quadrats of 1 m2, and the percent coverage value obtained for the sub-quadrats of the same 100 m2 quadrat were averaged. Two spatial variables, distance from coastline (Dist_coa) and mean quadrat slope (Mean_slo), were also derived using the ArcGIS® 10.0 software, by ESRI.
Data analysis
To define L. multiflorum habitat requirements we performed a principal component analysis (PCA) on the resulting data matrix using abiotic, biotic and spatially derived variables. In order to discriminate the vegetation structure and composition in the monitored quadrats, a cluster analysis using Euclidean distance and Ward clustering method (Ward 1963) was performed using the set of quadrats containing L. multiflorum. To ensure that the group of quadrats (clusters) had the same influence from the environmental variables, Kruskal–Wallis tests of variance were performed (Sokal and Rohlf 1997; Zar 2010). To complete habitat characterization of L. multiflorum, the most frequent and abundant plant species within each cluster was defined as described in Baumberger et al. (2012). The mean coverage of each species within each group was also calculated. All statistical analyses were conducted using the STATISTICA software (StatSoft V10). Bioclimatic and biogeographic nomenclature followed the proposals of Rivas-Martínez (2007). Taxonomic nomenclature followed Menezes de Sequeira et al. (2011).
Results
Influence of environmental variables on the presence/absence of L. multiflorum
On the western coast most L. multiflorum populations occur at inaccessible cliff sites and/or show very small size (Róis et al. 2013). Under these circumstances, this study was conducted at the best accessible site and on the largest known population for this species, in Raso cape. Study site rocks were bare of vegetation up to ∼7–8 m asl, due to strong exposure to Atlantic storms and waves over the years.
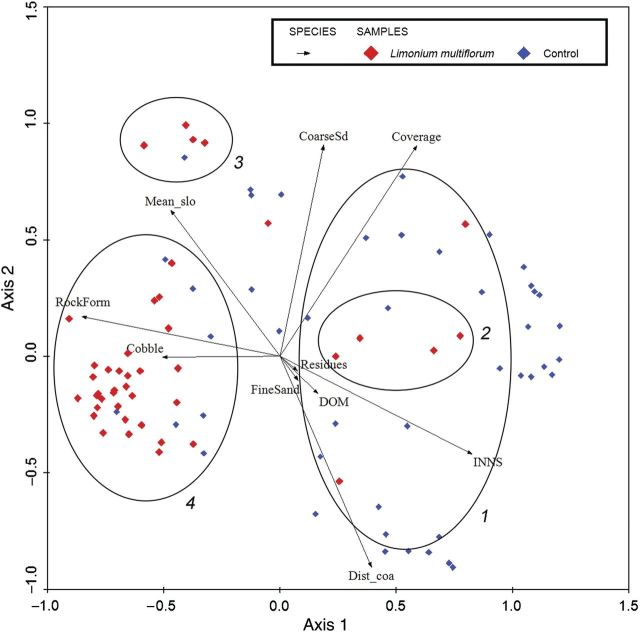
Of the 88 quadrats sampled, only 43 contained at least one L. multiflorum individual (presence) while control quadrats did not show any individual from this species (absence). Considering the presence quadrats, L. multiflorum mean coverage was very low (about 0.67 %). Only one of the variables measured in each quadrat fitted a normal distribution (variable ‘Mean_slo’), and the other nine failed to do so, even after a logarithmic transformation. Therefore, the remaining analyses were performed using the original (untransformed) values. Remarkably, visual inspection of PCA revealed that in the first two represented ordinations, quadrats showing the presence of L. multiflorum individuals were highly correlated with rock formation (variable ‘RockForm’) and non-correlated with high coverage (variable ‘vegetation cover’) (Fig. 5). Considering the first two principal components of PCA, the percentage of accumulated variance was 64.4 % (Fig. 5). The first axis accounted for 44.8 % of the variance and was explained by vegetation cover, while the second axis accounted for 19.7 % of the variance explained by distance from coast (variable ‘Dist_coa’).
Figure 5.
Two first axes of PCA based on L. multiflorum presence and absence quadrats. Red symbols mean that L. multiflorum was recorded whereas blue symbols signify that it was absent. Axis 1 represents 44.8 % of variation, and Axis 2, 19.7 %. CoarseSd, coarse sand; Cobble, cobble; Coverage, vegetation cover; Dist_coa, distance from coast; DOM, dead organic matter cover; FineSand, fine sand; INNS, invasive non-native species cover; Mean_slo, mean slope; Residues, litter cover; RockForm, rock formation.
