Abstract
Aldehydes are electrophilic compounds to which humans are pervasively exposed. Despite a significant health risk due to exposure, the mechanisms of aldehyde toxicity are poorly understood. This ambiguity is likely due to the structural diversity of aldehyde derivatives and corresponding differences in chemical reactions and biological targets. To gain mechanistic insight, we have used parameters based on the hard and soft, acids and bases (HSAB) theory to profile the different aldehyde subclasses with respect to electronic character (softness, hardness), electrophilic reactivity (electrophilic index), and biological nucleophilic targets. Our analyses indicate that short chain aldehydes and longer chain saturated alkanals are hard electrophiles that cause toxicity by forming adducts with hard biological nucleophiles, e.g., primary nitrogen groups on lysine residues. In contrast, α,β-unsaturated carbonyl derivatives, alkenals, and the α-oxoaldehydes are soft electrophiles that preferentially react with soft nucleophilic thiolate groups on cysteine residues. The aldehydes can therefore be grouped into subclasses according to common electronic characteristics (softness/hardness) and molecular mechanisms of toxicity. As we will discuss, the toxic potencies of these subgroups are generally related to corresponding electrophilicities. For some aldehydes, however, predictions of toxicity based on electrophilicity are less accurate due to inherent physicochemical variables that limit target accessibility, e.g., steric hindrance and solubility. The unsaturated aldehydes are also members of the conjugated type-2 alkene chemical class that includes α,β-unsaturated amide, ketone, and ester derivatives. Type-2 alkenes are electrophiles of varying softness and electrophilicity that share a common mechanism of toxicity. Therefore, exposure to an environmental mixture of unsaturated carbonyl derivatives could cause “type-2 alkene toxicity” through additive interactions. Finally, we propose that environmentally derived aldehydes can accelerate diseases by interacting with endogenous aldehydes generated during oxidative stress. This review provides a basis for understanding aldehyde mechanisms and environmental toxicity through the context of electronic structure, electrophilicity, and nucleophile target selectivity.
Introduction
Aldehydes are a large class of electrophilic carbonyl compounds that have at least one hydrogen atom substituent on the carbonyl carbon atom (Table 1). Chemicals in this family can be divided into subclasses based on corresponding structures that incorporate additional functional moieties: (1) short chain, unhindered aldehydes, formaldehyde, acetaldehyde; (2) long chain alkanals, nonanal; (3) aromatic aldehydes, benzaldehyde, vanillin; (4) α,β-unsaturated aldehydes that include numerous subclasses,aromatic alkenals, short and long chain alkenals, and hydroxy or oxoalkenals; and (5) α-oxoaldehydes, glyoxal and glycolaldehyde.1,2 Aldehydes present in the environment are derived from both natural and anthropogenic sources.1−5 For example, formaldehyde and acetaldehyde are normal dietary constituents and pervasive environmental contaminants due to their broad natural sources and high-volume use in a variety of industrial and manufacturing processes (Table 1). α,β-Unsaturated aldehyde derivatives such as acrolein and crotonaldehyde (Table 1) are significant components of air pollution due to petrochemical combustion5−7 and smoke from cigarette, wood, and coal combustion.4,8,9 Many aldehydes are contaminants of the U.S. water supply, and more than 300 unsaturated aldehydes (e.g., crotonaldehyde, citral, and cinnamaldehyde) are natural constituents of various foods (e.g., cheese, fish, and potatoes). Aldehyde derivatives are produced during the cooking of fats, oils, and sugars; e.g., 2-pentenal, acrolein, 2,4-nonadienal,2,3,10 and low concentrations of α,β-unsaturated aldehydes are used for flavor enhancement in the food and beverage industries, e.g., trans-2-hexenal.11 On the sole basis of dietary consumption, it is estimated that the daily α,β-unsaturated aldehyde burden in humans is 5.0 mg/kg-body weight.12,13 Human exposure to aldehydes can also occur through the formation of reactive intermediates during drug metabolism, e.g., metabolism of the chemotherapeutic agent cyclophosphamide to acrolein.14
Table 1. Classification and Hardness/Softness Values for Selected Aldehdyesa.
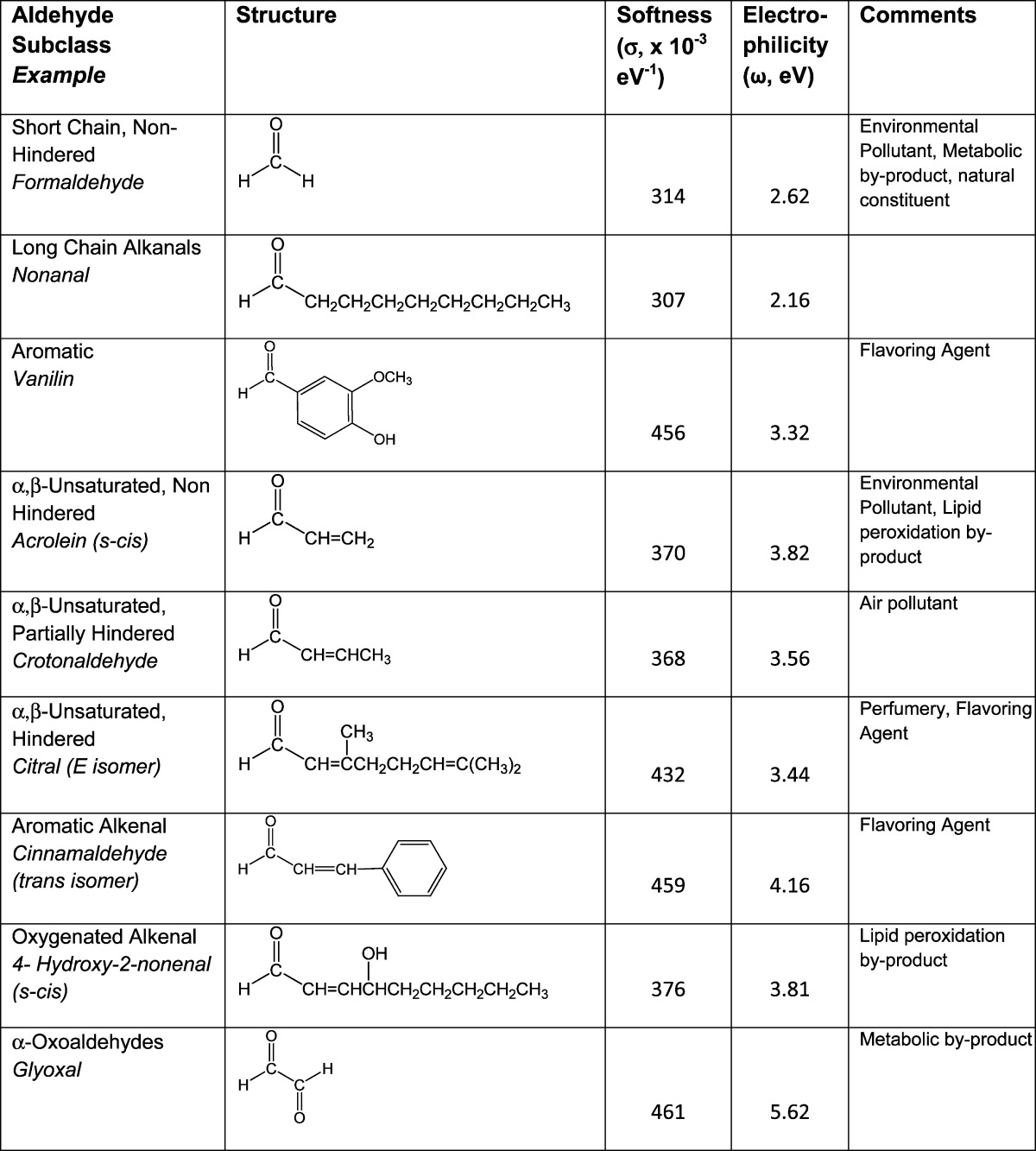
Ground state equilibrium geometries were calculated for each structure with DF B3LYP6-31G* in water from 6-31G* initial geometries. Values obtained were used to calculate σ and ω (see text).
Aldehydes are pervasive components of the environment, and human exposure represents a potential health risk given the well-documented toxicity of these chemicals.3,5,6 Despite the potential risks of aldehyde exposure, the toxic mechanisms are only understood in general terms, i.e., formation of covalent adducts with nucleophilic residues on macromolecules.1,15−18 This deficient understanding is likely due to the structural diversity of aldehyde derivatives and their correspondingly different biological targets and chemical reactions. In this perspective, we have used Hard and Soft, Acids and Bases (HSAB) parameters derived from quantum chemical calculations to characterize members of the different aldehyde subclasses with respect to their electronic nature (softness/hardness), electrophilic reactivity, and respective nucleophilic targets. This level of analysis can provide detailed mechanistic information regarding the different aldehyde subclasses and corresponding chemical reactions with specific nucleophilic sites on macromolecules.
