Abstract
Deficits in axonal transport are thought to contribute to the pathology of many neurodegenerative diseases. Expressing the slow Wallerian degeneration protein (WldS) or related nicotinamide mononucleotide adenyltransferases (NmNATs) protects axons against damage from a broad range of insults, but the ability of these proteins to protect against inhibition of axonal transport has received little attention. We set out to determine whether these proteins can protect the axons of cultured hippocampal neurons from damage due to hydrogen peroxide or oxygen-glucose deprivation (OGD) and, in particular, whether they can reduce the damage that these agents cause to the axonal transport machinery. Exposure to these insults inhibited the axonal transport of both mitochondria and of the vesicles that carry axonal membrane proteins; this inhibition occurred hours before the first signs of axonal degeneration. Expressing a cytoplasmically targeted version of NmNAT1 (cytNmNAT1) protected the axons against both insults. It also reduced the inhibition of transport when cells were exposed to hydrogen peroxide and enhanced the recovery of transport following both insults. The protective effects of cytNmNAT1 depend on mitochondrial transport. When mitochondrial transport was inhibited, cytNmNAT1 was unable to protect axons against either insult. The protective effects of mitochondrially targeted NmNAT also were blocked by inhibiting mitochondrial transport. These results establish that NmNAT robustly protects the axonal transport system following exposure to OGD and reactive oxygen species and may offer similar protection in other disease models. Understanding how NmNAT protects the axonal transport system may lead to new strategies for neuroprotection in neurodegenerative diseases.
Keywords: NmNAT1, mitochondria, axonal transport, Miro, oxidative stress, oxygen-glucose deprivation
Introduction
The unique geometry of neurons makes them particularly vulnerable to injuries that disrupt the microtubule-based transport of organelles between the cell body and the distal axon. Not surprisingly, genetic mutations that affect components of the axonal transport system, such as kinesins and dynein, lead to diseases that are characterized by axonal degeneration (Blackstone et al., 2011; Holzbaur and Scherer, 2011). Deficits in axonal transport may also contribute to the axonal degeneration that occurs following insults such as inflammation (Fang et al., 2012a; Stagi et al., 2005; Takeuchi et al., 2005) and are thought to contribute to the pathology in many neurodegenerative diseases (Bilsland et al., 2010; Decker et al., 2010; Her and Goldstein, 2008). Studies of cultured neurons show that inhibition of axonal transport is among the earliest signs of damage following exposure to reactive oxygen species or other insults (Fang et al., 2012a; Stagi et al., 2005). Thus there is a strong motivation to identify agents that can protect against damage to the axonal transport machinery.
The Wallerian degeneration slow (WldS) mutation, which arose spontaneously in a line of C57Black mice, greatly prolongs the survival of axons that have been cut off from their cell bodies (Coleman et al., 1998). The mutation leads to the expression of a chimeric protein that is a fusion of the first 70 amino acids from Ube4b, an E4 ubiquitin ligase, and the full length sequence of nicotinamide mononucleotide adenyltransferase 1 (NmNAT1), one of three isoforms of the adenylating enzyme responsible for the biosynthesis of NAD+ (Coleman, 2005). Neurons of mice carrying the WldS mutation are resistant to damage from a variety of insults (Adalbert et al., 2005; Gillingwater et al., 2006; Mi et al., 2005; Wang et al., 2002) and expressing the WldS protein confers neuronal protection in other species and in cultured neurons (Press and Milbrandt, 2008; Tokunaga and Araki, 2012; Wang et al., 2005). The mechanisms by which WldS protein protects axons against damage have been the subject of intensive research. In many cases, the protective effects of WldS can be mimicked by overexpressing one or another form of NmNAT and axonal protection is often prevented by mutations in WldS or NmNAT that interfere with enzymatic activity, but changes in basal NAD+ levels do not correlate with neuroprotection (Coleman and Freeman, 2010). Although WldS protein and NmNAT1 are concentrated in the nucleus, mutating their nuclear localization signals enhances their neuroprotective effects, suggesting that they act within the cytoplasm (Babetto et al., 2010). The exact subcellular sites of action of these proteins remain controversial, although recent evidence suggests that WldS increases mitochondrial transport in axons and enhances the calcium buffering capacity of axonal mitochondria (Avery et al., 2012).
We set out to determine whether NmNAT can protect axons in a culture model that mimics the insults that occur in neural diseases and, in particular, whether NmNAT can reduce the inhibition in axonal transport caused by these agents. We subjected neurons to two different insults: hydrogen peroxide, an inflammatory mediator, and oxygen-glucose deprivation (OGD), a model for ischemia/stroke. We examined two of the major organelle populations that undergo rapid axonal transport, vesicles that convey proteins from the Golgi complex to the axonal membrane and mitochondria. Our results show that expressing cytNmNAT1 (a cytosolic version of NmNAT1) reduces inhibition to the axonal transport system and protects axons from degeneration due to these insults. We further show that mitochondrial transport is critical for cytNmNAT1’s protective effects.
Materials and Methods
DNA constructs
All constructs were expressed from the CAG plasmid vector, which contains a chicken beta-actin promoter (Niwa et al., 1991). NgCAM-mCherry was constructed by fusing mCherry to the C-terminus of chicken NgCAM (Buchstaller et al., 1996; Sampo et al., 2003). cytNmNAT1-GFP was prepared by fusing eGFP to the C-terminus of NmNAT1 that lacked its nuclear localization signal in FUGW vector (Sasaki et al., 2006)(a generous gift from Dr. Jeffrey Milbrandt at Washington University). Mitochondrially targeted NmNAT1-GFP (mitoNmNAT-GFP) was constructed by fusing the mitochondrial targeting sequence from subunit VIII of human cytochrome C oxidase (Rizzuto et al., 1995) to the N-terminus of cytNmNAT1-GFP. ssNPY-mCherry and mito-GFP have been described previously (Fang et al., 2012a). All constructs were verified by DNA sequencing.
Cell culture and plasmid expression
Primary hippocampal cultures were prepared from E18 rat embryos of either sex as described previously (Banker and Goslin, 1988; Goslin K, 1998; Kaech and Banker, 2006). GFP-tagged NmNAT constructs or GFP alone (0.5–1 μg of DNA) were introduced into neurons by electroporation prior to plating using an Amaxa® Nucleofector® (Lonza, Basel, Switzerland). In experiments that required mitochondrial labeling, mito-GFP was also expressed by electroporation. Cells were plated onto poly-L-lysine-treated glass coverslips at a density of 15–25,000 cells/cm2 and maintained in N2 medium in the presence of astrocytes (prepared by differentiating neural stem cells). To evaluate axonal transport of Golgi-derived vesicles, appropriate constructs (1–2 μg of DNA) were expressed by transfection with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) at 7–8 days in vitro and transport was imaged 8–20 hours later.
Hydrogen peroxide exposure and oxygen–glucose deprivation
Neuronal cultures were subjected to hydrogen peroxide by replacing the culture medium with HibernateE® medium (Invitrogen, Carlsbad, CA) containing 100 μM hydrogen peroxide (Sigma, St Louis, MO) for 1 hour (for axonal degeneration study) or for 30 minutes (for axonal transport study). Then hydrogen peroxide was removed and the cells were returned to normal culture medium for 6 hours (for the axonal degeneration study) or for 1 hour (for analysis of axonal transport).
Neuronal cultures were subjected to oxygen–glucose deprivation (OGD) by replacing the culture medium with Earle’s BSS lacking glucose and containing 2-deoxglucose (10mM) and maintaining the cells in an atmosphere of 90% N2, 5% CO2, and 5% H2 (Airgas, Portland, OR) for 4 hours, as described by Okami et al. (2012). Then the cells were returned to normal culture medium and maintained in an atmosphere with 5% CO2 and ambient oxygen levels. Control cultures were transferred to Earles BSS containing 5.6 mM glucose and maintained at ambient oxygen levels for 4 hours, then returned to normal culture medium.