Influence of environmental variables in L. multiflorum persistence
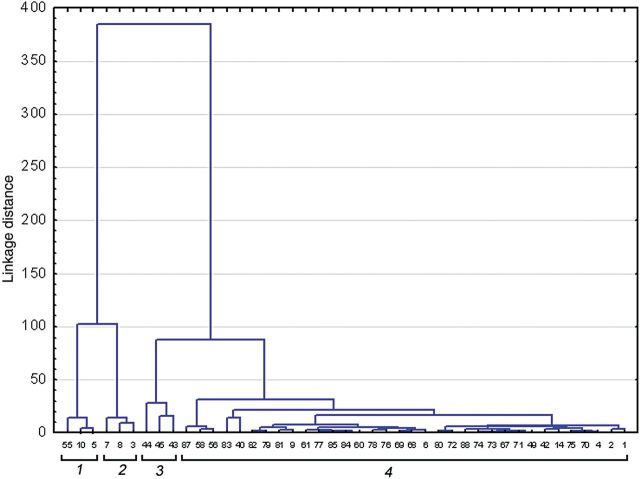
A cluster analysis was performed using the dataset of quadrats showing L. multiflorum presence. The data obtained revealed the existence of four floristic groups among the sampled quadrats (Fig. 6; Tables 1 and 2). Environmental conditions differed between Group 4 and the other three floristically defined groups. Clusters 3 and 4 were clearly individualized in PCA. The first groups (Groups 1, 2 and 3) presented high vegetation coverage percentage (variable ‘coverage’) combined with low rock outcrop percentages (variable ‘RockForm’) whereas the opposite occurred in Group 4, which showed the highest L. multiflorum cover percentage. Also, in this latter group low percentages of invasive non-native species (variable ‘INNS’) were found. Although in both Groups 1 and 2, ‘INNS’ percentages were greater than those in the other groups, Group 2 differed in terms of fine sand percentages (variable ‘FineSand’).
Figure 6.
Cluster dendogram of the four clusters containing L. multiflorum. The dissimilarity between groups was calculated based on species variables using Euclidean distance and the Ward aggregation method. Cluster definition 1 accounts for a high percentage of C. edulis (>65 %); Cluster 2 by a moderate percentage of C. edulis (<35 %); Cluster 3 is defined by A. welwitschii (9.67 %) and P. incurva (21.33 %); and Cluster 4 represents quadrats with the highest L. multiflorum frequency (0.84 %).
Table 1.
Mean values ± standard error of abiotic, biotic and spatial-related variables in each of four environmental groups based on the presence data of PCA. Bold letters within a row indicate non-significant with post hoc multiple comparison Tukey's test (α = 0.05; P < 0.001). P value <0.001. ns, non-significant; CoarseSD, coarse sand; Cobble, cobble; Coverage, vegetation cover; Dist_coa, distance from coast; DOM, dead organic matter cover; FineSd, fine sand; INNS, invasive non-native species cover; Mean_slo, mean slope; Residues, litter cover; RockForm, rock formation.
| Environmental groups |
||||
|---|---|---|---|---|
| 1 (n = 3) | 2 (n = 3) | 3 (n = 3) | 4 (n = 34) | |
| Coverage (%)* | 54.83 ± 47.54 (a) | 47.17 ± 11.07 (a) | 37.67 ± 11.68 (a) | 3.10 ± 7.44 (b) |
| FineSd (%)* | 0.00 ± 0.00 (a) | 3.17 ± 2.75 (b) | 0.00 ± 0.00 (a) | 0.00 ± 0.00 (a) |
| INNS (%)* | 73.00 ± 6.24 (a) | 33.00 ± 3.46 (b) | 0.00 ± 0.00 (c) | 0.88 ± 2.55 (c) |
| RockForm (%)* | 5.00 ± 4.00 (a) | 6.00 ± 5.29 (a) | 40.00 ± 11.79 (a,b) | 74.49 ± 23.58 (b) |
| CoarseSd (%) ns | 5.67 ± 8.14 | 0.00 ± 0.00 | 11.67 ± 4.16 | 3.37 ± 9.17 |
| Dist_coa (m) ns | 11.36 ± 8.24 | 16.51 ± 3.95 | 0.00 ± 0.00 | 16.31 ± 9.88 |
| Cobble (%) ns | 4.77 ± 6.60 | 45.00 ± 8.23 | 8.67 ± 10.017 | 13.63 ± 19.85 |
| DOM (%) ns | 2.67 ± 4.62 | 0.00 ± 0.00 | 5.33 ± 9.24 | 0.41 ± 1.23 |
| Mean_slo (m) ns | 16.63 ± 5.66 | 17.25 ± 0.82 | 68.92 ± 28.97 | 25.21 ± 21.50 |
| Residues (%) ns | 0.33 ± 0.58 | 0.42 ± 0.52 | 0.00 ± 0.00 | 0.09 ± 0.19 |
Table 2.
Mean coverage (%) of the most frequent species in each of the four environmental groups (clusters) based on the presence data of PCA.