Our HSAB analyses and review of the experimental literature indicate that aldehydes of a given subgroup cause toxicity via a common mechanism. This has significant regulatory implications since the environmental toxicology of aldehydes has been previously considered on an individual chemical basis with focus on specific aldehydes (e.g., acrolein or crotonaldehyde) of a subgroup (e.g., α,β-unsaturated aldehydes).3−5,10 Instead, it is evident that human populations are exposed to complex aldehyde mixtures, the chemical composition and corresponding concentrations of which depend upon several variables including geographical location, personal habits (diet, tobacco, and alcohol usage), and occupation. Therefore, in such mixtures, members of a subgroup could interact additively through their common chemical mechanism to cause environmentally derived toxicity. The toxicological impact of environmental aldehydes, however, can be considered in a much larger context since the α,β-unsaturated aldehydes are a subclass of the conjugated type-2 alkenes. This is a large chemical family of prominent environmental toxicants19−23 that have a common structure and mechanism of toxicity.15,18,24−26 Therefore, we will discuss the possibility that different environmental α,β-unsaturated carbonyl derivatives (including conjugated aldehydes) could act in an additive fashion to cause type-2 alkene toxicity. In addition to the risk of acquired toxicity, there is now evidence that exposure to environmental aldehydes can accelerate the onset and development of certain human ailments that involve oxidative stress, e.g., cardiovascular disease, diabetes, and Alzheimer’s disease.7,24,25,27 This is presumably due to the combined interactions of environmental toxicants with the endogenous unsaturated aldehydes (e.g., acrolein, 4-hydroxy-2-nonenal) and oxoaldehydes (e.g., glyoxal, methylglyoxal) generated during oxidative stress.28,29 Therefore, a final goal of this perspective is to discuss the possibility that environmental and endogenous aldehydes can interact additively and thereby amplify cellular oxidative damage associated with many pathogenic and toxicogenic processes. This review provides a basis for understanding aldehyde mechanisms and environmental toxicity through the context of electronic structure, electrophilicity, and nucleophile target selectivity. The common mechanism of toxicity for the different aldehyde subgroups suggests a significant potential for interaction among respective members.
Adduct Chemistry of Aldehydes
Hard and Soft, Acids and Bases Theory
The preceding discussion indicates that aldehydes are pervasive chemicals found in ambient environments (e.g., air, household, water, and soil), the diet, and intracellular milieu. Aldehydes are electrophiles (electron deficient species) that form covalent bonds with nucleophilic (electron rich) sites on biological targets. The resulting adduct formation can impair the functions of enzymes, DNA, structural proteins, and other macromolecules, thereby leading to the inhibition of cellular processes and eventual cytotoxicity.15,16,18,30 The potency of the aldehyde toxicant is governed by the second-order rate of adduct formation at cellular conditions. This rate is determined not only by the respective concentrations of the aldehyde toxicant and nucleophilic biological target but also by the electronic energies of these components (transition state energies) that influence the reaction rate constant (k; see ahead). This information is summarized by the following equation in which the aldehyde toxicant is an electrophile, and the biological target is a nucleophile:
Electrophile–nucleophile reactions, however, do not occur indiscriminately and instead exhibit a significant degree of selectivity as defined by the HSAB theory of Pearson.26,31−33 Thus, aldehyde electrophiles and their nucleophilic targets can be classified as either relatively hard or soft based on the distribution of electrons among specific atoms of a molecule. The ease with which electron density can be displaced or delocalized among atoms is termed polarizability. Thus, hard electrophilic aldehydes (e.g., formaldehyde and the alkanals) are relatively nonpolarizable since the low electron density (partial positive charge) exhibited is localized on the carbonyl carbon atom. In contrast, softer electrophiles (e.g., acrolein and crotonaldehyde) have multiple sites at which the electron density is low and are consequently more polarizable. With respect to nucleophiles, anionic side-chain sulfur atoms of cysteine residues, for example, have large atomic radii with highly polarizable valence electrons and are therefore soft nucleophiles. Other biological nucleophiles such as the primary and secondary nitrogen groups of lysine and histidine are relatively harder due to the more localized charge that results from their smaller atomic radii and greater electronegativity. The central theme of the HSAB concept is that electrophiles preferentially and more rapidly form covalent adducts with nucleophiles of comparable softness or hardness. Whereas electrophile–nucleophile reactions that differ significantly in softness or hardness are possible, they are less favorable and occur at slower rates. The HSAB parameters of softness and hardness describe the electronic character of a given toxicant, and as discussed in the next section, such descriptors can be used in conjunction with parameters of reactivity to determine electrophilic strength and hence toxic potency.22,26,32,33
Descriptors of Aldehyde Electrophilicity
Quantitative Measures of Electrophile Reactivity
Thus, far, we have described aldehyde electrophiles and their potential nucleophilic targets as either soft or hard depending upon the polarizability of their outermost electrons. According to the frontier molecular orbital (FMO) theory, covalent bond (adduct) formation can be approximated by describing the overlap between the outermost (frontier) orbitals of the reacting molecules. Frontier orbitals consist of the lowest energy orbital of the electrophile that is vacant (lowest unoccupied molecular orbital or LUMO) and the highest energy orbital of the nucleophile holding electrons (highest occupied molecular orbital or HOMO). Energy values for frontier orbitals can be obtained from quantum mechanical calculations and used in different algorithms to determine HSAB parameters such as hardness (η = [ELUMO – EHOMO]/2) and softness (σ = 1/η). These HSAB parameters are related to the ease with which electron redistribution occurs during the formation of covalent adducts (i.e., nucleophile donation of electrons and reciprocal electron acceptance by the electrophile), which, in turn, is related to the rate of the adduct-forming reaction.
As indicated above, molecular hardness and softness have general significance, but there is no simple quantitative relationship between these characteristics and reaction rates. However, values of σ or η can be combined with other HSAB descriptors to estimate the propensity of an electrophile or a nucleophile to undergo an adduct reaction. Specifically, the electrophilic index (ω)34 is a comprehensive measure of electrophilicity that combines softness and chemical potential (μ): ω = 1/2 σμ2. The latter parameter (μ = [ELUMO + EHOMO]/2) represents the ability of an electrophilic or nucleophilic species to undergo chemical change. Calculations of electrophilicity can provide quantitative information about the transition state energies involved in toxicant–protein covalent bond formation. Thus, values for ω correspond to the rate constant (k) of these adduct reactions and, as a consequence, are directly related to toxicant potency.35−37 Table 1 presents the respective softness (σ) and electrophilicity (ω) values for various aldehydes. As the data indicate, glyoxal and conjugated unsaturated aldehyde derivatives such as acrolein, for example, are soft, highly reactive electrophiles (i.e., relatively large ω and σ values). Indeed acrolein38,39 and glyoxal40 both react rapidly with proteins to form adducts at nucleophilic amino acid sites (preferentially Cys). In general, aldehydes are more electrophilic than ketones, esters, and amides with similar structures, e.g., acrolein (CH2=CHCHO, ω = 3.81) > methyl vinyl ketone (CH2=CHCOCH3, ω = 3.38) > methyl acrylate (CH2=CHCO2CH3, ω = 3.20) > acrylamide (CH2=CHCONH2, ω = 2.62). Reactivity toward nucleophiles and toxicity both closely corresponded to this rank order for electrophilicity in several experimental systems.35−37 It is also noteworthy that minor alterations in molecular structure can change ω and therefore toxic potency. Thus, a study by Schultz and colleagues41,42 showed that ethyl acrylate (EA) was substantially more toxic than methyl methacrylate (MMA). However, both toxicants are esters with the same molecular mass as well as comparable solubility and ELUMO values. Although the difference in toxicity could be the result of steric hindrance, the α methyl group of MMA is not attached at the site of electrophilic activity (i.e., the β carbon atom), and therefore, hindrance is of limited importance. More significant is the additional electron density contributed by the methyl group of MMA at the α-carbon of the double bond (CH2=CCH3CO2CH3). This is in contrast to EA, which possess an alkenyl hydrogen atom at the double bond (CH2=CHCO2CH2CH3). The increase in electron density reduces the relative electrophilicity of MMA,36,43−45 and therefore, the differential toxicity of EA (ω = 3.2 eV) and MMA (ω = 3.0 eV) can be explained by differences in respective electrophilic reactivity. On a molar basis, the 0.2 eV difference in ω values corresponds to almost 5 kcal (∼20 kJ/mol). This is significant since the rate constants (k) and corresponding reaction rates are exponentially related to energy differences in the transition states, i.e., relatively small changes in energy have a large effect on the rate constant.
Physicochemical Features: Influence on HSAB Application and Toxic Outcomes
Whereas ω values can directly reflect the toxic potencies of aldehyde electrophiles, the exact correspondence of toxicity to this parameter can be lower than expected since other physicochemical characteristics of the toxicant can modify the rate of the adduct reaction.
