Abstract
Background and Objective:
Tissue effects of vascular lesion laser treatment are incompletely understood. Injury caused by pulsed dye laser (PDL) treatment may result in altered expression of mediators associated with angiogenesis.
Materials and Methods:
Eight human subjects had one angioma treated with PDL (7 mm, 1.5 millisecond pulse duration, 9 J/cm2, cryogen spray cooling of 30 millisecond with a 30 millisecond delay). One week later, three biopsies were taken: normal skin, untreated angioma, angioma post-PDL. Tissue was frozen and sections processed for immunohistochemistry staining of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), matrix metalloproteinase 9 (MMP-9), and angiopoietin 2 (ANG-2). Images were graded in a blinded fashion by a board certified dermatopathologist.
Results:
There were no clear trends in VEGF expression in the epidermis, dermis, or endothelial cells. As compared to normal skin, angiomas demonstrated the following: bFGF was decreased in the epidermis; MMP-9 was decreased or unchanged in the epidermis and increased in the endothelial cells; ANG-2 was increased in the endothelial cells. When comparing normal skin to angiomas + PDL, bFGF was decreased in the epidermis and increased in the dermis; MMP-9 was decreased or unchanged in the epidermis; ANG-2 was again increased in the endothelial cells. Comparison of staining in angioma to angioma + PDL samples revealed increased dermal bFGF expression.
Conclusion:
Alterations in angiogenesis mediators were noted after PDL. Angiogenesis mediator changes associated with PDL treatment differed from those previously reported for incisional biopsies. This pilot study can guide future work on laser-induced alterations in vascular lesions and such information may ultimately be used to optimize treatment outcomes. Lasers Surg. Med. 44:205–210, 2012.
Keywords: angiopoietin (ANG), basic fibroblast growth factor (bFGF), matrix metalloproteinase (MMP), vascular endothelial growth factor (VEGF), vascular malformation
INTRODUCTION
The flash-lamp pumped pulsed dye laser (PDL) has been used for decades to safely and effectively treat a wide range of cutaneous vascular lesions including angiomas, telangiectasia, and vascular birthmarks [1]. The theory of selective photothermolysis describes the general mechanism by which the PDL damages vessels and allows lesion removal: selective absorption of a tissue chromophore, such as the hemoglobin in vessels, results in damage to the targeted vessel wall, clot formation and subsequent vessel removal [2]. However, other tissue effects of PDL devices are incompletely understood.
Angiogenesis is an important process in normal physiology, as well as disease pathogenesis. In a healthy body, it is controlled by a balance between angiogenic growth factors and inhibitors. An imbalance of these factors can lead to unwanted vessel formation and cutaneous disease including vascular malformations and tumors [3,4]. Our group and others have hypothesized that laser therapy of vascular lesions stimulates production of angiogenesis growth factors, promoting recurrence of targeted vascular lesions and limiting treatment effects. As such, post-treatment angiogenesis could be one explanation for incomplete lesion removal and/or recurrence, each of which is often seen post-PDL.
The presence of angiogenesis mediators following PDL has not been evaluated. However, there is evidence that light based treatment can stimulate angiogenesis. Solban et al. [5] demonstrated an increase in the synthesis and secretion of VEGF following subcurative photodynamic therapy (PDT) in prostate cancer.
The objective of this pilot project is to take the first step towards understanding the relationship between angiogenesis and laser therapy. Our ultimate goal is to use such knowledge to develop improved, targeted therapy regimens combining laser and anti-angiogenic agents. In this study, we determine the presence of specific angiogenesis mediators in angiomas before, and 7 days after, PDL. We chose to study angiomas, as they are common and easily treated with laser therapy. Previous literature demonstrated a peak in vascular endothelial growth factor (VEGF) production at 7 days post-incisional injury [6]. This prompted us to select the 7-day point for evaluation. Based on previous literature [3,4,6,7], four factors were selected for study: VEGF, basic fibroblast growth factor (bFGF), matrix metalloproteinase 9 (MMP-9), and angiopoietin 2 (ANG-2).
VEGF is thought to be the most potent and predominant angiogenesis stimulator. VEGF binds tyrosine kinase receptors in endothelial cells, thereby (1) increasing endothelial cell migration, endothelial cell mitoses, and vascular permeability, (2) promoting chemotaxis for granulocytes and macrophages, and (3) indirectly promoting vasodilatation via nitric oxide release. bFGF is produced by keratinocytes and acts as a mitogen for endothelial cells and keratinocytes. It increases both epithelialization and capillary infiltration in dermal wound healing. MMPs are enzymes that digest components of the extracellular matrix to permit the formation of new blood vessels. Several MMPs have been linked to angiogenesis, most notably MMP-9. ANG, like VEGF, binds to tyrosine kinase receptors in endothelial cells. ANG-2 has been reported to contribute both to angiogenesis and vascular regression of angiogenesis depending on the presence or absence of VEGF [4].