| Floristic list |
Mean coverage (%) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Psammophilous | Armeria welwitschii Boiss. | 1.17 | 2.34 | 9.67 | 0.03 |
| Inula crithmoides L. | 0.00 | 0.17 | 0.12 | 1.33 | |
| Herniaria maritima Link | 0.00 | 0.67 | 0.00 | 0.07 | |
| Lobularia maritima (L.) Desv. subsp. maritima | 0.00 | 0.00 | 0.20 | 0.00 | |
| Parapholis incurva (L.) C.E.Hubb. | 0.50 | 0.00 | 21.33 | 0.70 | |
| Helichrysum italicum (Roth) G.Don subsp. picardi (Boiss. & Reut.) Franco | 0.33 | 0.00 | 0.17 | 0.03 | |
| Andryala arenaria (DC.) Boiss. & Reut. subsp. arenaria | 0.17 | 0.33 | 0.58 | 0.06 | |
| Otanthus maritimus (L.) Hoffmanns. & Link | 0.00 | 2.75 | 0.00 | 0.02 | |
| Elymus farctus (Viv.) Runemark ex Melderis subsp. boreo-atlanticus (Simonet & Guin.) Melderis | 3.33 | 0.77 | 1.00 | 0.98 | |
| Lotus creticus L. | 0.00 | 0.01 | 0.25 | 0.40 | |
| Medicago minima (L.) L. | 0.00 | 0.50 | 1.00 | 0.03 | |
| Crucianella maritima L. | 0.13 | 0.00 | 0.00 | 0.03 | |
| Cakile maritima Scop. | 0.00 | 0.00 | 0.00 | 0.01 | |
| Dactylis smithii Link subsp. marina (Borrill) Parker | 0.02 | 0.01 | 0.25 | 0.09 | |
| Chamosphilous | Limonium ovalifolium (Poir.) Kuntze | 0.00 | 0.00 | 1.33 | 0.03 |
| Limonium virgatum (Willd.) Fourr. | 0.17 | 0.00 | 0.07 | 0.07 | |
| Plantago coronopus L. | 0.02 | 0.17 | 0.75 | 0.07 | |
| Limonium multiflorum Erben | 0.03 | 0.05 | 0.11 | 0.84 | |
| Frankenia laevis L. | 0.83 | 1.67 | 1.00 | 1.03 | |
| Crithmum maritimum L. | 0.30 | 3.67 | 0.23 | 0.66 | |
| Catapodium marinum (L.) C.E.Hubb. | 0.00 | 0.00 | 0.00 | 0.03 | |
| Nitrophilous | Carpobrotus edulis (L.) N.E.Br. | 73.00 | 33.00 | 0.00 | 0.88 |
| Beta maritima L. | 0.08 | 0.33 | 0.50 | 0.28 | |
| Daucus halophilus Brot. | 0.00 | 0.00 | 0.00 | 0.01 | |
| Leontodon taraxacoides (Vill.) Mérat subsp.taraxacoides | 0.00 | 0.00 | 0.00 | 0.01 | |
| Parapholis filiformis (Roth) C.E.Hubb. | 0.50 | 0.00 | 0.00 | 0.06 | |
| Polypogon maritimus Willd. | 0.00 | 0.00 | 0.00 | 0.02 | |
Vegetation composition also varied between clusters. Cluster 1 was dominated by Carpobrotus edulis with a coverage percentage >65 % whereas Cluster 2 presented a moderate coverage of C. edulis (<35 %). In this cluster significant mean coverage was observed for psammophilous species like the perennials Armeria welwitschii and Elymus farctus subsp. boreo-atlanticus. As for Cluster 3, significant frequencies of the perennial Limonium ovalifolium and the therophyte Parapholis incurva were revealed. Finally, Cluster 4 which had the greatest coverage percentage of L. multiflorum (0.835 %) of all clusters appeared to be associated with chasmophytic species such as C. maritimum, and other perennials like Frankenia laevis and Inula crithmoides.
Discussion
L. multiflorum displays narrow habitat specificity
In this study we show that L. multiflorum grows on sea-cliffs preferentially up to 50 m asl in karstic crevices within exposed rocks more or less filled with substrate, or on shallow soil above the rock strata and on scree slopes. The presence of L. multiflorum was favoured by the presence of rock formation, cobble, low percentage of vegetation coverage, low frequency of invasive non-native species and closest distance to coast. In fact, as pointed out by Warming (1909), L. multiflorum's preference for salt-rich rock crevices seems to be a strategy to use such locations as refuges, thus avoiding competition with many other coastal plants that are unable to colonize such habitats. Furthermore, as it happens in endemic Limonium spp., populations tend to be fragmented and usually show low population sizes as a consequence of many social and economic activities like urbanization, tourism and traffic (Rodríguez et al. 2003; Palop-Esteban et al. 2007; Suárez-García et al. 2009; Khan et al. 2012; Róis et al. 2013) on their habitats. This is also the case of L. multiflorum (ICNF 2013), in particular, in the Raso cape population (A.F. and V.S., unpubl. res.).
Vegetation composition significantly differs among the four floristically defined groups. Cluster 1 is dominated by the alien plant C. edulis with E. farctus subsp. boreo-atlanticus's due to nitrophilous coarse sand dunes corresponding to Elytrigietum junceo-boreoatlanticae association (Costa et al. 2012). The presence of L. multiflorum in this cluster can be explained by 5 % of rock outcrop, uncovered with sand. Cluster 2 includes the farthest quadrats from the sea where rock depressions filled up with fine sand and cobble appear. In this cluster A. welwitschii, F. laevis and L. multiflorum predominate. This cluster presents a poor floristic composition, as it happens in the previous cluster, due to invasion of C. edulis, possibly related with the proximity to roads (higher nitrification). Cluster 3 is typically formed by species of rocky substrate, with a high slope and direct influence of wind loaded with salt. Here L. ovalifolium, C. maritimum, A. welwitschii, F. laevis, L. multiflorum, P. coronopus form the perennial community Limonietum multifloro-virgati (Costa et al. 1998b, 2012). In their clearings P. incurva, Medicago minima, Andryala arenaria form the poor, annual association Parapholido incurvae–Catapodietum marini. Also, the presence of Lotus creticus is due to coarse sand. Cluster 4 also exhibits species that constitute the Limonietum multifloro-virgati association (Costa et al. 1998b, 2012), namely C. maritimum, L. multiflorum, I. crithmoides, F. laevis, P. coronopus. The referred species are at their ecological optimum in this rocky sea-cliff cluster although a small percentage of coarse sand explains the presence of E. farctus subsp. boreo-atlanticus and L. creticus. Plant survival in this ecological context is difficult due to low availability of fresh water, low level of essential nutrients and the abrasive action of strong winds laden with salt and increasing conditions of extreme dryness (Costa et al. 1998b). To overcome this some plants respond with adaptations, such as adopting the hemicryptophyte or therophyte life forms, presenting succulent leaves and/or salt glands in the leaves (Warming 1909; Costa 2001; Grigore et al. 2014).