Steric Hindrance
Structural steric hindrance diminishes target accessibility and hence raises the transition state energy of the interacting species. This ultimately results in a decreased rate constant (k) for the adduct reaction that is unrelated to the orbital energies from which ω is derived. As a consequence, the slower adduct formation does not correspond to aldehyde electrophilicity. Seiner et al.38 demonstrated that acrolein and a series of structurally related unsaturated aldehydes inhibited protein tyrosine phosphatase 1B (PTP1B) activity via covalent modification of a specific cysteine residue (Cys215). The order of potency was as follows: CH2=CHCHO (acrolein) ≫ CH3CH=CHCHO (crotonaldehyde) > (CH3)2C=CHCHO (3-methyl-2-butenal) ≈ CH3CH2CHO (propanal). When the respective aqueous ω values were calculated, the greater ability of acrolein to inactivate PTP1B was clearly related to correspondingly higher electrophilicity (ω = 3.82ev), and the reduced potency of crotonaldehyde was a function of lower electrophilicity (ω = 3.56 eV). However, the ω value for 3-methyl-2-butenal (ω = 3.53 eV) is only slightly lower than that of crotonaldehyde but significantly higher than that of propanal (ω = 2.32 eV), a very weak electrophile. Furthermore, 3-methyl-2-butenal is a much stronger electrophile than propanal, and consequently, the order of unsaturated aldehyde electrophilicity was not consistent with the corresponding rank order of toxic potency. As the authors point out, however, the lower than expected potency for 3-methyl-2-butenal is related to hindrance imposed by the bulky disubstituted alkene terminus. Nonetheless, 3-methyl-2-butenal is a significant electrophile, which could potentially inactivate PTP1B if higher concentrations were used to overcome the attenuated rate constant. This is not the case for propanal, however, since it is not structurally capable of irreversible sulfhydryl adduction.
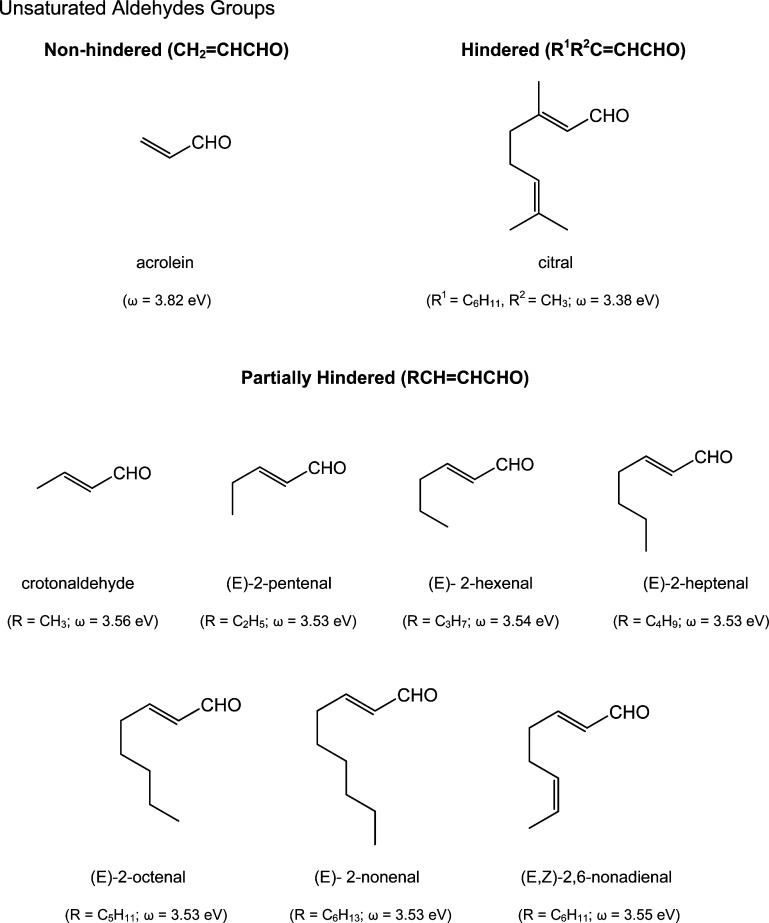
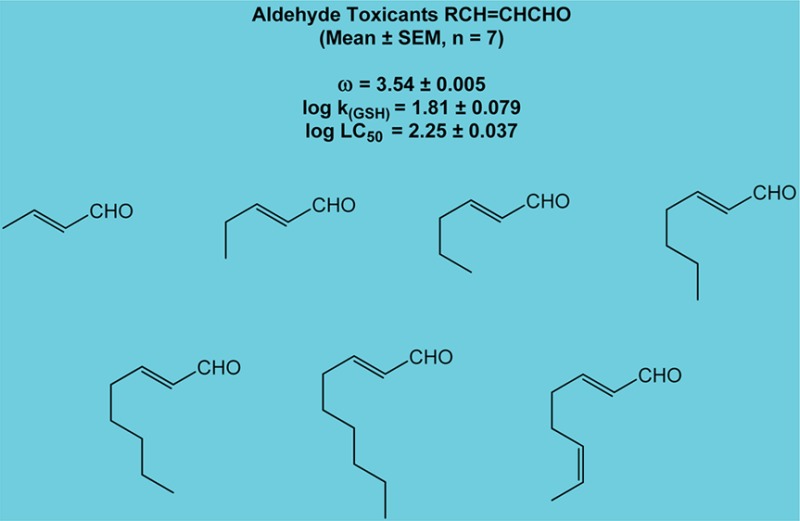
Data selected from Chan et al.43 offer a more comprehensive illustration of the effects of electrophilicity and steric hindrance on reaction rates. In this study, rates of glutathione reactivity (kGSH) with several unsaturated aldehydes were measured. It was shown that acrolein, an unhindered aldehyde, reacted faster with GSH than any of the seven partially hindered analogues. Correspondingly, the partially hindered (i.e., monosubstituted) aldehydes reacted faster than the more hindered (disubstituted) aldehyde, citral (Table 2). Figure 1 depicts the structures of nine of the compounds used in the study and includes the structures of seven monosubstituted analogues with virtually identical values for electrophilicity. Data given in Table 3 show little variability in reactivity toward GSH or the associated hepatocyte toxicity for these seven compounds. Thus, aldehydes with similar ω values and similar steric issues exhibit comparable rates of reaction and are essentially equivalent with respect to toxic behavior.
Table 2. Aldehyde Toxicants.
| aldehyde (scis) | ω (eV) | log ka (GSH) | log LC50a |
|---|---|---|---|
| acrolein CH2=CHCHO | 3.82 | 2.64 | 1.60 |
| monosubstitutedb RCH=CHCHO | 3.54 | 1.81 | 2.25 |
| citral C6H11(CH3)C=CHCHO | 3.38 | 0.29 | 2.35 |
Data are used with permission from ref (66). Copyright 2008 John Wiley & Sons Ltd. log k (GSH) = second-order rate constant for the reaction of selected aldehydes with glutathione (GSH); log LC50 = aldehyde concentration that produces 50% hepatocyte death at 2 h.
Mean values, n = 7 (see Table 3). For each aldehyde, respective orbital energies (ELUMO and EHOMO) were obtained from ground state equilibrium geometries with DF B3LYP6-31G* in water from 6-31G* initial geometries and were used to calculate the electrophilic index (ω) as described in LoPachin et al.26
Figure 1.
Unsaturated Aldehyde Groups.