MATERIALS AND METHODS
The study was approved by the Investigational Review Board at University of California, Irvine. In this initial pilot report, eight human subjects with multiple cherry angiomas participated. Verbal and written informed consent was obtained for all subjects. Each subject had one cherry angioma treated with PDL (7 mm; 1.5 millisecond; 9 J/cm2; 30 millisecond of cryogen with a 30 millisecond delay). The body sites were always on the trunk, including the abdomen, back, and chest. At day 7, each subject had three biopsies performed on normal skin, an untreated angioma (angioma), and the angioma treated with PDL (angioma + PDL). The normal skin was in the same area but several centimeters away from the two biopsied angiomas (one treated and one untreated). The two angiomas were also in a similar area but several centimeters away from each other.
Tissue was frozen and sections processed for immunohistochemistry staining. A total of 24 tissue samples were processed. Sections were stained with the mouse monoclonal antibody to VEGF (VF-1; Abcam, Cambridge, MA, detects isoforms 121, 165, 189), rabbit polyclonal antibody to bFGF (Anti-FGF basic antibody; Abcam), rabbit polyclonal antibody to MMP-9 (anti-MMP9 antibody – whole molecule; Abcam), and mouse monoclonal antibody to ANG-2 (ANGPT2; Novus, Littleton, CO). A customized protocol was followed for antibody application, adapted from the standard protocol recommended by Abcam®. Tissue obtained via 3 mm punch biopsy was submersed in phosphate-buffered sucrose until the tissue became saturated and sank to the bottom of the test tube (approximately 1 hour), then embedded in Tissue Tek* Optimal Cutting Temperature (OCT) compound (Fisher Scientific, Pittsburgh, PA) and frozen in liquid nitrogen. Samples were stored at −80°C and never allowed to reach above −15°C. Ten micron sections were cut and placed onto glass slides. Slides were washed with phosphate-buffer saline (1× PBS) and fixed in a 1:1 mixture of ice-cold methanol and acetone.
Slides were incubated for 1 hour with the appropriate primary blocking agent: goat serum for VEGF and ANG-2, and donkey serum bFGF and MMP-9 (Jackson ImmunoResearch, 005-000-121 and 017-000-121, concentration of 6 mg/ml). Following a 5-minute rinse in PBS, the slides were incubated overnight with the angiogenic antibodies in varying concentrations (VEGF at 0.0025 mg/ml, bFGF at 0.005 mg/ml, MMP-9 at 0.0025 mg/ml, and ANG-2 at 0.001 mg/ml). The antibody solution was rinsed off with PBS. The secondary antibody was applied and the sections were incubated in a dark humidified chamber for 1 hour: Alexa Fluor® 488 goat anti-mouse IgG (H + L) was used for VEGF and ANG-2, and Alexa Fluor® 594 donkey anti-rabbit IgG (H + L) was used for bFGF and MMP-9 (Invitrogen, A-11001 and A-21207, concentration of 0.005 mg/ml). The slides were then counterstained with 300 nM DAPI (Invitrogen, D3571) for 5 minutes. Slides were mounted in fluoro-gel (Electron Microscopy Sciences, Hatfield, PA) and stored in a light-free container at 4°C. Imaging was performed using a Leica DM4000B fluorescence microscope (Leica Microsystems, Inc., Buffalo Grove, IL), SPOT camera, and computer program (RTKE Diagnostic Instruments, Inc., Sterling Heights, MI). Fluorescent light intensity and camera exposure time were optimized over several representative samples and then standardized across samples. The digital images were captured using single band fluorescence. For each sample, antibody staining and DAPI staining images were merged to capture dual staining using SPOT imaging software. Staining was uniform throughout the tissue and one or two representative images were taken from each section which captured epithelium, endothelium, and dermis as well as tissue integrity. Each image was saved in a 24-bit RGB TIFF file format.
Resulting images were blinded and graded by a board certified dermatopathologist, who evaluated the specimens’ degrees of staining for each of the four antibodies in the epidermis, dermis, and endothelial cells. The sections were graded according to the proportion of cells stained and classified into one of four categories: 0%, <10%, 10–50%, >50%. A total of 192 images were included for analysis (24 tissue samples were stained with 4 different antibodies and negative controls were included for each section).