L. multiflorum appears to be intolerant to competition with invasive species
Our results show that both native and non-native vegetation cover are negatively correlated with L. multiflorum presence, emphasizing its preferences for sites where competition with other species is low. Among the species having a greater negative effect in their persistence is the exotic, invasive, crawling C. edulis. This species commonly invades coastal habitats in Mediterranean Europe forming mantles on maritime rocks, cliffs and sand dunes (D'Antonio 1990; Traveset et al. 2008; Pyšek et al. 2013) competing directly with native plant species, suppressing the growth and establishment of other plants (D'Antonio and Mahall 1991; Vilà et al. 2006), altering certain soil parameters (Vilà et al. 2006; Conser and Connor 2009) or creating litter accumulation on the soil surface (Molinari et al. 2007). Although C. edulis seems to develop well on rocky substrates at sites where seagulls' (Larus sp.) nutrient-rich droppings are frequent (M.D.E.-S., unpubl. res.), in our study it seems to compete mainly with species showing preference for sand substrates. Remarkably, some littoral species show strong resilience to C. edulis invasion, like the narrow endemic cliff-species Armeria pseudoarmeria (Pinto-Ricardo et al. 2010) or other Limonium spp. (Snogerup 1971; Suehs et al. 2003; Campos et al. 2004).
Conservation issues
For the effective protection of the rare and endemic L. multiflorum not only ecological data but also life story, demography and genetic data should be taken into account to ensure that maximum genetic variation is preserved. In this context, one of the factors to be considered in conserving L. multiflorum is related with chromosome polymorphisms since euploid and aneuploid cytotypes occur within populations (Róis et al. 2012). Although, it was first described as an unbalanced aneuploid tetraploid (2n = 4x = 35) (Erben 1978), both unbalanced and balanced tetraploid cytotypes (2n = 4x = 35, 36, 37) and diverse karyological polymorphisms were found within and among populations, with greater variability in the Raso cape population (Róis et al. 2012). This has also been observed in other Limonium taxa (e.g. Castro and Rosseló 2007), and even in a variety of genera from different plant families (e.g. Brassicaceae, Böcher 1954; Campanulaceae, James 1965; Onagraceae, Bloom 1974; Plumbaginaceae, Erben 1978; Poaceae, Sieber and Murray 1981; Ranunculaceae, Borchers-Kolb 1983). These cytological differences should be considered to ensure that maximum genetic variation is preserved. Furthermore, the resultant combination of incompatible cytotypes could result in the failure of reintroduced plants to reproduce and may bring reproductive instability to augmented populations (Young and Murray 2000; Ennos et al. 2005; Severns and Liston 2008).
Noticeably, male sterility and gynodioecism are found in plants from experimental collections (Róis et al. 2012) and field plants (Heike Sprenger and A.D.C., unpubl. res.). However, these plants are capable of producing large numbers of viable seeds with variable ploidy levels, as revealed by flow cytometry seed screening (Róis et al. 2012) and reproduce through apomixis (A.S.R. and A.D.C., unpubl. res.). This form of uniparental reproduction is found in ‘taxonomically complex groups’ like in other Limonium spp. (Erben 1978; Palacios et al. 2000; Lledó et al. 2005) and also in dandelions (Taraxacum spp.; van Dijk 2003), blackberries (Rubus fruticosus agg.; Salvini et al. 2006) and in Ranunculus spp. (Hörandl et al. 2009), which generates a diverse mixture of related individuals (Ennos et al. 2005).
While some L. multiflorum populations are located in natural parks (Parque Natural Sintra-Cascais) and other areas under legal protection (e.g. Site of Community Importance—SCI Sintra/Cascais PTCON0008; SCI Peniche/Sta Cruz PTCON0056), in the past decades no information on population size along its distribution range has been available. However, we hypothesized that population decline might have occurred due to increasing urban development in the coastal areas and the impact of tourism. For instance, in Raso cape, sightseeing tourists, sports fishing and other socio-economic activities, together with competition associated with invasion by non-native species are cumulatively leading to population decline (V.S., A.F. and A.D.C., unpubl. res.). Notably, methylation-sensitive amplified polymorphism (MSAP) data from L. multiflorum natural populations reveal low levels of genetic/epigenetic diversity (Róis et al. 2013). Altogether these data mean that none of the populations analysed could restore the genotypic diversity observed in any other L. multiflorum population. Hence, habitat protection emerges as the top priority to prevent population extinction of the narrow-specialist L. multiflorum. For the conservation of this species, management actions such as those referred by Laguna et al. (2013), namely limiting access to populations, for example, the experimental exclusion of herbivores by means of fences to protect populations of L. dufourii in ‘Marsh dels Moros’ (Spain) and to perform selective removal and control of invasive species (Laguna et al. 2013), are required. Other technical solutions could be population reinforcements, reintroduction or establishment of new populations, the goal of which is the introduction of new specimens. In the case of L. multiflorum, attempts to create new populations through experimental actions like sowing seeds directly in situ did not prove to be successful (A.D.C. and V.S., unpubl. res.) but population reinforcements by planting newly produced specimens using seed stock that originated from the same natural populations (e.g. Raso cape) appears to give better results (Caperta et al. 2013). Complementary ex situ conservation actions for this species such as collection and storage of seeds preserved in João do Amaral Franco Seed Bank (Ajuda Botanical Garden) have also been conducted (Caperta et al. 2013) with the hope that the genetic diversity contained in these collections could be representative of natural populations. Thus, to conserve L. multiflorum, it is preferable to develop small, localized experimental actions as Laguna et al. (2013) pointed out. In fact, only moderate success is reported for reintroduction projects due, for example, to insufficient long-term monitoring following reintroduction and/or lack of understanding of the underlying reasons for decline in existing plant populations (e.g. Godefroid et al. 2011).