Imaging and Analysis of Axonal Transport
Organelle transport was imaged following the methods described by Kaech et al. (Kaech et al., 2012a; Kaech et al., 2012b; Kaech et al., 2012c). Vesicle transport was imaged 8 h after expression of NgCAM-GFP or 20 h after expression of SS-mCherry. Mitochondrial transport was imaged 7–8 days after mito-GFP expression. Coverslips were placed in a heated chamber (Warner Instruments, Hamden, CT) containing Hibernate E medium (Brainbits LLC, Springfield, IL) and maintained at 37°. Imaging was performed using a Nikon TE2000/PFS microscope equipped with a Yokugawa CSU-10 spinning disk confocal head (Solamere Technology Group, Salt Lake City, Utah) and images were captured using an ORCA ER camera (Hammamatsu Instruments, Hammamatsu, Japan). Mitochondrial transport was imaged using a 40X 1.2 N.A oil immersion objective and vesicle transport was imaged with a 60X 1.35 N.A oil immersion objective. Mitochondrial transport was imaged at 1 frame every 2 sec for 2 min and vesicle transport was imaged at 2 frames/sec for 30 sec.
Transport was quantified by constructing kymographs of organelle movement, using Metamorph Image Analysis software (Molecular Devices, Sunnyvale, CA). This produced a graph in which the X-axis represents time and the Y-axis represents distance along the axon. Diagonal lines correspond to moving organelles. To determine the number of transport events, we counted all organelles that moved at least 5 μm in either the anterograde or retrograde direction, then normalized these values to correct for differences in axon length and imaging duration (events/min/100 μm). Statistical significance of the differences between groups was determined by performing student T-test.
Quantification of Axonal Degeneration
Axonal morphology was assessed by acquiring low-magnification images of cells expressing GFP or GFP-tagged NmNAT constructs (see Figure 1). These images typically included the proximal 200 μm of the primary axon along with numerous collateral branches. Only neurons that expressed the labeled constructs at high levels were selected for analysis. Images were collected from at least three separate experiments performed on different culture preparations and 65–150 cells were analyzed per condition. The extent of degeneration, as indicated by axonal beading or fragmentation, was assessed from these images by an observer blinded to the experimental condition, according to the following scoring system: 1--axon fully intact or with beading limited to less than 10% of the axon length; 2: beading and/or fragmentation over 10–50% of the axon; 3: beading and fragmentation over 50–90% of the axon; 4: fragmentation of the entire axon. For statistical analysis and for the figures, cells with a degeneration score of 1 or 2 were grouped together in a single category, referred to as “mild” degeneration, and those with scores of 3 and 4 were grouped together as “severe” degeneration. Statistical differences between experimental and control groups were analyzed using Fisher’s exact test.
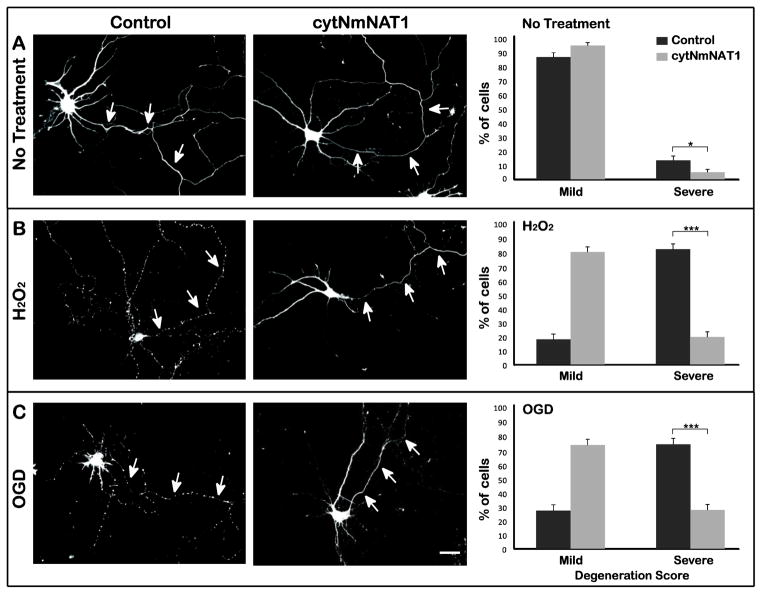
Figure 1. Expression of cytNmNAT1 protects the axons of cultured hippocampal neurons from hydrogen peroxide or OGD.
(A) Neurons were electroporated with either soluble GFP (control) or cytNmNAT1-GFP before plating. After 7–8 days in culture, neurons were challenged with either 100 μM hydrogen peroxide for 1 h (B) or oxygen glucose deprivation for 4 h (C). Axons from control neurons (i.e. expressing GFP alone) exhibited pronounced signs of degeneration following hydrogen peroxide exposure or OGD, as indicated by beading and fragmentation (B & C left column). In contrast, axons from neurons expressing cytNmNAT1-GFP were largely intact following the same challenges (B & C middle column). To quantify these results, the extent of degeneration was evaluated based on a scale ranging from 1 (minimal degeneration) to 4 (beading or fragmentation of the entire axonal arbor). Cells with a degeneration score of 1 or 2 were grouped together in a single category, referred to as “mild” degeneration, and those with scores of 3 and 4 were grouped together as “severe” degeneration. Exposure to hydrogen peroxide or OGD caused a large increase in the percentage of severely degenerated axons. In cells expressing cytNmNAT1-GFP, the percentage of severely degenerated axons was much reduced (B & C right column). Axonal degeneration was evaluated 6 h after hydrogen peroxide exposure and 24 h after OGD. * p < 0.05; *** P < 0.001. Scale bar: 30 μm.
Quantification of Mitochondrial Density
To quantify mitochondria occupancy, line scans were drawn on the axons of cells expressing mito-RFP and soluble GFP (control situation) or dnMiro-GFP, using the GFP signal as a guide to determine axons. Mitochondrial occupancy was calculated as the percentage of overall axonal length that contained at least one mitochondrion (based on maximum intensity levels at least twice background values. The length of axon measured varied between 60 and 120 μm per cell; a minimum of 20 cells were evaluated per condition.
Results
cytNmNAT1 protects axons from damage following hydrogen peroxide exposure and oxygen-glucose deprivation
The overall goal of these experiments was to ask whether expressing WldS -related proteins can protect primary hippocampal neurons against axonal transport inhibition induced by exposure to hydrogen peroxide or oxygen-glucose deprivation (OGD). In doing so, we focused initially on the effects of cytNmNAT1, a version of NmNAT1 that lacks its nuclear localization signal and hence is localized to the cytoplasm (Sasaki et al., 2006). Millbrandt and colleagues have demonstrated that expressing cytNmNAT1 in cultured dorsal root ganglion neurons provides robust protection against axonal degeneration following axotomy as well as a variety of toxic insults, including exposure to reactive oxygen species and vincristine (Press and Milbrandt, 2008; Sasaki and Milbrandt, 2010; Vohra et al., 2010).
We set out to test the effects of expressing cytNmNAT1 in hippocampal neurons exposed to two different insults—reactive oxygen species, which are associated with a variety of CNS lesions (Brown and Neher, 2010)--and OGD, a cell culture model for ischemia and stroke (Jones et al., 2011). Before plating, hippocampal neurons were electroporated with cytNmNAT1-GFP or soluble GFP (as a control) and then were challenged by exposure to injury 7 days later. We chose this strategy because electroporation results in much higher transduction efficiency than transfection; typically about 50–80% of electroporated cells expressed cytNmNAT1-GFP compared with less than 5% following Lipofectamine 2000-mediated transfection. Like soluble GFP, cytNmNAT1-GFP filled the entire cell, including the full extent of the axon. In neurons that were not injured, the axons extended over long distances and gave rise to many branches (Figure 1A); signs of axonal degeneration, such as beading, were minimal.
We first asked whether expressing cytNmNAT1-GFP affected the axons of neurons that were not exposed to insults (Fig. 1A). To quantify axonal damage, a scoring system was developed to assess degeneration, based on the GFP images of the axonal arbors. Cells were scored from 1 to 4, with 1 corresponding to minimal signs of axonal degeneration and 4 corresponding to complete or nearly complete axonal fragmentation (for details, see Materials and Methods). The quantification was performed by an observer blinded to the experimental conditions. In the case of cells that were not subjected to injury, about 90% of the axons were classified as having mild or no degeneration (scores of 1 or 2). There was a subtle but statistically significant difference between control cells (expressing GFP alone) and cells expressing cytNmNAT1-GFP. The axons of the latter cells were judged to be slightly less damaged than those of control neurons (Fisher’s exact test, p < 0.05). This result suggests that cells expressing cytNmNAT1 may be protected from some of the stresses inherent in maintaining cells in culture.