Table 3. Partially Hindered Aldehyde Toxicants.
| aldehyde (scis) | ω (eV) | log ka (GSH) | log LC50a |
|---|---|---|---|
| CH3CH=CHCHO | 3.56 | 2.2 | 2.18 |
| C2H5CH=CHCHO | 3.53 | 1.95 | 2.36 |
| C3H7CH=CHCHO | 3.54 | 1.73 | 2.38 |
| C4H9CH=CHCHO | 3.53 | 1.75 | 2.18 |
| C5H11CH=CHCHO | 3.53 | 1.64 | 2.27 |
| C6H13CH=CHCHO | 3.53 | 1.59 | 2.11 |
| C6H11CH=CHCHO | 3.55 | 1.82 | 2.26 |
| mean ± SEM | 3.54 ± 0.005 | 1.81 ± 0.079 | 2.25 ± 0.037 |
Data are used with permission from ref (66). Copyright 2008 John Wiley & Sons Ltd. log k (GSH) = second-order rate constant for the reaction of selected aldehydes with glutathione (GSH); log LC50 = aldehyde concentration that produces 50% hepatocyte death at 2 h. For each aldehyde, respective orbital energies (ELUMO and EHOMO) were obtained from ground state equilibrium geometries with DF B3LYP6-31G* in water from 6-31G* initial geometries and were used to calculate the electrophilic index (ω) as described in LoPachin et al.26
Solubility
Esters of acrylic acid and methacrylic acid are high volume industrial chemicals. These conjugated α,β-unsaturated compounds (CH2=CR1CO2R2) produce toxicity through adduction of protein sulfhydryl groups.45,46 Early studies by Tanii and Hashimoto47 showed that the lethal oral doses (LD50) in mice for a series of acrylates/methacrylates were related to both solubility (as a partition coefficient, log P) and the reaction rate (log k) of these esters with glutathione. When the respective ω values were calculated, we26 found that the corresponding rate constants (log k) were highly correlated (r2 = 0.97) to the ω parameter, whereas both log k (r2 = 0.65) and ω (r2 = 0.60) were only modestly correlated with the in vivo LD50 values. This discrepancy can be explained by the variable solubility of the acrylate and methacrylate compounds. Toxicant solubility is a physiochemical determinant of tissue distribution and, consequently, determines the effective concentration of the electrophile at the target. For example, 2-hydroxyethyl acrylate (CH2=CHCO2CH2CH2OH) is considerably more soluble (log P = −0.21 vs 1.33) than ethyl acrylate (CH2=CHCO2CH2CH3). Both have virtually identical ω values (∼3.2 eV) but significantly different in vivo toxic potencies (LD50 = 5.2 mmol/kg vs 18 mmol/kg, respectively). For these compounds, the structural factors that establish solubility are independent from those that determine electronic characteristics such as electrophilicity, i.e., although the hydroxyl group of 2-hydroxyethyl acrylate improves solubility, this substituent is distant from the carbon–carbon double bond and therefore has little effect on the electronic characteristics of the alkene. Consequently, the difference in electrophilicity between the two compounds is small, and it is evident from the toxicity data that physical properties, such as solubility, can affect the overall rate of adduct formation by modulating toxicant concentrations at the biological target.39,45,46
Acid–Base Equilibrium
In a recent structure–toxicity analysis of endogenous α,β-unsaturated aldehyde derivatives, McGrath et al.48 determined the half-lives (t1/2) and second-order rate constants (k) for the reactions of HNE, 4-oxo-2-nonenal (ONE) and other structurally related analogues with N-acetylcysteine (NAC). These electrophilic byproducts of cell membrane peroxidation cause cytotoxicity through irreversible modification of protein sulfhydryl groups.28,29,49 On the sole basis of their findings in aldehyde-exposed cell culture systems, the authors reported that analogue-induced changes in toxicity were not correlated to electrophilicity (measured by reactivity with NAC). Indeed, when the corresponding ω values were calculated for the HNE analogues, the kinetic parameters (t1/2, k) were only qualitatively correlated to ω. However, when the carboxylic acid structures (RCOOH) were replaced in the ω calculations with those of the dominant (at pH 7.4) anionic species (RCOO–), correlations of both k2 and t1/2 with ω improved from r2 = 0.553 to 0.906 and r2 = 0.752 to 0.911, respectively.
These examples from the literature illustrate the fact that a lack of correspondence is possible between the experimentally derived toxic potency of a given aldehyde and that predicted by the calculated electrophilicity (ω). Some discordance is expected since the HSAB algorithms do not consider all of the possible physicochemical attributes that can influence the rate of adduct formation and toxic outcome. These extenuating characteristics are recognizable from the chemical structure of the toxicant, and therefore, the experimental findings can be interpreted appropriately. In animal studies, certain physicochemical features directly influence toxicokinetics (e.g., tissue distribution and metabolism) and therefore toxicant delivery to the corresponding nucleophile target. At the molecular level, the short-range accessibility and covalent interaction of an aldehyde toxicant with the respective target is determined by electrophilicity, size (steric hindrance), and solubility in the corresponding microenvironment. For many aldehydes, relative electrophilicity (ω) is a useful predictor of toxic potential because the mechanistic basis of their toxicity involves the rate of covalent adduct formation reactions with various macromolecules. However, for some members in this chemical class, inherent physicochemical variables can influence predicted and experimental agreement because these variables also affect adduct-forming reaction rates.
Chemical Reactions of Soft and Hard Aldehyde Toxicants with Their Nucleophilic Targets
Hard Aldehydes: Nucleophile Targets and Chemical Reactions
According to HSAB definitions, formaldehyde, acetaldehyde, and the longer chain saturated alkanals are relatively hard electrophiles (Table 1). Specifically, the highly electronegative oxygen atom of the carbonyl (electronegativity = 3.44) moiety draws electron density from the less electronegative carbon (2.55) and hydrogen (2.20) atoms. Because of the resulting localized electron deficiency, the carbonyl carbon atom of these molecules is a hard electrophilic site that will react preferentially with a hard nucleophile. Both formaldehyde and acetaldehyde are recognized genotoxicants50−52 that cause nasopharyngeal cancer in humans and carcinomas of the nasal respiratory epithelium in rodent models. The toxicity of these aldehydes is related to their ability to undergo 1,2-addition reactions with amines. The initially formed aminols can then undergo dehydration to imines (Schiff base formation). Accordingly, formaldehyde and acetaldehyde have been shown to react preferentially with relatively hard nucleophiles such as the N2 nitrogen of deoxyguanosine.53,54 The alkanals can also induce the formation of deoxynucleoside–protein amino acid cross-links, which involves hard–hard interactions with ε-amino groups of lysine residues and exocyclic amino groups of DNA50,53,55 via the same type of addition reaction.
Soft Aldehydes: Nucleophile Targets and Chemical Reactions
The α,β-unsaturated carbonyl substructure of, for example, acrolein or crotonaldehyde, is a soft electrophile. The highly electronegative carbonyl oxygen atom can withdraw electron density from the electron-rich carbon–carbon double bond, thereby creating an electron deficient (electrophilic) center at the β-carbon atom. As a consequence of this polarization, such compounds can undergo addition reactions at the relatively hard carbonyl carbon atom (direct or 1,2-addition) or at the softer β-carbon atom (conjugate or 1,4-addition). The latter reaction constitutes the Michael reaction. In accordance with HSAB principles, members of the α,β-unsaturated aldehyde subclass readily form adducts via 1,4-Michael addition with soft nucleophiles, which in biological systems are sulfhydryl groups on cysteine residues.35−37,56−59 However, cysteine sulfhydryl groups (RSH) exist in equilibrium with their respective nonprotonated anionic thiolate (RS–) states.26 Cysteine thiolate sites on proteins are soft and highly nucleophilic, and therefore, these sites are the preferred nucleophile target of soft unsaturated aldehyde electrophiles. Whereas cysteine thiolates can add directly to the carbonyl group of aldehydes, the reaction is reversible at cellular conditions due to the instability of the resulting thiohemiacetal adducts.
The imidazole side chain of histidine and the ε-amino group of lysine contain nucleophilic nitrogen groups that are potential sites of 1,4-Michael addition for the unsaturated aldehydes. However, comparisons of respective values calculated for softness and nucleophilicity indicate that these harder nucleophilic sites are less likely than thiolates to be targets for soft unsaturated aldehydes.26 Although there is abundant in chemico evidence that aldehyde toxicants can form adducts with lysine and histidine residues on proteins,60−64 the relatively high aldehyde-to-protein ratios (e.g., 50:1) and long incubation times (≥24 h) needed to produce these adducts reflect the very slow rate of adduct formation between a soft aldehyde electrophile and a significantly harder amino acid nucleophile; see kinetic studies by Doorn and Petersen.57,65 On a quantitative basis, the cysteine preference of these soft electrophiles is several orders of magnitude greater than that for other nucleophiles37,57,66,67 This preference is consistent with the facile soft–soft reaction of an unsaturated aldehyde electrophile with a sulfhydryl nucleophile. Thus, in a biological system of mixed soft and hard nucleophile targets, the very rapid kinetics for cysteine adduction would preclude the much slower 1,4-Michael addition of a harder amine target.
The bifunctional nature of α,β-unsaturated aldehyde derivatives suggests an alternative chemical mechanism. As the soft β-carbon atom of acrolein forms an adduct with a soft nucleophile target on a protein (Michael addition), the harder carbonyl carbon atom of acrolein can potentially attack a hard nucleophile site (e.g., Lys) on a separate protein (1,2-addition). The resulting cross-linked protein (or DNA and RNA) is dysfunctional, which could mediate cytotoxicity.15,66,68,69 However, the toxicological relevance of the hard–hard 1,2-addition reaction is unclear since it is reversible at physiological conditions and inherently slow.35−37,70 For example, propanal, a hard alkanal that can undergo 1,2-addition reactions, did not cause toxicity in our previous synaptosomal studies.35,36 As an index of possible protein cross-links formed during in vitro acrolein exposure of synaptosomes, the electrophoretic migration of proteins was detected by immunoblot analysis.36 Results showed that slowly migrating higher molecular weight protein complexes (e.g., SNAP-25, N-ethylmaleimide sensitive factor) occurred only at relatively high (≥25 mM) acrolein concentrations. Considered together, these data indicate that the toxicity of unsaturated aldehydes is mediated by 1,4-Michael addition of a nucleophile at the β-carbon.