Conclusions
According to Rabinowitz' scheme (1981), in which species were classified into categories according to their geographic range, habitat specificity and local population size, L. multiflorum can be classified as a ‘classic rarity’ since it presents both narrow geographic range and narrow habitat specificity, thus being considered a restricted endemic. Furthermore, species' populations observed throughout its entire range consistently present small sizes and low levels of genetic diversity as revealed by population genetic and epigenetic studies using MSAP markers (Róis et al. 2013). Taking the above into consideration, as well as diverse social-economic impacts exerted on its populations, we considered that L. multiflorum deserves a status of ‘Vulnerable’ in the IUCN red list.
As already pointed out for other ‘taxonomically complex groups’ (Ennos et al. 2005), conservation of rare and endemic L. multiflorum is best achieved by facilitating evolutionary interactions among its members that generate and maintain their taxonomic biodiversity.
Sources of Funding
Our work was funded by Fundação para a Ciência e Tecnologia (FCT) (Portugal) (PEST-OE/AGR/UI0240/2011). A.D.C. (Researcher, CBAA/ISA) and A.S.R. (SFRH/BD/62542/2009 grant) were supported by FCT.
Contributions by the Authors
A.D.C. and P.A. designed and coordinated the study. M.D.E.-S. coordinated the fieldwork and A.D.C., M.D.E.-S., A.P.P. and A.S.R. performed the fieldwork. A.F. and V.S. processed the raw data and carried out statistical analysis. J.C.C. and M.D.E.-S. performed the phytosociological analysis. A.D.C. and P.A. drafted the manuscript. All authors read and approved the manuscript.
Conflicts of Interest Statement
None declared.
Acknowledgements
We thank Manuela Rodrigues (Volunteer), Ana Cortinhas (CBAA/ISA) and Vanessa Mendonça (CBAA/ISA) for help in field work, Jorge Cadima (ISA) for help in defining the experimental design, and the anonymous reviewers for all the helpful comments and suggestions. We thank the Instituto da Conservação da Natureza e das Florestas (ICNF) for providing permissions for field work.
Literature Cited
- Akeroyd J, Wyse Jackson P. A handbook for botanic gardens for reintroduction of plants to the wild. Richmond: BGCI; 1995. [Google Scholar]
- APA [The Environmental Protection Agency of Portugal] Guide for the assessment of imminent threats and environmental damages. Lisboa: The Ministry of Agriculture, the Sea, The Environment and Territorial Planning; 2011. [Google Scholar]
- Asensi A, Diez Garretas B, van der Maarel E. Dry coastal ecosystems of Portugal. In: van der Maarel E, editor. Dry coastal ecosystems: polar regions and Europe. Ecosystems of the World 2A. Amsterdam: Elsevier; 1993. pp. 342–347. [Google Scholar]
- Baumberger T, Affre L, Torre F, Vidal E, Dumas PJ, Tatoni T. Plant community changes as ecological indicator of seabird colonies' impacts on Mediterranean Islands. Ecological Indicators. 2012;15:76–84. [Google Scholar]
- Bloom WL. Origin of reciprocal translocations and their effects in Clarkia speciosa. Chromosoma. 1974;49:61–76. [Google Scholar]
- Böcher T. Experimental taxonomic studies in the Arabis holboellii and allied species. Biologiske Skrifter. 1954;14:1–74. [Google Scholar]
- Borchers-Kolb E. Ranunculus sect. Auricomus in Bayern und den angrenzenden Gebieten. I. Allgemeiner Teil. Mitteilungen der Botanischen Staatssammlung München. 1983;19:363–429. [Google Scholar]
- Braun-Blanquet J, Rozeira A, Pinto da Silva AR. Résultats de deux excursions géobotaniques à travers le Portugal septentrional et moyen IV. Esquisse sur la végétation dunale. Agronomia Lusitana. 1972;33:217–234. [Google Scholar]
- Brussard PF. The role of ecology in biological conservation. Ecological Applications. 1991;1:6–12. doi: 10.2307/1941843. [DOI] [PubMed] [Google Scholar]
- Campos JA, Herrera M, Biurrun I, Loidi J. The role of alien plants in the natural coastal vegetation in central-northern Spain. Biodiversity and Conservation. 2004;13:2275–2293. [Google Scholar]
- Caperta AD, Paes AP, Espírito-Santo MD, Silva V, Ferreira A, Saraiva S. Arribas da faixa costeira de Cascais. Projecto de Recuperação de Habitats. El Botânico. 2013;7:69–71. [Google Scholar]
- Castro M, Rosseló JA. Karyology of Limonium (Plumbaginaceae) species from the Balearic Islands and the western Iberian Peninsula. Botanical Journal of the Linnean Society. 2007;155:257–272. [Google Scholar]
- Conser C, Connor EF. Assessing the residual effects of Carpobrotus edulis invasion, implications for restoration. Biological Invasions. 2009;11:349–358. [Google Scholar]
- Costa JC. Tipos de Vegetação e adaptações das plantas do litoral de Portugal continental. In: Moreira ME, Moura A, Granja H, Noronha F, editors. Homenagem (In Honorium) Professor Doutor Gaspar Soares de Carvalho. Braga: Universidade do Minho; 2001. pp. 283–299. [Google Scholar]