To evaluate the effects of reactive oxygen species on axonal integrity, we exposed neurons to 100 μM hydrogen peroxide for 1 hour, following the approaches of Fang et al (Fang et al., 2012a). Immediately after this treatment, the axons of control neurons appeared normal, but 6 hours later extensive beading and fragmentation had appeared all along the axon and its branches. In contrast, neurons expressing cytNmNAT1-GFP showed much less damage (Figure 1B). Phase contrast images show the healthy axons of neurons expressing cytNmNAT1-GFP are surrounded by numerous untransfected neurons, whose axons have almost completely degenerated (Supplementary Figure 1). After hydrogen peroxide treatment, 82% of the axons of control neurons were severely damaged (score of 3 or 4), compared with only 19% of the axons in neurons expressing cytNmNAT1 (p<0.001).
Oxygen-glucose deprivation was initiated by placing cultured neurons in medium in which glucose was replaced with 2-deoxyglucose (a glucose analog that inhibits glycolysis), then maintaining the cultures in an oxygen-free atmosphere for 4 h. The axons of control neurons showed no signs of damage immediately after OGD, but over time their axons underwent extensive degeneration (although the time course of axonal degeneration was slower than that following hydrogen peroxide exposure). By 24 h after OGD, axons were almost entirely fragmented. In contrast, the axons of neurons over-expressing cytNmNAT1 were much less extensively damaged. Quantification shows a degree of protection similar to that seen in cells exposed to hydrogen peroxide. In control neurons, 73% of axons showed severe degeneration (score of 3 or 4) compared with only 27% of neurons expressing cytNmNAT1 (p<0.001) (Figure 1C). These results show that expression of cytNmNAT1 robustly protects the axons of hippocampal neurons against injury from exposure to both reactive oxygen species and to OGD. The axons of cytNmNAT1-expressing cells exposed to these conditions were similar to those of control neurons that were not subjected to any insult.
Expressing cytNmNAT1 with a point mutation in its substrate binding domain (Sasaki et al., 2009) failed to protect hippocampal axons against either hydrogen peroxide or OGD (data not shown). Thus the protective effect of expressing cytNmNAT1 depends on its enzymatic function.
CytNmNAT1 protects the axonal transport machinery from inhibition due to hydrogen peroxide and oxygen glucose deprivation
We next asked whether expression of cytNmNAT1 protects hippocampal neurons against inhibition to the axonal transport system. We examined the transport of two important organelle populations: vesicles that arise from the Golgi complex and deliver newly synthesized membrane proteins required to maintain axonal excitability and synaptic transmission; axonal mitochondria, which are transported to sites of high energy demand or where increased calcium buffering is required (Wang and Schwarz, 2009). We labelled Golgi-derived vesicles by transfecting one of the following constructs: GFP-tagged NgCAM, a transmembrane adhesion molecule that is delivered preferentially to the axonal plasma membrane (Sampo et al., 2003); SS-mCherry, a construct consisting of an ER-targeting signal sequence fused to mCherry (Fang et al., 2012a). After cleavage of the signal sequence in the ER, soluble mCherry fills the lumen of the ER, the Golgi complex and transport vesicles that arise from the Golgi. Since the label does not accumulate in the plasma membrane following vesicle exocytosis, transport of vesicles labeled by expressing SS-mCherry can be visualized even after long periods of expression. Mitochondria were labelled by expressing GFP fused to a mitochondrial targeting sequence (Fang et al., 2012a).
In cultured hippocampal neurons, vesicles derived from the Golgi complex undergo extensive, rapid transport into the axon (Burack et al., 2000; Kaether et al., 1997; Nakata and Hirokawa, 2003). In a 100 μm stretch of axon, there were on average 47±18 anterograde vesicle movements per minute and 23±14 retrograde movements per minute; the average velocity of vesicle transport was 1.60±0.41 μm/sec in the anterograde direction and 1.17±0.48 μm/sec in the retrograde direction. These values are similar to those from previous reports for membrane vesicle transport in axons (Fang et al., 2012a; Her and Goldstein, 2008).
As shown in Figure 2 (A & B), after exposure to hydrogen peroxide for 30 min, the number of anterograde vesicle movements decreased markedly (to 34±7% of control values) while there was no change in the number of vesicles moving in the retrograde direction (Figure 2A & B). There was also a significant inhibition in the velocity of transport in both directions. This result is consistent with our previous findings (based on following individual cells that were exposed to hydrogen peroxide), which showed that the earliest indication of damage was a reduction in transport velocity, which was followed by a decrease in the number of vesicles moving in the anterograde direction (Fang et al., 2012a). Expression of cytNmNAT1 significantly reduced the inhibition of anterograde transport; the number of anterograde movements declined by only about one-third (to 67±13% of control values). Moreover, when hydrogen peroxide-treated cells were returned to normal medium and examined 1 h later, vesicle transport was fully restored to normal levels in neurons expressing cytNmNAT1. In control neurons, anterograde transport recovered only partially. Similar effects of expressing cytNmNAT1 were seen when Golgi-derived vesicles were labeled by expressing SS-mCherry (data not shown). Thus expression of cytNmNAT1 protected neurons from inhibition to the transport system during the period of hydrogen peroxide exposure and enabled transport to recover to normal levels following peroxide exposure.
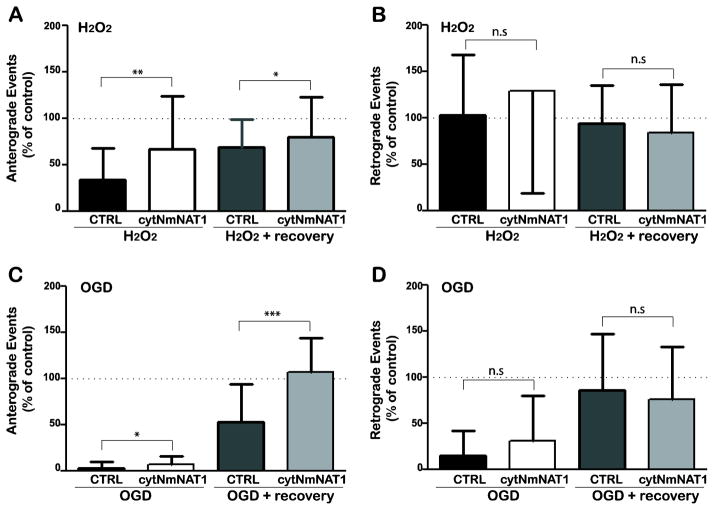
Figure 2. Expression of cytNmNAT1 protects against inhibition of the transport of Golgi-derived vesicles.
Cultured hippocampal neurons were electroporated with either soluble GFP (control) or cytNmNAT1-GFP before plating, then were transfected with constructs to label Golgi-derived vesicles (NgCAM-GFP or ssNPY-GFP) 7 days later. (A, B) Neurons were treated with hydrogen peroxide (100 μM for 30min), then imaged immediately (H2O2) or returned to normal medium and allowed to recover for 1 h before imaging (H2O2 + recovery). Hydrogen peroxide caused a profound reduction in the number of anterograde vesicle movements (> 65% inhibition compared with untreated neurons), which recovered only partially (33% residual inhibition) (A). There was no reduction in the number of retrograde movements (B). In neurons expressing cytNmNAT1, the inhibition of anterograde vesicle transport was significantly reduced and recovery was significantly improved. (C, D) Neurons were subject to OGD for 4 h and imaged immediately (OGD) or returned to normal medium and imaged after a 2 h recovery period (OGD + recovery). Immediately after OGD, anterograde vesicle transport (C) was almost completely eliminated in control neurons. Expression of cytNmNAT1 reduced this inhibition to a small, but statistically significant degree. When cells were returned to normal medium, vesicle transport in control neurons recovered only partially (47% residual inhibition); in contrast, anterograde vesicle transport was fully restored to normal levels in neurons expressing cytNmNAT1. Expressing cyNmNAT1 also reduced the inhibition of retrograde transport observed immediately after OGD (D), but this reduction was not statistically significant. Neurons were imaged either 8 h after expression of NgCAM-GFP (A, B) or 20 h after expression of ssNPY-mCherry (C, D). Only neurons that co-expressed vesicles markers and cytNmNAT1-GFP or GFP were imaged. Bars show means and standard deviations. * p < 0.05; ** p<0.01; *** p < 0.001
We used a similar approach to test whether expression of cytNmNAT1 protects vesicle transport when neurons were subjected to OGD (Figure 2C & D). In control neurons after 4 h of OGD, transport in both anterograde and retrograde directions was almost completely abolished. The number of anterograde events was reduced to only 3±1% of controls and retrograde event numbers dropped to only 15±5% of control. Vesicle transport was also severely inhibited in neurons expressing cytNmNAT1, but the inhibition was not quite as severe as in control cells. The difference between control and cytNmNAT1-expressing cells was statistically significant only for anterograde transport. The protective effects of expressing cytNmNAT1 were more apparent when cells were allowed to recover after OGD. In neurons expressing cytNmNAT1, anterograde vesicle transport fully recovered 2 h after their being return to normal conditions, whereas in control neurons without cytNmNAT1 anterograde transport remained depressed (at 53±10% of normal levels; p<0.001). Retrograde vesicle transport fully recovered in both control- and cytNmNAT1-expressing neurons (Figure 2D). These results make three important points. First, they show that anterograde vesicle transport is particularly vulnerable to inhibition by both reactive oxygen species and OGD. Second, they establish that expressing cytNmNAT1 reduces the inhibition of anterograde vesicle transport following exposure to hydrogen peroxide and, to a lesser extent, OGD. Finally, they demonstrate that in neurons expressing cytNmNAT1 vesicle transport is restored to near-normal levels when neurons exposed to these insults are returned to normal conditions.