α-Oxoaldehydes, such as glyoxal (Table 1), are lipid peroxidation products that can react with nucleophilic groups on proteins to form advanced glycation end products (AGEs). These end products have been linked to both micro- and macrovascular conditions in the pathogenesis of diabetes.71 The oxoaldehyde derivatives have been shown to form adducts with hard nitrogen groups on lysine and arginine side chains.72 However, more recent studies indicate that these aldehydes preferentially target cysteine thiolate sites on proteins through carbonyl addition with subsequent formation of S-(carboxymethyl)cysteine (CMC) adducts. The CMC adduct results from rearrangement of the initially formed thiohemiacetal via an internal Cannizzaro reaction.40 A thiolate predilection is consistent with calculations of HSAB parameters, which indicate that glyoxal is a soft (σ), highly electrophilic (ω) aldehyde derivative that readily reacts with anionic sulfur sites (Table 1).
Molecular Mechanisms of α,β-Unsaturated Aldehydes
Detailed proteomic studies have demonstrated that unsaturated aldehyde derivatives impair cellular protein function by targeting specific cysteine residues, e.g., HNE inhibited mitochondrial sirtuin 3 (SIRT3) activity by targeting Cys280;73 Michael-type adduct formation at Cys47 by a series of α,β-unsaturated aldehydes and ketones impaired glutathione S-transferease P1-1 (GSTP1-1) activity;59 and acrolein inhibited glyceraldehyde 3-phosphate dehydrogenase (GAPDH) function by forming adducts with Cys152 in the enzyme active site.39 As discussed in the preceding section, this targeting should reflect the reaction of soft unsaturated aldehydes with the highly nucleophilic sulfhydryl thiolate sites on cysteine residues. However, the pKa of the cysteine sulfhydryl side-chain is 8.4, and therefore, at intracellular pH ranges (7.0–7.4), these groups exist mostly in the non-nucleophilic thiol state. Nonetheless, anionic thiolate groups are present in pKa-lowering microenvironments such as cysteine-centered catalytic triads74,75 that are located within the active sites of critical cellular enzymes and proteins, e.g., N-ethylmaleimide sensitive factor (NSF), GAPDH, and vesicular monoamine transporter. These sulfhydryl thiolate sites regulate protein activity by playing a direct role in the enzymatic catalytic process (e.g., Cys152 of human GAPDH) and by acting as acceptors for nitric oxide (NO), hydrogen peroxide (H2O2), and other redox modulators that transiently regulate enzyme function (e.g., Cys91 and Cys264 of NSF)74,76,77 Therefore, unsaturated aldehydes appear to act via a common molecular mechanism involving irreversible adduct formation at regulatory cysteine thiolate residues of functionally critical proteins.35−37,39,67,78,79 However, the ensuing cytotoxicity is unlikely to be mediated by focal inhibition of a single protein. Rather, a confluence of data now indicate that aldehydes and other unsaturated carbonyl toxicants inhibit an electrophile-responsive proteome29,78 that is composed of cell-specific proteins that are cysteine-directed. The ultimate toxicological manifestations of proteome inhibition are influenced by physicochemical characteristics that determine toxicokinetic outcomes (e.g., electrophilicity, metabolism, and tissue distribution)17,22,26 and accessibility to individual protein targets (e.g., steric hindrance). In addition, cell-level responses to electrophile intoxication such as the activation of cytoprotective signaling pathways (e.g., Nrf2/Keap1 pathway) and gene expression can also shape the development of toxicity.
Aldehyde Mixtures and Environmental Toxicity
Additive Toxicity and Type-2 Alkenes
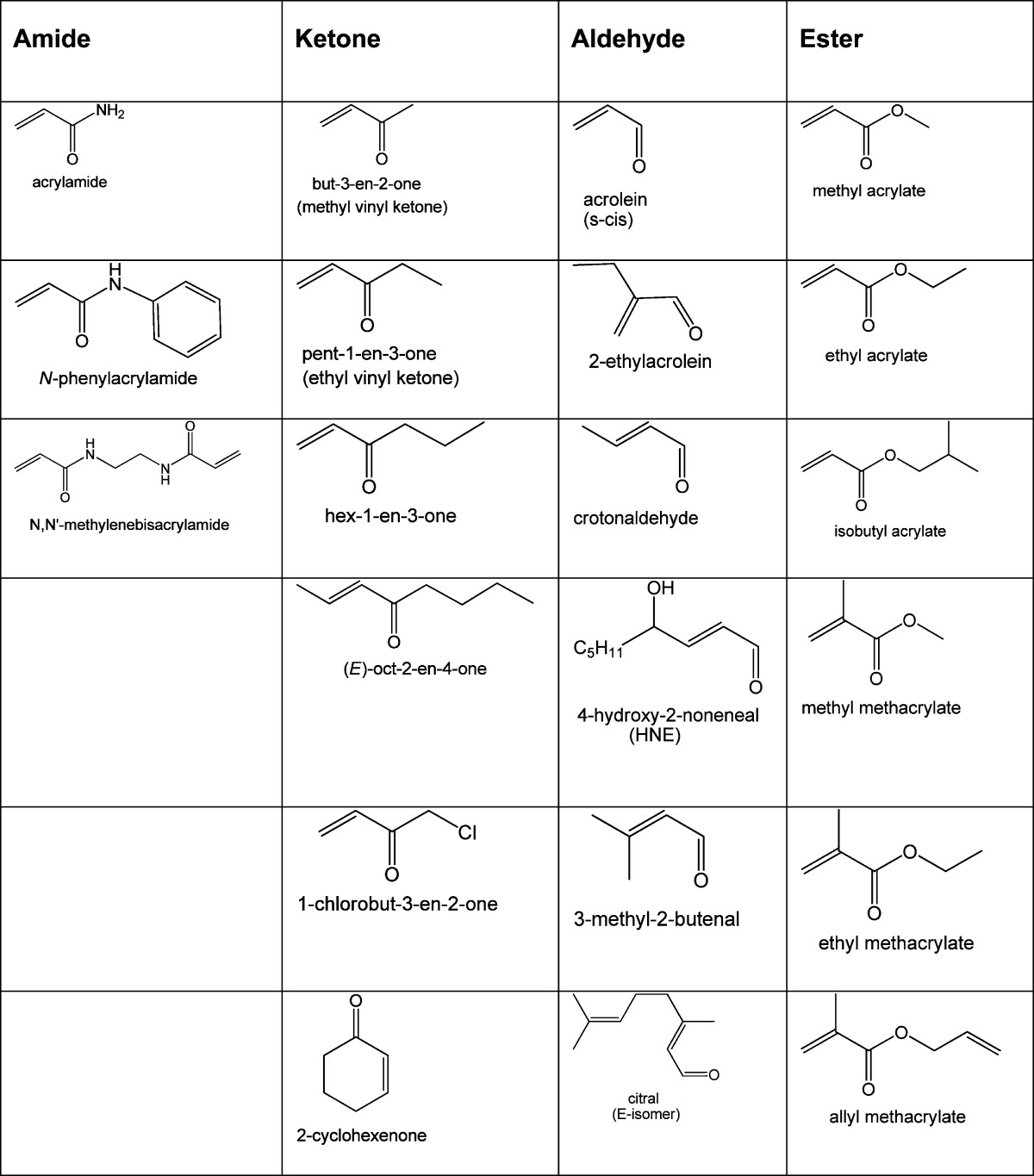
As a result of natural and anthropogenic production, aldehydes such as formaldehyde, acrolein, acetaldehyde, and crotonaldehyde have a ubiquitous presence in the environment. Human populations are, therefore, exposed to aldehyde mixtures, the respective compositions of which depend upon variables such as occupation, geographical location, and personal habits. However, despite the obvious relevance of mixtures to real world environmental toxicity,80 most research and governmental legislation have focused on the toxicity of individual aldehydes.3−5,10,12 The problem with this chemical-by-chemical approach is the possible underestimation of toxic risk.81 Thus, for example, the environmental concentrations of many α,β-unsaturated aldehydes are below corresponding NOAEL concentrations,1,2 and when considered on an individual basis, it might be concluded that a given aldehyde does not pose a significant human health risk. However, combined with other unsaturated aldehydes that have a common molecular mechanism of action, it is possible that subchronic exposure to the resulting environmental mixture is toxic due to additive interactions among subgroup members.81−83 Furthermore, when considering the toxic potential of an environmental aldehyde mixture it is critical to recognize that these toxicants are members of a much larger chemical class known as the conjugated type-2 alkenes. This class includes, not only aldehyde analogues but also amide (e.g., acrylamide), ketone (e.g., methylvinyl ketone), and acrylate (e.g., methyl acrylate) derivatives, many of which are significant environmental and/or dietary toxicants (Table 4).19−23 Like the unsaturated aldehyde subgroup, many type-2 alkene members are characterized by α,β-unsaturated carbonyl substructures and are therefore soft electrophiles. Accordingly, type-2 alkene toxicity is mediated via a common mechanism involving adduct formation with soft nucleophilic thiolate sulfhydryl groups on cysteine residues of cellular proteins.15,18,24,84 Schwobel et al.84 introduced a computational model that incorporates electrophilicity and steric hindrance to predict the reaction rates of unsaturated carbonyl derivatives with glutathione (GSH) across a variety of functional groups. Since the rates of adduct formation with protein sulfhydryl groups are linked to toxic potency, applications of this type illustrate the potential for additive toxicity by type-2 alkenes and suggest that the different subclasses of α,β-unsaturated carbonyl compounds can interact additively. The potential toxicity induced by exposure to a complex mixture of unsaturated aldehydes and other type-2 alkenes is theoretically due to combined individual rates of protein adduct formation, which would differ among the constituents as a function of their respective differences in electrophilicity (ω) and steric hindrance. However, regardless of the reacting soft electrophile, once the thiolate adduct has formed the consequential inhibitory effects on protein function will be equivalent to the protein inhibition caused by other members of the mixture; see additional discussions in LoPachin et al.35−37 Each aldehyde component therefore might contribute to collective adduct formation, which could result in an additive pattern of toxicity. Therefore, we propose that exposure to environmental type-2 alkene mixtures could constitute a significant risk potential.