- Costa JC, Aguiar C, Capelo JH, Lousã M, Neto C. Biogeografia de Portugal Continental. Quercetea. 1998a;0:5–56. [Google Scholar]
- Costa JC, Capelo J, Lousã M, Espírito-Santo MD. Sintaxonomia da vegetação halocasmofítica das falésias marítimas portuguesas (Crithmo-Staticetea Br.-Bl. 1947) Itinera Geobotanica. 1998b;11:227–247. [Google Scholar]
- Costa JC, Capelo J, Aguiar C, Neto C, Lousã M, Espírito-Santo MD. An overview of the Pegano harmalae-Salsoletea vermiculatae Br.-Bl. and. O. Bolòs 1958, vegetation class in continental Portugal. Colloques Phytosociologiques. 2000;27:81–93. [Google Scholar]
- Costa JC, Monteiro-Henriques T, Neto C, Arsénio P, Aguiar C. The application of the Habitats Directive in Portugal. Fitosociologia. 2007;44:23–28. [Google Scholar]
- Costa JC, Neto C, Aguiar C, Capelo J, Espírito-Santo MD, Honrado J, Pinto-Gomes C, Monteiro-Henriques T, Menezes de Sequeira M, Lousã M. Vascular plant communities in Portugal (Continental, the Azores and Madeira) Global Geobotany. 2012;2:1–180. [Google Scholar]
- Council Directive 92/43/EEC. On the conservation of natural habitats and of wild fauna and flora. Official Journal of the European Union L. 21 May 1992;206:7–50. [Google Scholar]
- D'Antonio CM. Seed production and dispersal in the non-native, invasive succulent Carprobrotus edulis (Aizoaceae) in coastal strand communities of central California. Journal of Applied Ecology. 1990;27:693–702. [Google Scholar]
- D'Antonio CM, Mahall BE. Root profile and competition between the invasive, exotic perennial, Carprobrotus edulis and two native species in California coastal shrubs. American Journal of Botany. 1991;78:885–894. [Google Scholar]
- Domínguez Lozano F, Moreno Saiz JC, Sainz Ollero H. Rarity and threat relationships in the conservation planning of Iberian flora. Biodiversity and Conservation. 2003;12:1861–1882. [Google Scholar]
- EC. European Commission: seventh updated list of sites of community importance for the Mediterranean biogeographical region. Official Journal of the European Union L. 2013;350:101–198. [Google Scholar]
- Ennos RA, French GC, Hollingsworth PM. Conserving taxonomic complexity. Trends in Ecology and Evolution. 2005;20:164–168. doi: 10.1016/j.tree.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Erben M. Die gattung Limonium im südwestmediterranen Raum. Mitteilungen der Botanischen Staatssammlung München. 1978;14:361–631. [Google Scholar]
- Erben M. Flora Iberica, Vol. 3. Plumbaginaceae (partim)–Capparaceae. Madrid: Real Jardín Botánico, CSIC; 1993. Limonium Miller; pp. 2–143. Castroviejo S, Aedo C, Cirujano S, Laínz M, Montserrat P, Morales R, Muñoz Garmendia F, Navarro C, Paiva J, Soriano C. [Google Scholar]
- Espírito-Santo MD, Alves HN, Caperta AD, Moreira I. Plantas endémicas do litoral de Portugal Continental. In: Monteiro A, Gomes da Silva F, Jorge R, editors. Gestão e Conservação da Flora e da Vegetação de Portugal e da África Lusófona. In Honorium do Professor Catedrático Emérito Ilídio Rosário dos Santos Moreira. Lisboa: ISA Press; 2012. pp. 267–302. [Google Scholar]
- Farnsworth EJ. Plant life history traits of rare versus frequent plant taxa of sandplains: implications for research and management trials. Biological Conservation. 2007;136:44–52. [Google Scholar]
- Frederiksen L, Kollmann J, Vestergaard P, Bruun HH. A multivariate approach to plant community distribution in the coastal dune zonation of NW Denmark. Phytocoenologia. 2006;36:321–342. [Google Scholar]
- Godefroid S, Piazza C, Rossi G, Buord S, Stevens AD, Aguraiuja R, Cowell C, Weekley CW, Vogg G, Iriondo J, Johnson I, Dixon B, Gordon D, Magnanon S, Valentin B, Bjureke K, Koopman R, Vicens M, Virevaire M, Vanderborght T. How successful are plant species reintroductions? Biodiversity and Conservation. 2011;144:672–682. [Google Scholar]
- Grigore M-N, Ivanescu L, Toma C. Halophytes: an integrative anatomical study. New York: Springer; 2014. [Google Scholar]
- Heywood VH, Iriondo JM. Plant conservation: old problems, new perspectives. Biological Conservation. 2003;113:321–335. [Google Scholar]
- Hörandl E, Greilhuber J, Klimova K, Paun O, Temsch E, Emadzade K, Hodálová I. Reticulate evolution and taxonomic concepts in the Ranunculus auricomus complex (Ranunculaceae): insights from morphological, karyological and molecular data. Taxon. 2009;58:1194–1215. [PMC free article] [PubMed] [Google Scholar]
- ICNF. Relatório Nacional de Aplicação da Directiva Habitats (2007–2012) Lisboa: Instituto da Conservação da Natureza e das Florestas; 2013. [Google Scholar]
- IUCN. 2013. IUCN red list of threatened species. Version 2013.2 http://www.iucnredlist.org. 5 February 2014.