We next examined whether expression of cytNmNAT1 can protect mitochondrial transport from peroxide and OGD. Mitochondria move slower than Golgi-derived vesicles and a greater fraction of mitochondria are stationary at any point in time (Fang et al., 2012a; Morris and Hollenbeck, 1995; Shidara and Hollenbeck, 2010). Expressing WldS in Drosophila neurons increases the number of moving mitochondria and reduces the number of stationary mitochondria (Avery et al., 2012). In cultured hippocampal neurons, expressing cytNmNAT1 also slightly increased the number of mitochondrial movements (by about 20% for anterograde events and 10% for retrograde events), but this change did not reach statistical significance.
When control neurons (not expressing cytNmNAT1) were exposed to hydrogen peroxide (100 μM for 30 min), mitochondrial transport was almost completely abolished in both anterograde and retrograde directions (Figure 3A & B). The number of transport events was reduced to less than 5% of control levels. This result is consistent with previous reports indicating that mitochondrial transport is particularly susceptible to peroxide (Fang et al., 2012a). After 1h of recovery, transport recovered to only about 40% of normal in control neurons. Expressing cytNmNAT1 significantly protected mitochondrial transport, as assessed immediately after the insult, and significantly improved recovery following return to normal medium. Both anterograde and retrograde transport were protected to a similar degree.
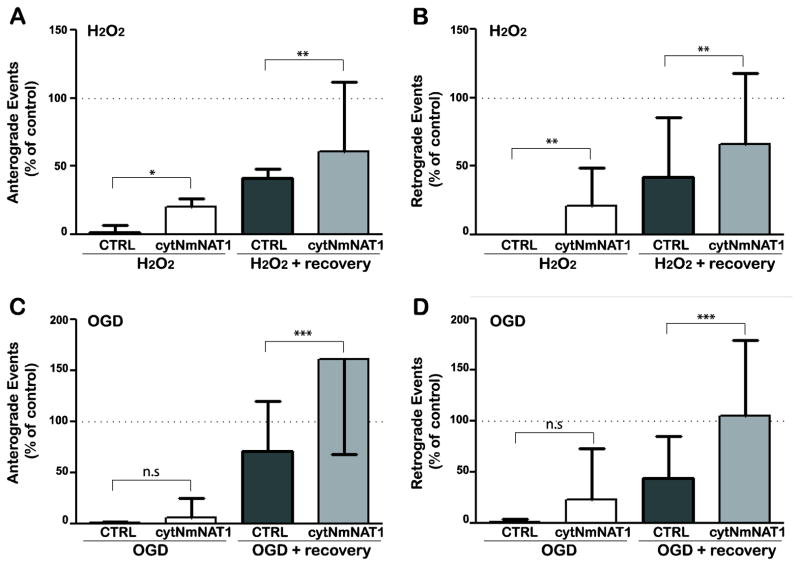
Figure 3. Expression of cytNmNAT1 protects against inhibition of mitochondrial transport.
Prior to plating, hippocampal neurons were co-electroporated with a mitochondrial marker (mitoRFP) and either soluble GFP (control) or cytNmNAT1-GFP. (A, B) In control neurons immediately after exposure to 100 μM hydrogen peroxide for 30 min (H2O2), mitochondrial transport was severely inhibited in both the anterograde (A) and retrograde (B) directions. After the hydrogen peroxide was removed and the cultures returned to normal medium for 1 h (H2O2 + recovery), mitochondrial transport was still severely inhibited (to about 50% of normal levels). Expression of cytNmNAT1 significantly reduced the inhibition of transport immediately after peroxide exposure and significantly improved recovery after the peroxide was removed. (C, D) Immediately after OGD for 4 h, mitochondrial transport was almost completely inhibited (to <2% of control values) in both anterograde and retrograde directions. Expression of cytNmNAT1 improved mitochondrial transport only slightly (differences not statistically significant). When cultures were returned to normal medium after OGD (OGD + recovery), mitochondrial transport was fully restored in cells expressing cytNmNAT1 but not in control neurons. Only neurons that co-expressed the mitochondrial marker and cytNmNAT1-GFP or GFP were imaged. Bars show means and standard deviations * p < 0.05; ** p<0.01; *** p < 0.001.
Using a similar approach, we tested whether cytNmNAT1 can also protect mitochondrial transport following OGD (Figure 3C & D). Like hydrogen peroxide, OGD severely inhibited mitochondrial transport in control neurons. The number of anterograde and retrograde transport events was reduced to less than 5% of normal. Even 2 h after return to medium containing normal levels of glucose and oxygen, neurons that had been subjected to OGD continued to show a significant deficit in mitochondrial transport (71±9% of normal in the anterograde direction and 44±8% of normal in the retrograde direction). Expressing cytNmNAT1 had a slight protective effect on transport after 4 h of OGD, but this effect did not reach statistical significance. By comparison, the effects of cytNmNAT1 on recovery from OGD were striking. Mitochondrial transport in both the anterograde and retrograde directions was fully restored to normal levels after 2 h.
Taken together, these data demonstrate that mitochondrial transport is particularly susceptible to hydrogen peroxide and OGD and that expressing cytNmNAT1 is able to protect against this inhibition. NmNAT expression also significantly enhanced the recovery of mitochondrial transport when cells were returned to normal conditions.