Table 4. α,β-Unsaturated Carbonyl (Type-2 Alkene) Derivatives.
Interactions of Environmental Type-2 Alkenes with Endogenous Unsaturated Aldehydes
In addition to environmental sources, highly reactive endogenous unsaturated aldehydes (e.g., acrolein, HNE, and 4-oxo-2-nonenal) are generated during lipid peroxidation and subsequently mediate the cytotoxicity associated with oxidative stress injury.28,29 These cellular unsaturated aldehydes are relatively reactive soft electrophiles that cause toxicity via the type-2 alkene mechanism.28,29,37,49,85 Therefore, environmentally derived aldehydes and other type-2 alkene derivatives might act additively with endogenously generated α,β-unsaturated aldehydes. In fact, research has shown that dietary exposure to acrolein exacerbates myocardial ischemic injury and atherosclerosis in mice by interacting with endogenous unsaturated aldehydes generated during ongoing oxidative stress.12,13,86−88 On the sole basis of these studies, it has been proposed that chronic environmental exposure to unsaturated aldehydes is a significant risk factor for cardiovascular diseases.7,13,87 Also generated during lipid peroxidation are oxaldehydes such as glyoxal and methylglyoxal. These lipid byproducts appear to play a role in many age-related diseases, presumably through their ability to form adducts with cysteine-containing proteins40 and their ability to form inter- and intramolecular cross-links that also inactivate proteins (reviewed in O’Brien et al.1).
Summary and Research Needs
Human exposure to aldehydes represents a significant toxicological concern, and therefore, understanding the corresponding molecular mechanism of toxicity is important for accurate risk assessment and remediation. In this perspective, we have shown that environmental and endogenous aldehydes can be described by their relative softness (σ) and electrophilicity (ω), which are important electronic determinants of the respective second-order reaction rates with nucleophilic targets on macromolecules. Corresponding analyses of nucleophilicity indicate that soft unsaturated aldehydes react selectively with soft nucleophilic thiolate sites on specific cysteine residues located in the active sites of enzymes (reviewed in LoPachin et al.37). In contrast, hard alkanals preferentially form adducts with hard nucleophiles such as nitrogen atoms of ε-amino groups on lysine residues or the N2 nitrogen of deoxyguanosine.54,89 These soft–soft and hard–hard adduct reactions appear to mediate toxicity by impairing the function of macromolecules (e.g., proteins, DNA, and RNA) that play critical roles in cytophysiological processes.15−18 However, more research is needed to broaden our understanding of how these specific covalent reactions disable macromolecular targets. Also critical to understanding toxicity is the recognition that human populations are exposed to complex mixtures that occur due to the ubiquitous environmental distribution of aldehydes. On the basis of their common mechanism, the individual aldehyde components of the mixture can interact, either additively or synergistically, to produce toxicity. Perhaps of more concern, the unsaturated aldehydes are a subgroup the conjugated type-2 alkenes. These toxicants share a common mechanism, which suggests that different family members found in the environment could interact to cause type-2 alkene toxicity. Therefore, based on the ability of these toxicants to interact collectively, more research emphasis should be placed on mixture toxicology. Finally, highly reactive unsaturated aldehydes (e.g., acrolein, 4-hydroxy-2-nonenal) are also generated endogenously during the oxidative stress associated with many pathogenic and toxicogenic processes. Thus, exploring how environmental and endogenous aldehydes might interact to accelerate disease or tissue injury processes represents an exciting area of future investigation. Finally, we focused on the electronic structure of aldehyde toxicants as revealed by the selected HSAB descriptors and demonstrated how this structure was related to corresponding toxic reactivities and molecular targets. As we gain a better understanding of this relationship, it should be possible to use these HSAB descriptors, in conjunction with knowledge of physicochemical features, to accurately predict the potential toxicity of a given chemical structure.
Glossary
Abbreviations
- HSAB
hard and soft, acids and bases
- HIV
human immunodeficiency virus
- DNA
DNA
- RNA
ribonucleic acid
- FMO
frontier molecular orbital
- LUMO
lowest unoccupied molecular orbital
- HOMO
highest occupied molecular orbital
- Cys
cysteine
- EA
ethyl acrylate
- MMA
methyl methacrylate
- ELUMO
LUMO energy
- PTP1B
protein tyrosine phosphate 1B
- LD50
lethal oral dose for 50% of the population
- HNE
4-hydroxy-2-nonenal
- ONE
4-oxy-2-nonenal
- NAC
N-acetylcysteine
- NSF
N-ethylmaleimide sensitive factor
- SNAP-25
synaptosomal-associated protein of 25 kDa
- AGEs
advanced glycation end products
- CMC
S-(carbonxymethyl)cysteine
- SIRT3
mitochondrial sirtuin3
- GSTP1-1
glutathione S-transferase P1-1
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- NO
nitric oxide
- H2O2
hydrogen peroxide
- Nrf2/Keap1
nuclear factor erythroid 2-related factor 2/kelch-like erythroid cell-derived protein with CNS homology-associated protein 1
- eV
electronvolt
The research discussed in this perspective was supported by NIH grants from the National Institutes of Environmental Health Sciences RO1 ES03830-25 and RO1 ESO7912-11.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- O’Brien P. J.; Diraki A. G.; Shangari N. (2005) Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 35, 609–662. [DOI] [PubMed] [Google Scholar]
- Feron V. J.; Til H. P.; de Vrijer F. (1991) Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 259, 363–385. [DOI] [PubMed] [Google Scholar]
- Abraham K.; Andres S.; Palavenskas R.; Berg K.; Appel K. E. (2011) Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 55, 1277–1290. [DOI] [PubMed] [Google Scholar]
- Faroon O.; Roney N.; Taylor J. (2008) Acrolein environmental levels and potential for human exposure. Toxicol. Ind. Health 24, 543–564. [DOI] [PubMed] [Google Scholar]
- Woodruff T. J.; Wells E. M.; Holt E. W.; Burgin D. E.; Axelrad D. A. (2007) Estimating risk from ambient concentrations of acrolein across the United States. Environ. Health Perspect. 115, 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikauf G. D. (2002) Hazardous air pollutants and asthma. Environ. Health Perspect. 110(suppl.4), 505–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole T. E.; Conklin D. J.; Bhatnagar A. (2008) Environmental risk factors for heart disease. Res. Environ. Health 23, 167–202. [DOI] [PubMed] [Google Scholar]
- Fujioka K.; Shibamoto T. (2006) Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 21, 47–54. [DOI] [PubMed] [Google Scholar]
- Werley M. S.; Freelin S. A.; Wrenn S. E.; Gerstenberg B.; Roemer E.; Schramke H.; van Miert E.; Vanscheeuwijck P.; Weber S.; Coggins C. R. E. (2008) Smoke chemistry, in vitro and in vivo toxicology evaluations of the electrically heated cigarette smoking system series K. Reg. Toxicol. Pharmacol. 52, 122–139. [DOI] [PubMed] [Google Scholar]
- Stevens J. F.; Maier C. S. (2008) Acrolein: sources, metabolism and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 52, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T. B.; Gavin C. L.; Taylor S. V.; Waddell W. J.; Cohen S. V.; Feron V. J.; Goodman; Rietjens I. M. C. M.; Marnett L. J.; Portoghese P. S.; Smith R. L. (2008) The FEMA GRAS assessment of α,β-unsaturated aldehydes and related substances used as flavor ingredients. Food Chem. Toxicol. 46, 2935–2967. [DOI] [PubMed] [Google Scholar]