- James SH. Complex hybridity in Isotoma petraea. 1. The occurrence of interchange heterozygosity, autogamy and a balanced lethal system. Heredity. 1965;20:341–353. [Google Scholar]
- Khan Z, Santpere G, Traveset A. Breeding system and ecological traits of the critically endangered endemic plant Limonium barceloi (Gil and Llorens) (Plumbaginaceae) Plant Systematics and Evolution. 2012;298:1101–1110. [Google Scholar]
- Kubitzki K. Plumbaginaceae. In: Kubitzki K, Rohwer JG, Bittrich V, editors. The families and genera of vascular plants. Vol. 2. Berlin: Springer; 1993. pp. 523–530. [Google Scholar]
- Laguna E, Deltoro V, Ferrer PP, Navarro A, Ferrando I, Escribá MC, Albert FJ. Relevant plant recovery programmes. Conservation management of plant micro-reserves and ecological restoration. In: Kadis C, Thanos CA, Laguna E, editors. Plant micro-reserves: from theory to practice. Experiences gained from EU LIFE and other related projects. Athens: Utopia Publishing; 2013. pp. 127–140. [Google Scholar]
- Lledó MD, Crespo MB, Fay MF, Chase MW. Molecular phylogenetics of Limonium and related genera (Plumbaginaceae): biogeographical and systematic implications. American Journal of Botany. 2005;92:1189–1198. doi: 10.3732/ajb.92.7.1189. [DOI] [PubMed] [Google Scholar]
- Magos-Brehm J, Maxted N, Ford-Lloyd BV, Martins-Loução MA. National inventories of crop wild relatives and wild harvested plants: case-study for Portugal. Genetic Resources and Crop Evolution. 2008;55:779–796. [Google Scholar]
- Martins MC, Neto C, Costa JC. The meaning of mainland Portugal beaches and dunes' psammophilic plant communities: a contribution to tourism management and nature conservation. Journal of Coastal Conservation. 2013;17:279–299. [Google Scholar]
- Maun MA. Burial of plants as a selective force in sand dunes. In: Martínez ML, Psuty NP, editors. Coastal dunes. Ecology and conservation. Heidelberg: Springer; 2004. pp. 119–135. [Google Scholar]
- Menezes de Sequeira M, Espírito-Santo MD, Aguiar C, Capelo J, Honrado J, coords . Checklist da Flora de Portugal (Continental, Açores e Madeira) Lisboa: ALFA [Associação Lusitana de Fitossociologia]; 2011. [Google Scholar]
- Mesquita S, Sousa AJ. Bioclimatic mapping using geostatistical approaches: application to mainland Portugal. International Journal of Climatology. 2009;29:2156–2170. [Google Scholar]
- Molinari HBC, Marur CJ, Daros E, Campos MKF, Carvalho JFRP, Filho JCB, Pereira LFPP, Vieira LGE. Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp): osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiologia Plantarum. 2007;130:218–229. [Google Scholar]
- Monteiro-Henriques T. Lisboa: Universidade Técnica de Lisboa, Instituto Superior de Agronomia; 2010. Fitossociologia e Paisagem da Bacia Hidrográfica do Rio Paiva/Landscape and phytosociology of the Paiva River's hydrographical basin. PhD Thesis. [Google Scholar]
- Mucina L. Europe, ecosystems of. In: Levin SA, editor. Encyclopedia of biodiversity. Waltham, MA: Academic Press; 2013. pp. 333–346. [Google Scholar]
- Palacios C, Rosselló JA, González-Candelas F. Study of the evolutionary relationships among Limonium species (Plumbaginaceae) using nuclear and cytoplasmic molecular markers. Molecular Phylogenetics and Evolution. 2000;14:232–249. doi: 10.1006/mpev.1999.0690. [DOI] [PubMed] [Google Scholar]
- Palop-Esteban M, Segarra-Moragues JG, González-Candelas F. Historical and biological determinants of genetic diversity in the highly endemic triploid sea lavender Limonium dufourii (Plumbaginaceae) Molecular Ecology. 2007;16:3814–3827. doi: 10.1111/j.1365-294X.2007.03449.x. [DOI] [PubMed] [Google Scholar]
- Pärtel M, Kalamees R, Reier U, Tuvi E-L, Roosaluste E, Vellak A, Zobel M. Grouping and prioritization of vascular plant species for conservation: combining natural rarity and management need. Biological Conservation. 2005;123:271–278. [Google Scholar]
- Pinto-Ricardo C, Espírito-Santo MD, Carrasquinho I, Veloso C, Ricardo CP. Impact of the invasive Carpobrotus edulis on Armeria pseudarmeria at Cabo da Roca. Proceedings of the XIII OPTIMA Meeting; 2010. p. 85. [Google Scholar]
- Pyšek P, Genovesi P, Pergl J, Monaco A, Wild J. Plant invasions in protected areas: patterns, problems and challenges, invading nature. Vol. 7. Dordrecht: 2013. Plant invasions of protected areas in Europe: an old continent facing new problems. Foxcroft LC, Pys̆ek P, Richardson DM, Genovesi P Springer Series in Invasion Ecology. [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www.R-project.org . [Google Scholar]
- Rabinowitz D. Seven forms of rarity. In: Synge H, editor. The biological aspects of rare plant conservation. New York: John Wiley & Sons Ltd; 1981. pp. 205–217. [Google Scholar]
- Rivas-Martínez S. Mapa de series, geoseries y geopermaseries de vegetación de España. Memoria del mapa de vegetación potencial de España. Itinera Geobotanica. 2007;17:5–436. [Google Scholar]