Inhibiting mitochondrial transport reduces the protective effects of expressing cytNmNAT1
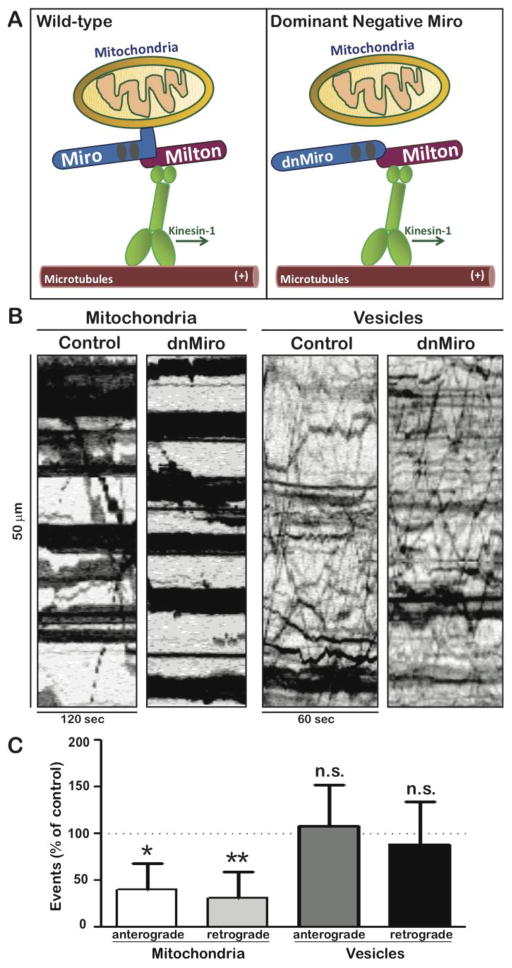
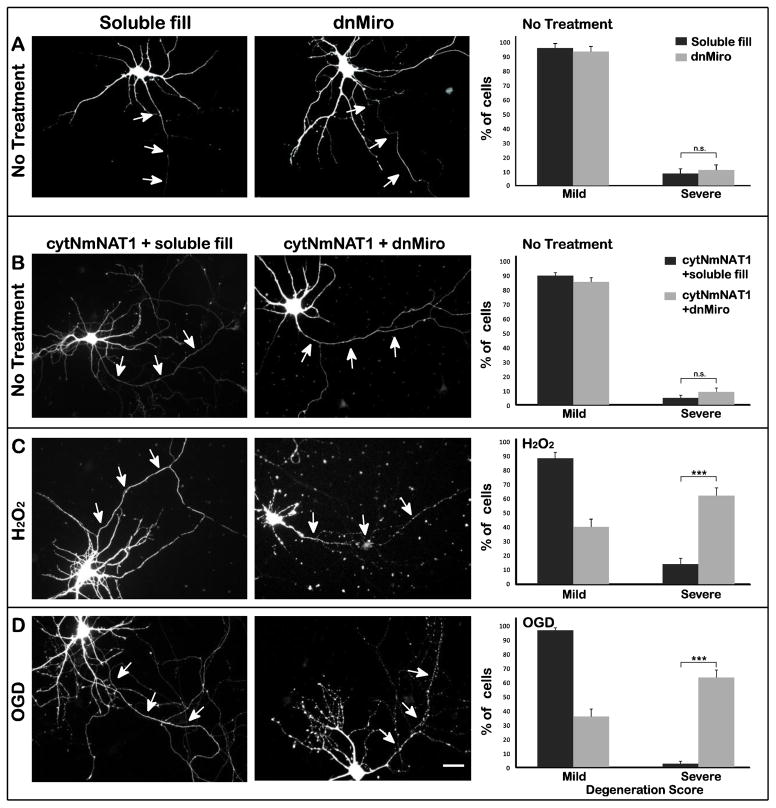
Based on experiments in Drosophila neurons, it has been suggested that mitochondrial transport is important for enabling expressed NmNAT to prolong the survival of transected axons (Avery et al., 2012). To explore the role of mitochondrial transport in NmNAT’s protection against other insults, we developed a dominant negative strategy to selectively inhibit mitochondrial transport in cultured neurons (Figure 4A). Miro is a mitochondrial membrane protein that binds Milton, the cytoplasmic adaptor that links mitochondria to the motor protein Kinesin-1 (Glater et al., 2006). The dominant negative Miro construct (dnMiro) includes the Milton binding domain, but lacks the transmembrane domain that links Miro to mitochondria (Saotome et al., 2008; Wang and Schwarz, 2009). When this construct was expressed by lipfofectamine-mediated transfection, mitochondrial transport was markedly reduced when assayed 24 later (Figure 4B). The number of anterograde mitochondrial movements was reduced to 40±7.3% of control values and the number of retrograde movements to 31.6±7% of controls, based on recordings of 18 cells (both differences were significant at p < 0.002). Despite this inhibition of transport, the density and appearance of axonal mitochondrial was similar to that in control neurons. The percentage of axonal length occupied by mitochondria was 38±17% in control neurons and 39±20% in neurons expressing dnMiro (For details, see Materials & Methods). The inhibition of transport following expression of dnMiro was highly specific, as the transport of Golgi-derived vesicles was unaffected. The number of anterograde vesicles movements was 108 ± 44% of control and the number of retrograde movement was 89 ± 45% of control (Figure 4C). The axons of neurons expressing this dnMiro construct were indistinguishable from those of control neurons, based on morphology and on the degeneration index (Figure 5A).
Figure 4. Expression of dominant-negative Miro specifically inhibits mitochondrial transport.
(A) Miro, a transmembrane protein present in the outer mitochondrial membrane, links mitochondria to the adaptor protein Milton, which in turn binds to the tail of Kinesin-1. The dominant negative construct used in these experiments, dnMiro-RFP, lacks a transmembrane domain but retains the Milton binding region. When the dominant negative construct binds to Milton, Milton cannot bind to mitochondria. (B) To test the efficacy of dnMiro-RFP, the construct was expressed by transfection and axonal transport was assayed 24 h later. Representative kymographs illustrate the transport of mitochondria or Golgi-derived vesicles in neurons expressing dnMiro-RFP or in control neurons (expressing soluble RFP). In the kymographs, horizontal lines represent stationary organelles and diagonal lines show moving organelles. Mitochondrial transport was significantly inhibited in neurons expressing dnMiro-RFP while vesicle transport was unaffected. (C) Quantification showed that mitochondrial transport event number were dropped to 40 ± 29% of control in anterograde direction and 32 ± 27% of control in retrograde direction when co-expressed with dnMiro. Vesicle transport remained 108 ± 44% in anterograde direction and 89 ± 45% in retrograde direction with dnMiro compared with control (P>0.5). Bars show means and standard deviations (n=18–23 cells per condition). * p<0.05; ** p<0.01.
Figure 5. Inhibition of mitochondrial transport interferes with the ability of cytNmNAT1 to protect axons against hydrogen peroxide or OGD.
(A) Neurons were transfected with either soluble GFP (control) or dominant-negative Miro (dnMiro) construct and their axonal integrity was examined 20 h later. Transiently expressing dnMiro did not affect axonal integrity (8 and 10% severely damaged respectively P>0.05). (B) Neurons were electroporated with cytNmNAT1-GFP before plating. After 7 days in culture they were transfected with either dnMiro-RFP or soluble RFP alone, were then challenged with either 100 μM hydrogen peroxide for 1 h (C) or OGD for 4 h (D). The axons of neurons expressing cytNmNAT1 and a soluble fill showed minimal signs of damage following either insult (C & D left column). In marked contrast, the axons of neurons expressing dominant-negative Miro showed extensive beading and fragmentation (C & D middle column). When neurons were exposed to hydrogen peroxide, 61% of the axons were severely degenerated in neurons expressing dominant-negative Miro and cytNmNAT1, compared with only 14% in neurons expressing cytNmNAT1 alone. Similarly, in neurons expressing both dnMiro and cytNmNAT1, 63% of axons were severely degenerated after OGD, compared with only 2.5% in neurons with cytNmNAT1 alone (C & D right column). Axonal damage was assessed 6 h after hydrogen peroxide and 24 h after OGD. *** p< 0.001. Scale bar=30 μm.
We then used this dominant negative strategy to ask if inhibiting mitochondrial transport affects the ability of cytNmNAT1 to protect axons against reactive oxygen species and OGD (Figure 5C & D). After 7–8 days in vitro, we transfected dnMiro (or, as a control, soluble RPF) into neurons that had been electroporated with cytNmNAT1 at the time of plating, and then tested their response to these insults. In neurons that were not exposed to any insult, expressing dnMiro had no effect on axonal integrity, either in normal cells (data not shown) or in cells expressing cytNmNAT1 (Figure 5B). This result was somewhat surprising, but presumably the mitochondrial transport that remained (roughly 30–40% of normal) was sufficient for maintaining the axon under control conditions. Consistent with the results described earlier, the axons of neurons expressing cytNmNAT1 and soluble RFP showed little sign of degeneration when exposed to hydrogen peroxide or OGD (Figure 5 C & D left column). Their degeneration scores were not significantly different from those of neurons that were not subject to insult (compare Fig. 5 C & D right column and Fig. 1B). However, when dnMiro was expressed in neurons that also expressed cytNmNAT1, this greatly reduced the ability of cytNmNAT1 to protect axons against hydrogen peroxide and OGD (Fig 5 C & D middle column). In neurons expressing dnMiro and cytNmNAT1, more than 60% of axons exhibited severe degeneration (degeneration scores of 3 or 4) when exposed to these insults, compared with less than 20% of axons in cells expressing cytNmNAT1 alone (p < 0.001) (Figure 5 C & D right column). These data strongly indicate that the ability of cytNmNAT1 to protect axons against hydrogen peroxide and OGD requires that the mitochondrial transport system be intact.