- Conklin D. J.; Barski O. A.; Lesgards J.-F.; Juvan P.; Rezen T.; Rozman D.; Prough R. A.; Vladykovskaya E.; Liu S.; Srivastava S.; Bhatnagar A. (2010) Acrolein consumption induces systemic dyslipidemia and lipoprotein modification. Toxicol. Appl. Pharmacol. 24, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-W.; Guo Y.; Vondriska T. M.; Zhang J.; Zhang S.; Tsai L. L.; Zong N. C.; Bolli R.; Bhatnagar A.; Prabhu S. D. (2008) Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCε signaling and cardioprotection. J. Mol. Cell. Cardiol. 44, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Gurtoo H. L.; Hipkens J. H.; Sharma S. D. (1981) Role of glutathione in the metabolism-dependent toxicity and chemotherapy of cyclophosphamide. Cancer Res. 41, 3584–3591. [PubMed] [Google Scholar]
- Esterbauer H.; Schaur R. J.; Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med. 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Kehrer J. P.; Biswal S. S. (2000) The molecular effects of acrolein. Toxicol. Sci. 57, 6–15. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T.; Petersen D. R.; Barber D. S. (2009) Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem. Res. Toxicol. 22, 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witz G. (1989) Biological interactions of α,β-unsaturated aldehydes. Free Radical Biol. Med. 7, 333–340. [DOI] [PubMed] [Google Scholar]
- Andrews L. S.; Clary J. J. (1986) Review of the toxicity of multifunctional acrylates. J. Toxicol. Environ. Health 19, 149–164. [DOI] [PubMed] [Google Scholar]
- Bisesi M. S. (1994) Esters. 3. Esters of Alkenylcarboxylic Acids and Monoalcohols, in Patty’s Industrial Hygiene and Toxicology, 4th ed. (Clayton G. D., and Clayton F. E., Eds.) Vol. 11, pp 2999–3007, John Wiley and Sons, New York. [Google Scholar]
- Friedman M. (2003) Chemistry, biochemistry and safety of acrylamide. A review. J. Agric. Food Chem. 51, 4504–4526. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T. (2012) Molecular mechanism of acrylamide neurotoxicity: lessons learned from organic chemistry. Environ. Health Perspect. 120, 1650–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucek M.; Tenglerova J.; Kollarova B. (2002) Effect of acrylate chemistry on human health. Int. Arch. Occup. Environ. Health 75, S67–S72. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Barber D. S.; Gavin T. (2008) Molecular mechanisms of the conjugated α,β-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 104, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T.; Barber D. S. (2008) Type-2 alkenes mediate synaptotoxicity in neurodegenerative diseases. NeuroToxicology 29, 871–882. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T.; DeCaprio A.; Barber D. S. (2012) Application of the hard and soft, acids and bases (HSAB) theory to toxicant-target interactions. Chem. Res. Toxicol. 25, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A. (2006) Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ. Res. 99, 692–705. [DOI] [PubMed] [Google Scholar]
- Fritz K. S.; Petersen D. R. (2013) An overview of the chemistry and biology of reactive aldehydes. Free Radical Biol. Med. 59, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon A. N.; Landar A.; Barnes S.; Darley-Usmar V. M. (2012) The electrophile responsive proteome: Integrating proteomics and lipidomics with cellular function. Antiox. Redox Signaling 17, 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. O.; Andjelkovich D. A.; Kligerman A. D.; Morgan K. T.; Heck H.d’A. (1985) A critical review of the literature on acrolein toxicity. Crit. Rev. Toxicol. 14, 309–380. [DOI] [PubMed] [Google Scholar]
- Pearson R. G. (1990) Hard and soft acids and bases – the evolution of a chemical concept. Coord. Chem. Rev. 100, 403–425. [Google Scholar]
- Schultz T. W.; Carlson R. E.; Cronin M. T. D.; Hermens J. L. M.; Johnson R.; O’Brien P. J.; Roberts D. W.; Siraki A.; Wallace K. B.; Veith G. D. (2006) A conceptual framework for predicting the toxicity of reactive chemicals: modeling soft electrophilicity. SAR QSAR Environ. Res. 17, 413–428. [DOI] [PubMed] [Google Scholar]
- Schwobel J. A. H.; Koleva Y. K.; Enoch S. J.; Bajot F.; Hewitt M.; Madden J. C.; Roberts D. W.; Schultz T. W.; Cronin M. T. D. (2011) Measurement and estimation of electrophilic reactivity for predictive toxicology. Chem. Rev. 111, 2562–2596. [DOI] [PubMed] [Google Scholar]
- Chattaraj P. K.; Sarkar U.; Roy D. R. (2006) Electrophilicity index. Chem. Res. Toxicol. 106, 511–513. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Barber D. S.; Geohagen B. C.; Gavin T.; He D.; Das S. (2007) Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity. Toxicol. Sci. 95, 136–146. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T.; Geohagen B. C.; Das S. (2007) Neurotoxic mechanisms of electrophilic type-2 alkenes: soft-soft interactions described by quantum mechanical parameters. Toxicol. Sci. 98, 561–570. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; Gavin T.; Geohagen B. C. (2009) Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal. Toxicol. Sci. 107, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiner D. R.; LaButti J. N.; Gates K. S. (2007) Kinetics and mechanism of protein tyrosine phosphatase B inactivation by acrolein. Chem. Res. Toxicol. 20, 1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk C. J.; Fang B.; Koomen J. M.; Gavin T.; LoPachin R. M.; Barber D. S. (2011) Molecular mechanisms of α,β-unsaturated carbonyl toxicity: cysteine-adduct formation correlates with loss of enzyme function. Chem. Res. Toxicol. 24, 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J.; Davies M. J. (2005) Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of α-dicarbonyl compounds with thiol groups on amino acids, peptides and proteins. Chem. Res. Toxicol. 18, 1232–1241. [DOI] [PubMed] [Google Scholar]
- Schultz T. W.; Yarbrough J. W. (2004) Trends in structure – activity relationships for carbonyl-containing α,β–unsaturated compounds. SAR QSAR Environ. Res. 15, 139–146. [DOI] [PubMed] [Google Scholar]
- Schultz T. W.; Netzeva T. I.; Roberts D. W.; Cronin M. T. D. (2005) Structure-toxicity relationships for the effects to Tetrahymena pyriformis of aliphatic, carbonyl-containing α,β-unsaturated chemicals. Chem. Res. Toxicol. 18, 330–341. [DOI] [PubMed] [Google Scholar]
- Chan K.; O’Brien P. J. (2008) Structure-activity relationships for hepatocyte toxicity and electrophilic reactivity of α,β-unsaturated esters, acrylates and methacrylates. J. Appl. Toxicol. 28, 1004–1015. [DOI] [PubMed] [Google Scholar]
- Freidig A. P.; Verhaar H. J. M.; Hermens J. L. (1999) Quantitative structure-activity relationships for the chemical reactivity of acrylates and methacrylates. Environ. Toxicol. Chem. 18, 1133–1139. [Google Scholar]
- McCarthy T. J.; Hayes E. P.; Schwartz C. S.; Witz G. (1994) The reactivity of selected acrylates esters toward glutathione and deoxyribonucleosides in vitro: structure-activity relationships. Fundam. Appl. Toxicol. 22, 543–548. [DOI] [PubMed] [Google Scholar]
- Bohme A.; Thaens D.; Paschke A.; Schuurmann G. (2009) Kinetic glutathione chemoassay to quantify thiol reactivity of organic electrophiles – application to α,β-unsaturated ketones, acrylates and propiolates. Chem. Res. Toxicol. 22, 742–750. [DOI] [PubMed] [Google Scholar]
- Tanni H.; Hashimoto K. (1985) Effect of acrylamide and related compounds on glycolytic enzymes of rat brain. Toxicol. Lett. 26, 79–84. [DOI] [PubMed] [Google Scholar]
- McGarth C. E.; Tallman K. A.; Proter N. A.; Marnett L. J. (2011) Structure-activity analysis of diffusible lipid electrophiles associated with phospholipid peroxidation: 4-hydroxnonenal and 4-oxononenal analogues. Chem. Res. Toxicol. 24, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer F. J.; Cipollina C.; Freeman B. A. (2011) Formation and signaling actions of electrophilic lipids. Chem. Rev. 111, 5997–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuykendall J. R.; Bogdanffy M. S. (1994) Formation and stability of acetaldehyde-induced crosslinks between poly-lysine and poly-deoxyguanosine. Mutat. Res. 311, 49–56. [DOI] [PubMed] [Google Scholar]