- Rodríguez S, Palop ML, Palacios C, González-Candelas F. Molecular and morphological differentiation in Limonium dufourii (Plumbaginaceae), an endangered Mediterranean plant. Conservation Genetics. 2003;4:383–391. [Google Scholar]
- Róis AS, Teixeira G, Sharbel TF, Fuchs J, Martins S, Espírito-Santo MD, Caperta AD. Male fertility versus sterility, cytotype, and DNA quantitative variation in seed production in diploid and tetraploid sea lavenders (Limonium sp., Plumbaginaceae) reveal diversity in reproduction modes. Sexual Plant Reproduction. 2012;25:305–318. doi: 10.1007/s00497-012-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róis AS, López CMR, Cortinhas A, Erben M, Espírito-Santo MD, Wilkinson M, Caperta AD. Epigenetic rather than genetic factors may explain phenotypic divergence between coastal populations of diploid and tetraploid (Limonium sp.; Plumbaginaceae) in Portugal. BMC Plant Biology. 2013;13:205. doi: 10.1186/1471-2229-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury E. Downs and dunes. Their plant life and environment. London: G. Bell and Sons; 1952. [Google Scholar]
- Salvini D, Fineschi S, Pastorelli R, Sebastiani F, Vendramin G. Absence of geographic structure in European populations of Rubus fruticosus L. complex using chloroplast DNA microsatellites. Journal of the American Society for Horticultural Science. 2006;131:616–621. [Google Scholar]
- Scheffers A, Kelletat D. Tsunami relics on the coastal landscape west of Lisbon, Portugal. International Journal of the Tsunami Society. 2005;1:3–16. [Google Scholar]
- Schemske DW, Husband BC, Ruckelshaus MH, Goodwillie C, Parker I, Bishop JG. Evaluating approaches to the conservation of rare and endangered plants. Ecology. 1994;75:584–606. [Google Scholar]
- Severns PM, Liston A. Intraspecific chromosome number variation: a neglected threat to the conservation of rare species. Conservation Biology. 2008;22:1641–1647. doi: 10.1111/j.1523-1739.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- Sieber VK, Murray BG. Structural and numerical chromosomal polymorphism in natural populations of Alopecurus L. (Poaceae) Plant Systematics and Evolution. 1981;139:121–136. [Google Scholar]
- Simberloff D. The contribution of population and community to conservation science. Annual Review of Ecology and Systematics. 1988;19:473–511. [Google Scholar]
- Snogerup S. Evolutionary and plant geographical aspects of chasmophytic communities. In: Davis PH, Harper PC, Hedge IC, editors. Plant life of South West Asia. Aberdeen: Botanical Society of Edinburgh UK; 1971. pp. 157–170. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. 3rd edn. New York: WH Freeman and Co; 1997. [Google Scholar]
- Suárez-García C, de Paz JP, Febles R, Caujapé-Castells J. Genetic diversity and floral dimorphism in Limonium dendroides (Plumbaginaceae), a woody Canarian species on the way of extinction. Plant Systematics and Evolution. 2009;280:105–117. [Google Scholar]
- Suehs CM, Médail F, Affre L. Invasion by South African Carpobrotus (Aizoaceae) taxa in the Mediterranean Basin: the effects of insularity on plant reproductive systems. In: Child L, Brock JH, editors. Plant invasions: ecological threats and management solutions. Leiden: Backhuys Publishers; 2003. pp. 247–263. [Google Scholar]
- Traveset A, Brundu G, Carta L, Mprezetou I, Lambdon P, Manca M, Médail F, Moragues E, Rodríguez-Pérez J, Siamantziouras AD, Suehs CM, Troumbis AY, Vilà M, Hulme PE. Consistent performance of invasive species within and among islands of the Mediterranean basin. Biological Invasions. 2008;10:847–858. [Google Scholar]
- van der Maarel E. Dry coastal ecosystems: polar regions and Europe. The Netherlands: Elsevier Science Publishers; 1993. [Google Scholar]
- van der Maarel E, van der Maarel-Versluys M. Distribution and conservation status of littoral vascular plant species along the European coasts. Journal of Coastal Conservation. 1996;2:73–92. [Google Scholar]
- van Dijk PJ. Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358:1113–1121. doi: 10.1098/rstb.2003.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà M, Tessier M, Suehs CM, Brundu G, Carta L, Galanidis A, Lambdon P, Manca M, Médail F, Moragues E, Traveset A, Troumbis AY, Hulme PE. Local and regional assessment of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. Journal of Biogeography. 2006;33:853–861. [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58:236–244. [Google Scholar]
- Warming E. Oecology of plants: an introduction to the study of plant-communities. Oxford: Clarendon Press; 1909. [Google Scholar]
- Young AG, Murray BG. Genetic bottlenecks and dysgenic gene flow into re-established populations of the grassland daisy, Rutidosis leptorrhynchoides. Australian Journal of Botany. 2000;48:409–416. [Google Scholar]
- Zar JH. Biostatistical analysis. Prentice-Hall: Upper Saddle River; 2010. [Google Scholar]