The protective effect of mitochondrially targeted NmNAT also requires mitochondrial transport
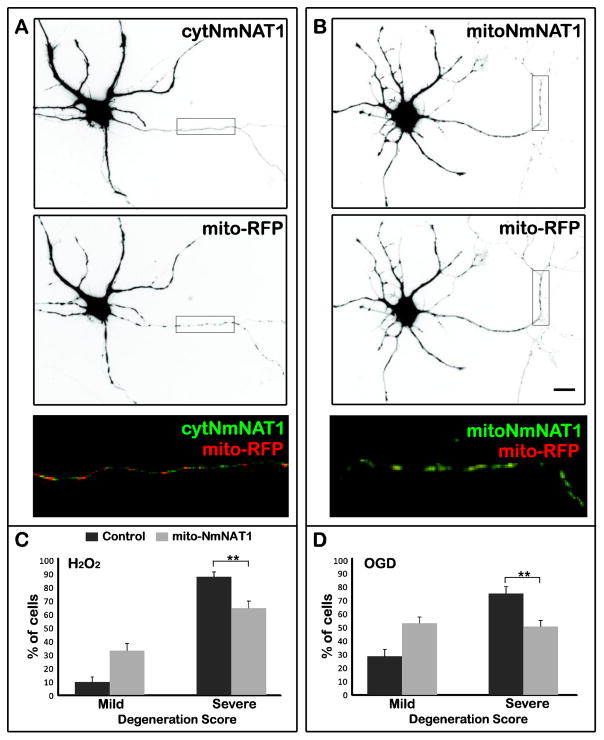
In Drosophila neurons, WldS enhances mitochondrial calcium buffering in axotomized axons, which may be crucial for its ability to prolong axonal viability (Avery et al., 2012), and overexpressing NmNAT3, which is localized to mitochondria, can protect mammalian neurons against some insults (Press and Milbrandt, 2008; Sasaki et al., 2006). These results might also explain our finding that mitochondrial transport is important for NmNAT’s protection against reactive oxygen species and OGD, leading us to ask whether mitochondrially targeted NmNAT could protect axons against these insults. Rather than adding the complication of comparing the effects of expressing two different NmNATs, we created a mitochondrially targeted form of NmNAT1 (mitoNmNAT1) by adding the mitochondrial matrix targeting signal from cytochrome C oxidase to the N-terminal of cytNmNAT1. Figure 6A shows that mitoNmNAT1 co-localizes with a mitochondrial marker, in contrast to cytNmNAT1, which is diffusely distributed throughout the axon. We then tested whether electroporation of mitoNmNAT1 prior to plating could protect axons against hydrogen peroxide or OGD. As shown in Figure 6C and D, this construct was quite effective in protecting axons against both insults. For example, in cultures exposed to hydrogen peroxide, 90% of axons from control cells were severely degenerated, compared with only 65% in neurons expressing mitoNmNAT1 (p < 0.01). mitoNmNAT1 appeared to be somewhat less effective than cytNmNAT1 in preventing axonal degeneration (compare Figures 1 and 6), but we do not know whether expression levels are similar for the two constructs.
Figure 6. Mitochondrially targeted NmNAT1 protects axons against damage due to hydrogen peroxide and OGD.
(A, B) cytNmNAT1-GFP was present throughout the axon, whereas mitoNmNAT1-GFP was present in elongated organelles that resemble mitochondria and that co-localized with a mitochondrial marker (mito-RFP). (C, D) Expressing mitoNmNAT1-GFP reduced axonal damage following exposure to hydrogen peroxide (100 μM for 1 h) and OGD (for 4 h). mitoNmNAT1-GFP was expressed by electroporation prior to plating. Cultures were exposed to insults after 7–8 days in vitro and axonal degeneration was evaluated 6 h after hydrogen peroxide exposure and 24 h after OGD. ** p< 0.01. Scale bar=30 μm.
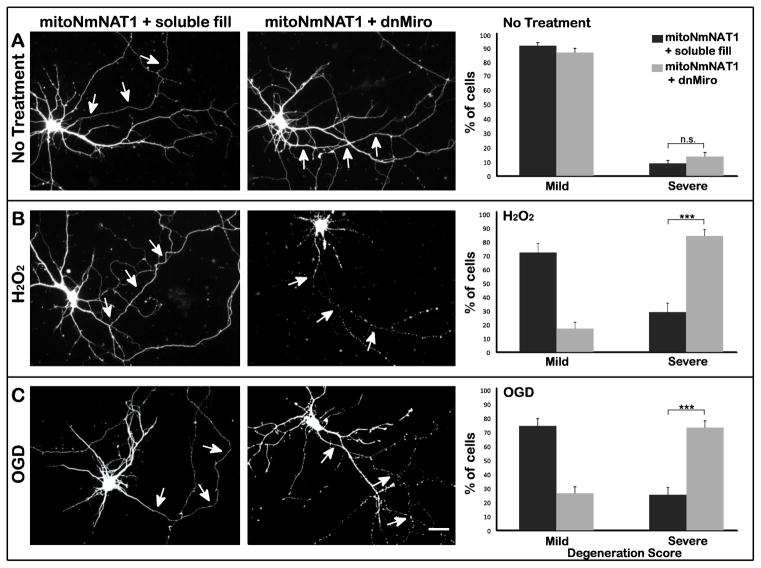
To determine whether mitochondrial transport is required for the protective effects of mitochondrially-targeted NmNAT, we transfected dnMiro-RFP into cells that expressed mitoNmNAT1-GFP (Figure 7). dnMiro abolished the protective effects of expressing mitoNmNAT following exposure to both hydrogen peroxide and OGD (Figure 7 B & C). dnMiro induced a significant shift in the percentage of neurons with severely damaged axons (score 3 & 4) following hydrogen peroxide exposure, from 28% to 83% (p < 0.001) and a shift from 24% to 73% after OGD (P<0.001) (Figure 7 B & C). These data highlight the importance of mitochondrial transport for NmNAT function, whether NmNAT is localized in the cytoplasm or in mitochondria.
Figure 7. Inhibition of mitochondrial transport abolishes the axon-protective effects of mitochondrially targeted NmNAT1.
(A) Neurons were electroporated with mitoNmNAT1-GFP before plating, and then were transfected with either dnMiro-RFP or RFP alone as a control (soluble fill) at DIV 7. Neurons were subjected to hydrogen peroxide (B) or OGD (C) 20h after transfection. In neurons expressing mitoNmNAT1 and a soluble fill, axons showed little damage after either insult (B & C left column). In contrast, the axons of cells expressing dnMiro were badly fragmented (B & C middle column). Quantification confirmed that without injury, expressing dnMiro did not affect axonal integrity (P=0.2) (A right column). When treated with hydrogen peroxide, 83% of the axons were severely degenerated (scores of 3 or 4) in neurons expressing dnMiro, compared with only 28% in neurons expressing mitoNmNAT1 (B right column). After OGD, 73% of the axons of cells expressing dnMiro were severely degenerated, compared with 25% of axons in neurons expressing mitoNmNAT1 (C right column). *** p < 0.001. Scale bar=30 μm.
Discussion
These results show that cytNmNAT1 protects cultured hippocampal neurons from axonal degeneration induced by two different insults, hydrogen peroxide and oxygen glucose deprivation. Expressing cytNmNAT1 also reduced hydrogen peroxide-induced transport inhibition and enhanced the recovery of axonal transport following both insults. Expression of a dominant negative construct that specifically inhibited mitochondrial transport interfered with cytNmNAT1 protection. Together, these results establish that NmNAT can protect axonal transport and conversely that mitochondrial transport is crucial for NmNAT protection.
NmNAT Reduces Inhibition of Axonal Transport
One key finding of this report is that expression of cytNmNAT1 protects the axonal transport machinery from inhibition due to both hydrogen peroxide and OGD, although the nature of the protection was slightly different for the two insults. Previously, we reported that axonal transport inhibition was one of the earliest signs of axonal damage after hydrogen peroxide insult (Fang et al., 2012a). Here we showed that inhibition of axonal transport is also an early consequence of oxygen-glucose deprivation. Expression of cytNmNAT1 reduced the inhibition of transport that occurred during hydrogen peroxide exposure and enhanced the recovery of transport after cells were returned to control medium. These effects were observed both for vesicle transport and mitochondrial transport. cytNmNAT1 did not prevent the inhibition of vesicle or mitochondrial transport that occurred when cells were exposed to OGD, but it significantly enhanced the recovery of transport when cells were returned to normal medium and oxygen levels. For cells exposed to either insult, expression of cytNmNAT1 allowed both vesicle and mitochondrial transport to recover to near-normal levels. Thus, protection of the axonal transport machinery may be an important factor in explaining the ability of NmNAT/WldS to protect axons against a wide variety of environmental insults. Deficits in axonal transport are also thought to contribute to the axonal degeneration that is a common component in many neurodegenerative diseases (Adalbert and Coleman, 2012; Coleman and Perry, 2002; Millecamps and Julien, 2013). Our demonstration that NmNAT1 protects against axonal transport inhibition offers a promising new direction for developing strategies to protect the transport machinery and hence confer axonal protection.