- Recio L.; Sisk S.; Pluta L.; Bermudez E.; Gross E. A.; Chen Z.; Morgan K. (1992) p53 mutations in formaldehyde-induced nasal squamous cell carcinomas in rats. Cancer Res. 52, 6113–6116. [PubMed] [Google Scholar]
- Swenberg J. A.; Dems W. D.; Mitchell R. I.; Gralla E. J.; Pavkov K. L. (1980) Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 40, 3398–3402. [PubMed] [Google Scholar]
- Lu K.; Collins L. B.; Ru H.; Bermudez E.; Swenberg J. A. (2010) Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol. Sci. 116, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller B. C.; Recio L.; Green A.; Sun W.; Wright F. A.; Bodnar W. M.; Swenberg J. A. (2013) Biomarkers of exposure and effect in human lymphoblastoid TK6 cells following [13C2]-acetaldehyde exposure. Toxicol. Sci. 133, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S.; Laso Y.; Yang I. Y.; Hecht S. S.; Moriya M. (2006) Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1-N2-propanodeoxyguanosine DNA adducts in human cells. Mutat. Res. 608, 1–7. [DOI] [PubMed] [Google Scholar]
- Dalle-Donne I.; Vistoli G.; Gamberoni L.; Giustarini D.; Colombo R.; Facino R. M.; Rossi R.; Milzani A.; Aldini G. (2007) Actin Cys374 as a nucleophilic target of α,β-unsaturated aldehydes. Free Radical Biol. Med. 42, 583–598. [DOI] [PubMed] [Google Scholar]
- Doorn J. A.; Petersen D. R. (2003) Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem-Biol. Interact. 143–144, 93–100. [DOI] [PubMed] [Google Scholar]
- Ishii T.; Tatsuda E.; Kumazawa S.; Nakayama T.; Uchida K. (2003) Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 42, 3473–3480. [DOI] [PubMed] [Google Scholar]
- Van Iersel M. L.; Ploemen J.-P.; LoBello M.; Federici G.; van Bladeren P. J. (1997) Interactions of α,β-unsaturated aldehydes and ketones with human glutathione S-transferase P1–1. Chem.-Biol. Interact. 108, 67–78. [DOI] [PubMed] [Google Scholar]
- Aldini G.; Gamberoni L.; Orioli M.; Beretta G.; Regazzoni L.; Facino R. M.; Carini M. (2006) Mass spectrometric characterization of covalent modification of human serum albumin by 4-hydroxy-trans-2-nonenal. J. Mass Spectrom. 41, 1149–1161. [DOI] [PubMed] [Google Scholar]
- Friguet B.; Stadtman E. R.; Szweda L. I. (1994) Modification of glucose-6-phophate dehydrogenase by 4-hydroxy-2-nonenal. J. Biol. Chem. 269, 21639–21643. [PubMed] [Google Scholar]
- Uchida K.; Stadtman E. R. (1993) Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 268, 6388–6393. [PubMed] [Google Scholar]
- Uchida K.; Kanematsu M.; Morimitsu Y.; Noriko T.; Noguchis N.; Niki E. (1998) Acrolein is product of lipid peroxidation reaction. J. Biol. Chem. 273, 16058–16066. [DOI] [PubMed] [Google Scholar]
- Uchida K.; Kanematsu M.; Sakai K.; Matsuda T.; Hattori N.; Mizuno Y.; Suzuki D.; Miyata T.; Noguchi N.; Niki E. (1998) Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 95, 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorn J. A.; Petersen D. R. (2002) Covalent modification of amino acid nucleophiles by the lipid peroxidation products 4-hydroxynonenal and 4-oxo-2-nonenal. Chem. Res. Toxicol. 15, 1445–1450. [DOI] [PubMed] [Google Scholar]
- Chan K.; Poon R.; O’Brien P. J. (2008) Application of structure-activity relationships to investigate the molecular mechanisms of hepatocyte toxicity and the electrophilic reactivity of α,β-unsaturated aldehydes. J. Appl. Toxicol. 28, 1027–1039. [DOI] [PubMed] [Google Scholar]
- Friedman M.; Cavins J. F.; Wall J. S. (1965) Relative nucleophilic reactivities of amino groups and mercaptide ions in addition reactions with α,β-unsaturated compounds. J. Am. Chem. Soc. 87, 3672–3682. [Google Scholar]
- Kurtz A. J.; Llyod R. S. (2003) 1,N2-Deoxyquanosine adducts of acrolein, crotonaldehyde and trans-4-hydroxynonenal cross-link to peptides via Schiff based linkage. J. Biol. Chem. 278, 5970–5976. [DOI] [PubMed] [Google Scholar]
- Nadkarni D.; Sayre L. M. (1995) Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem. Res. Toxicol. 278, 284–291. [DOI] [PubMed] [Google Scholar]
- Petersen D. R.; Doorn J. A. (2004) Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radical Biol. Med. 37, 937–945. [DOI] [PubMed] [Google Scholar]
- Baynes J. W.; Thorpe S. R. (2000) Glycoxidation and lipoxidation in atherogenesis. Free Radical Biol. Med. 28, 1708–1716. [DOI] [PubMed] [Google Scholar]
- Lo T. W.; Westwood M. E.; McLellan A. C.; Selwood T.; Thornalley P. J. (1994) Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N-alpha-acetylarginine, N-alpha-acetylcysteine and N-alpha-acetyllysine and bovine serum albumin. J. Biol. Chem. 269, 32299–32305. [PubMed] [Google Scholar]
- Fritz K. S.; Galligan J. J.; Smathers R. L.; Roede J. R.; Shearn C. T.; Peigan P.; Petersen D. R. (2011) 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem. Res. Toxicol. 24, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin R. M.; Barber D. S. (2006) Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol. Sci. 94, 240–255. [DOI] [PubMed] [Google Scholar]
- LoPachin R. M.; DeCaprio A. P. (2005) Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicol. Sci. 86, 214–225. [DOI] [PubMed] [Google Scholar]
- Jones D. P. (2010) Redox sensing: orthogonal control in cell cycle and apoptosis signaling. J. Int. Med. 268, 432–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C.; Hampton M. B. (2008) Thiol chemistry and specificity in redox signaling. Free Radical Biol. Med. 45, 549–561. [DOI] [PubMed] [Google Scholar]
- Barber D. S.; Stevens S.; LoPachin R. M. (2007) Proteomic analyses of rat striatal synaptosomes during acrylamide intoxication at a low dose-rate. Toxicol. Sci. 100, 156–167. [DOI] [PubMed] [Google Scholar]
- Cavins J. F.; Friedman M. (1968) Specific modification of protein sulfhydryl groups with α,β-unsaturated compounds. J. Biol. Chem. 243, 3357–3360. [PubMed] [Google Scholar]
- Yang R. S. H.; Thomas R. S.; Gustafson D. L.; Campain J.; Benjamin S. A.; Verhaar J. J. M.; Mumatz M. M. (1998) Approaches to developing alternative and predictive toxicology based on PBPK/PD and QSAR modeling. Environ. Health Perspect. 106(Suppl), 1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus T.; Faust M. (2012) Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ. Sci. Technol. 46, 2564–2573. [DOI] [PubMed] [Google Scholar]
- Altenburger R.; Nendza M.; Schuurmann G. (2003) Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ. Toxicol. Chem. 22, 1900–1915. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. (2007) Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 115(Suppl 1), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwobel J. A. H.; Wondrousch D.; Koleva Y. K.; Madden J. C.; Cronin M. T. D.; Schuurmann G. (2010) Prediction of Michael-type acceptor reactivity toward glutathione. Chem. Res. Toxicol. 23, 1576–1585. [DOI] [PubMed] [Google Scholar]
- Long E. K.; Picklo M. J. (2010) Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radical Biol. Med. 49, 1–8. [DOI] [PubMed] [Google Scholar]
- Ismahil M. A.; Hamid T.; Haberzetti P.; Gu Y.; Chandrasekar B.; Srivastava S. (2011) Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 301, H2050–H2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Hill B. G.; Gu Y.; Cai J.; Srivastava S.; Bhatnagar A.; Prabhu S. D. (2007) Mechanisms of acrolein-induced myocardial dysfunction: implications of environmental and endogenous aldehyde exposure. Am. J. Physiol. Heart Circ. Physiol. 293, H3673–H3684. [DOI] [PubMed] [Google Scholar]
- Srivastava S.; Sithu S. D.; Vladykovskaya E.; Haberzetti P.; Hoetker D. J.; Siddiqui M. A.; Conklin D. J.; D’Souza S. E. (2011) Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 215, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Gavin T.; DeCaprio A. P.; LoPachin R. M. (2010) γ-Diketone axonopathy: analysis of cytoskeletal motors and highways in CNS myelinated axons. Toxicol. Sci. 117, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]