It is noteworthy that mitochondrial transport is more vulnerable to inhibition by both of these agents than is vesicular transport. This differential susceptibility has been noted previously in studies of oxidative stress (Fang et al., 2012a; Kim-Han et al., 2011). Since both mitochondria and vesicles are transported primarily by the same kinesins, the selective vulnerability of mitochondrial transport may reflect differences in how vesicle and mitochondrial transport is regulated (Wang and Schwarz, 2009) or differences in their reliance on oxidative versus glycolytic energy production (Zala et al., 2013).
How does cytNmNAT1 protect the axonal transport system against reactive oxygen species and OGD? There are several possibilities. It may be that transport inhibition arises from the calcium increases that occur following exposure to hydrogen peroxide and OGD (Barsukova et al., 2012; Nikic et al., 2011). High levels of cytoplasmic calcium inhibit mitochondrial transport by disrupting the interaction between Miro and the cytoplasmic adaptor protein Milton, which links mitochondria to kinesin motors (Macaskill et al., 2009; Wang and Schwarz, 2009). In addition, increases in calcium stimulate stress-activated kinases (Arundine and Tymianski, 2003; Ermak and Davies, 2002), which can phosphorylate kinesin motor domains (Morfini et al., 2013; Morfini et al., 2009) and kinesin adaptors (Arundine and Tymianski, 2003; Horiuchi et al., 2007; Morfini et al., 2002), thereby inhibiting transport. Mice carrying the WldS mutation and Drosophila over-expressing WldS protein or various NmNAT isoforms showed enhanced mitochondrial calcium buffering and reduced calcium increases following axotomy (Adalbert et al., 2012; Avery et al., 2012). A second possibility is that transport inhibition is due to the decrease in ATP levels that follows exposure to reactive oxygen species and OGD (De Cristobal et al., 2002; Karuppagounder et al., 2013), since the motors that mediate axonal transport are ATP-dependent. NmNAT1 catalyzes the synthesis of NAD, a key substrate in ATP production. Moreover, mitochondria from WldS mice have an enhanced ability to generate ATP (Yahata et al., 2009) and expression of the WldS protein or various forms of NmNAT in cultured neurons reduces the decline in ATP levels that occur following axotomy (Wang et al., 2005). On the other hand, Press and colleagues (2008) report that over-expressing NmNAT3 in DRG neurons does not reduce the ATP depletion that occurs following exposure to reactive oxygen species (Press and Milbrandt, 2008). Yet another possibility is that the protective effects of the WldS protein on axonal transport derive from its ability to prevent activation of Sarm (sterile alpha/armadillo/Toll-interleukin receptor homology domain protein). Sarm activation initiates a local apoptotic pathway in the axon (Osterloh et al., 2012). Whether Sarm is involved in the pathways of axonal degeneration induced by agents such as hydrogen peroxide or OGD is not yet known.
The Role of Mitochondria and Mitochondrial Transport in NmNAT Protection
There is emerging evidence that mitochondria play a key role in mediating many of the protective effects of WldS/NmNAT, although they do not participate in every instance of WldS protection (Avery et al., 2012; Court and Coleman, 2012; Fang et al., 2012b; Kitay et al., 2013). Mitochondria isolated from WldS mice mutation exhibit increased capacity for calcium buffering and in Drosophila neurons expressing the WldS protein a higher proportion of mitochondria undergo axonal transport (Avery et al., 2012). It is less clear whether WldS/NmNAT must be localized to mitochondria in order to exert its protective effects. Over-expressing NmNAT3, a mitochondrially localized homolog of NmNAT isoform, delays axonal degeneration in mouse and fly models and protects the axons of cultured neurons against rotenone toxicity (Avery et al., 2012; Press and Milbrandt, 2008; Yahata et al., 2009). On the other hand, other forms of NmNAT that are not targeted to mitochondria also exert protective effects in a variety of models (Babetto et al., 2010; Feng et al., 2010; Ljungberg et al., 2012; Yan et al., 2010). Our results show that expressing mitochondrially targeted NmNAT1 protects axons against OGD and reactive oxygen species, as does cytoplasmically targeted NmNAT1. Expressing a form of NmNAT1 that is membrane bound (via the KRas membrane targeting signal) also protects hippocampal axons against damage from reactive oxygen species and OGD (unpublished observation).
Whether or not the mitochondrial localization of WldS/NmNAT is responsible for axonal protection, it is becoming increasingly clear that the protection it confers requires microtubule-based transport of mitochondria. The ability of WldS to markedly prolong the survival of axotomized Drosophila neurons is reduced with mutations in the Milton gene, which cause a reduction in mitochondria motility (Avery et al., 2012). Here we show that the ability of cytNmNAT1 to protect the axons of cultured hippocampal neurons against damage following exposure to reactive oxygen species and OGD is blocked when mitochondrial transport is reduced by transiently expressing dominant negative Milton. The protective effects of mitochondrially targeted NmNAT1 also require mitochondrial transport. It is quite remarkable that in these two very different model systems (Drosophila olfactory neurons in situ and cultured rat hippocampal cells) subjected to three different kinds of axonal damage, the protective effects of WldS/NmNAT depend on mitochondrial transport.
It is not yet clear why mitochondrial transport is so important for NmNAT protection. In cultured hippocampal neurons, the density of axonal mitochondria is quite high. Mitochondria occupy about 40% of the length of the axon and the distance between adjacent mitochondria seldom is more than a few micrometers. Why then is mitochondrial transport critical for the protective effects of WldS/NmNAT? One possibility, as suggested by Avery et al. (2012), is that motile mitochondria traverse more “axonal space”, so that they are exposed to more calcium than stationary mitochondria (Avery et al., 2012). It is also possible that sites of calcium entry are quite localized and motile mitochondria can be more accurately positioned to calcium channel “hot spots” (Barsukova et al., 2012). Motility could also be required to facilitate docking between mitochondria and the endoplasmic reticulum, another factor that can be important for efficient mitochondrial calcium buffering (Kornmann, 2013). Decreased mitochondrial transport could also interfere with mitochondrial fission and fusion, which are important for maintaining normal mitochondrial functions (Chan, 2007; Chen and Chan, 2009; Detmer and Chan, 2007).
Taken together, our results show that exogenous NmNAT1, targeted either to the cytoplasm or to mitochondria, protects the axons of hippocampal neurons against damage from exposure to reactive oxygen species or OGD and reduces the axonal transport inhibition that these agents cause. Neurons express endogenous NmNAT1 as well as other NmNAT isoforms, but these are unable to prevent the inhibition of axonal transport, either because they are not present at high enough levels or because they do not reach the appropriate intracellular compartments. Given that mitochondrial transport is required for the protective effects of exogenously expressed NmNAT, it may be that the mitochondrial transport inhibition that occurs after axonal injury also interferes with the ability of endogenous NmNAT to protect against axonal damage. If so, identifying strategies to maintain axonal transport could enhance the protective effects of endogenous NmNATs and reduce the axonal damage that is characteristic of so many neural diseases.
Conclusions
This study shows that expressing a cytoplasmically targeted version of NmNAT1 (cytNmNAT1) robustly protects cultured rat hippocampal neurons against axonal degeneration caused by either exposure to hydrogen peroxide and oxygen-glucose deprivation. Both agents cause an inhibition of axonal transport, which occurs hours before any visible signs of axonal damage. Expressing cytNmNAT1 reduces the inhibition of vesicle and mitochondrial transport due to hydrogen peroxide and enhances the recovery of axonal transport following exposure to hydrogen peroxide or oxygen-glucose deprivation. Mitochondrial transport plays a critical role in the protective effects of cytNmNAT1. Selectively inhibiting mitochondrial transport by expressing a dominant-negative Miro construct abolishes the ability of cytNmNAT1 to protect axons against hydrogen peroxide or oxygen-glucose deprivation. Expressing a mitochondrially targeted form of NmNAT1 also protects axons against both insults and this protection is also abolished by inhibition of mitochondrial transport.
Supplementary Material
Highlights.
Cytoplasmically targeted NmNAT1 protected cultured hippocampal neurons from axonal degeneration following exposure to oxidative stress and oxygen-glucose deprivation.
Cytoplasmically targeted NmNAT1 reduced inhibition of axonal transport following exposure to oxidative stress or oxygen-glucose deprivation.
Mitochondrial transport was critical for the protective effects of NmNAT1.
Acknowledgments
This research was supported by the National Multiple Sclerosis Society (MS Center Grant CA 1055-A-3). Cheng Fang was supported by a postdoctoral fellowship from the National Multiple Sclerosis Society and Helena Decker was supported by a postdoctoral fellowship from the Multiple Sclerosis International Federation. Live-cell imaging was conducted in the Advanced Light Microscopy Core @ The Jungers Center, which is supported in part by NIH P30-NS06180 (S. Aicher, P.I.). We are grateful to Julie Luisi and Barbara Fisher Smoody for their expert technical assistance, to Dr. Marvin Bentley for helpful advice throughout the course of this work, and to Dr. Mary Logan for helpful comments on the manuscript.
Abbreviations
- WldS
Wallerian degeneration slowed protein
- cytNmNAT1
cytoplasmically targetred NmNAT1
- mitoNmNAT1
mitochondrially targeted NmNAT1
- OGD
oxygen-glucose deprivation
- dnMiro
dominant negative Miro
- GFP
green fluorescent protein
- RFP
red fluorescent protein
Footnotes
Conflict of Interest: The authors have declared that no competing interests exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adalbert R, Coleman MP. Axon pathology in age-related neurodegenerative disorders. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Gillingwater TH, Haley JE, Bridge K, Beirowski B, Berek L, Wagner D, Grumme D, Thomson D, Celik A, Addicks K, Ribchester RR, Coleman MP. A rat model of slow Wallerian degeneration (WldS) with improved preservation of neuromuscular synapses. The European journal of neuroscience. 2005;21:271–277. doi: 10.1111/j.1460-9568.2004.03833.x. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Morreale G, Paizs M, Conforti L, Walker SA, Roderick HL, Bootman MD, Siklos L, Coleman MP. Intra-axonal calcium changes after axotomy in wild-type and slow Wallerian degeneration axons. Neuroscience. 2012;225:44–54. doi: 10.1016/j.neuroscience.2012.08.056. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Avery MA, Rooney TM, Pandya JD, Wishart TM, Gillingwater TH, Geddes JW, Sullivan PG, Freeman MR. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E, Beirowski B, Janeckova L, Brown R, Gilley J, Thomson D, Ribchester RR, Coleman MP. Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J Neurosci. 2010;30:13291–13304. doi: 10.1523/JNEUROSCI.1189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Goslin K. Developments in neuronal cell culture. Nature. 1988;336:185–186. doi: 10.1038/336185a0. [DOI] [PubMed] [Google Scholar]
- Barsukova AG, Forte M, Bourdette D. Focal increases of axoplasmic Ca2+, aggregation of sodium-calcium exchanger, N-type Ca2+ channel, and actin define the sites of spheroids in axons undergoing oxidative stress. J Neurosci. 2012;32:12028–12037. doi: 10.1523/JNEUROSCI.0408-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland LG, Sahai E, Kelly G, Golding M, Greensmith L, Schiavo G. Deficits in axonal transport precede ALS symptoms in vivo. Proc Natl Acad Sci U S A. 2010;107:20523–20528. doi: 10.1073/pnas.1006869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C, O’Kane CJ, Reid E. Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nat Rev Neurosci. 2011;12:31–42. doi: 10.1038/nrn2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, Sonderegger P. Cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial dynamics in disease. N Engl J Med. 2007;356:1707–1709. doi: 10.1056/NEJMp078040. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, Brown MC, Lyon MF, Perry VH. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, V, Perry H. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- Court FA, Coleman MP. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012;35:364–372. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- De Cristobal J, Cardenas A, Lizasoain I, Leza JC, Fernandez-Tome P, Lorenzo P, Moro MA. Inhibition of glutamate release via recovery of ATP levels accounts for a neuroprotective effect of aspirin in rat cortical neurons exposed to oxygen-glucose deprivation. Stroke. 2002;33:261–267. doi: 10.1161/hs0102.101299. [DOI] [PubMed] [Google Scholar]
- Decker H, Lo KY, Unger SM, Ferreira ST, Silverman MA. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J Neurosci. 2010;30:9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Fang C, Bourdette D, Banker G. Oxidative stress inhibits axonal transport: implications for neurodegenerative diseases. Mol Neurodegener. 2012a;7:29. doi: 10.1186/1750-1326-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel Drosophila model of nerve injury reveals an essential role of nmnat in maintaining axonal integrity. Curr Biol. 2012b;22:590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Yan T, Zheng J, Ge X, Mu Y, Zhang Y, Wu D, Du JL, Zhai Q. Overexpression of Wld(S) or Nmnat2 in mauthner cells by single-cell electroporation delays axon degeneration in live zebrafish. J Neurosci Res. 2010;88:3319–3327. doi: 10.1002/jnr.22498. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Ingham CA, Parry KE, Wright AK, Haley JE, Wishart TM, Arbuthnott GW, Ribchester RR. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain: a journal of neurology. 2006;129:1546–1556. doi: 10.1093/brain/awl101. [DOI] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin KAH, Banker G. Rat hippocampal neurons in low density culture. Cambridge: 1998. [Google Scholar]
- Her LS, Goldstein LS. Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J Neurosci. 2008;28:13662–13672. doi: 10.1523/JNEUROSCI.4144-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur EL, Scherer SS. Microtubules, axonal transport, and neuropathy. N Engl J Med. 2011;365:2330–2332. doi: 10.1056/NEJMcibr1112481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–1317. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Novak AE, Elliott JP. Primary culture of cellular subtypes from postnatal mouse for in vitro studies of oxygen glucose deprivation. J Neurosci Methods. 2011;199:241–248. doi: 10.1016/j.jneumeth.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kaech S, Huang CF, Banker G. General considerations for live imaging of developing hippocampal neurons in culture. Cold Spring Harb Protoc. 2012a;2012:312–318. doi: 10.1101/pdb.ip068221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Huang CF, Banker G. Long-term time-lapse imaging of developing hippocampal neurons in culture. Cold Spring Harb Protoc. 2012b;2012:335–339. doi: 10.1101/pdb.prot068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Huang CF, Banker G. Short-term high-resolution imaging of developing hippocampal neurons in culture. Cold Spring Harb Protoc. 2012c;2012:340–343. doi: 10.1101/pdb.prot068247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Salm T, Glombik M, Almers W, Gerdes HH. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur J Cell Biol. 1997;74:133–142. [PubMed] [Google Scholar]
- Karuppagounder SS, Basso M, Sleiman SF, Ma TC, Speer RE, Smirnova NA, Gazaryan IG, Ratan RR. In vitro ischemia suppresses hypoxic induction of hypoxia-inducible factor-1alpha by inhibition of synthesis and not enhanced degradation. J Neurosci Res. 2013;91:1066–1075. doi: 10.1002/jnr.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Han JS, Antenor-Dorsey JA, O’Malley KL. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLDS-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed]
- Kornmann B. The molecular hug between the ER and the mitochondria. Curr Opin Cell Biol. 2013 doi: 10.1016/j.ceb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Ali YO, Zhu J, Wu CS, Oka K, Zhai RG, Lu HC. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 2012;21:251–267. doi: 10.1093/hmg/ddr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, Grumme D, Osaka H, Conforti L, Arnhold S, Addicks K, Wada K, Ribchester RR, Coleman MP. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain: a journal of neurology. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, Tavassoli E, Bagnato C, Tiwari A, Hayward LJ, Pigino GF, Watterson DM, Huang CF, Banker G, Brown RH, Jr, Brady ST. Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS One. 2013;8:e65235. doi: 10.1371/journal.pone.0065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131:1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D, Misgeld T, Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995;5:635–642. doi: 10.1016/s0960-9822(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Milbrandt J. Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem. 2010;285:41211–41215. doi: 10.1074/jbc.C110.193904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara Y, Hollenbeck PJ. Defects in mitochondrial axonal transport and membrane potential without increased reactive oxygen species production in a Drosophila model of Friedreich ataxia. J Neurosci. 2010;30:11369–11378. doi: 10.1523/JNEUROSCI.0529-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagi M, Dittrich PS, Frank N, Iliev AI, Schwille P, Neumann H. Breakdown of axonal synaptic vesicle precursor transport by microglial nitric oxide. J Neurosci. 2005;25:352–362. doi: 10.1523/JNEUROSCI.3887-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Araki T. Wallerian degeneration slow mouse neurons are protected against cell death caused by mechanisms involving mitochondrial electron transport dysfunction. Journal of neuroscience research. 2012;90:664–671. doi: 10.1002/jnr.22792. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. The Journal of cell biology. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Annals of neurology. 2002;52:442–447. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Feng Y, Zheng J, Ge X, Zhang Y, Wu D, Zhao J, Zhai Q. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.