RESULTS
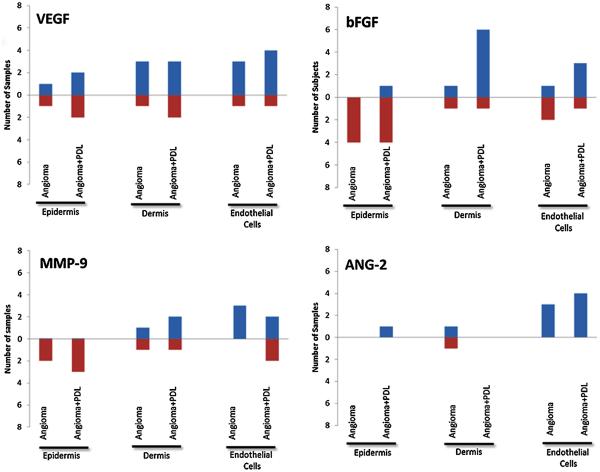
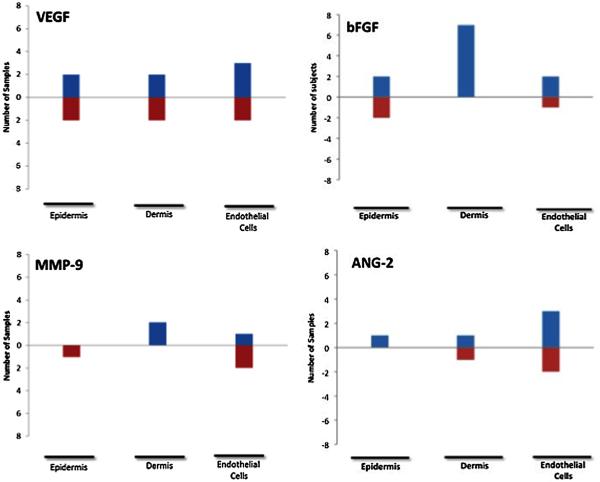
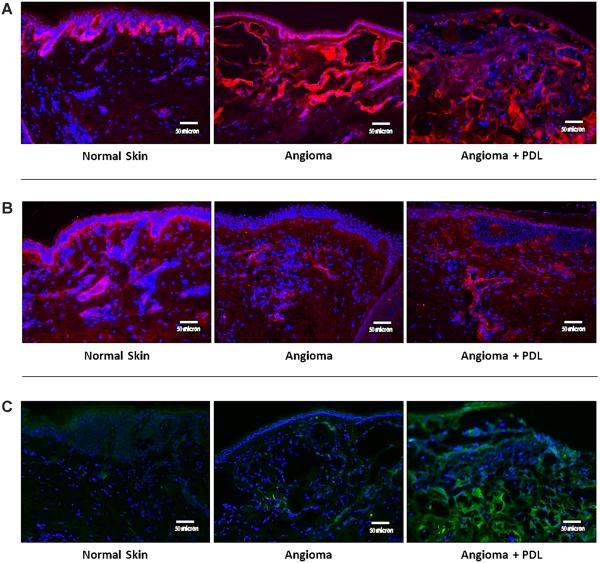
Figure 1 shows the number of samples with a positive or negative change for each measured factor when comparing normal skin to angiomas and angiomas + PDL. There were no clear trends in VEGF expression in the epidermis, dermis, or endothelial cells. As compared to normal skin, angiomas demonstrated the following: bFGF was decreased in the epidermis; MMP-9 was decreased or unchanged in the epidermis and increased in the endothelial cells; ANG-2 was increased in the endothelial cells. When comparing normal skin to angiomas + PDL, bFGF was decreased in the epidermis and increased in the dermis; MMP-9 was decreased or unchanged in the epidermis; ANG-2 was again increased in the endothelial cells. Comparison of staining in angiomas to angiomas + PDL samples (Fig. 2) revealed increased dermal bFGF expression. Figure 3 shows representative immunofluorescent images that illustrate our results.
Fig. 1.
The number of samples with a positive or negative change for each measured factor when comparing normal skin to angioma and angioma + PDL. Note: Total number of subjects is not 8 as there was no difference in compared conditions for some subjects.
Fig. 2.
The number of samples with a positive or negative change for each measured factor when comparing angioma + PDL to untreated angioma. Note: Total number of subjects is not 8 as some subjects demonstrated no difference in compared conditions.
Fig. 3.
A: Representative images of bFGF. Immunofluorescence of bFGF is represented by red color. As compared to normal skin, both angiomas and angiomas + PDL demonstrated decreased bFGF staining in the epidermis but angiomas + PDL alone showed an increase in the dermis. When comparing angiomas to angioma + PDL, there was a clear dermal increase of bFGF expression. B: Representative images of MMP-9. Immunofluorescence of MMP-9 is represented by red color. As compared to normal skin, both angiomas and angiomas + PDL showed decreased or unchanged expression in the epidermis but angiomas alone had increased expression in the endothelial cells. C: Representative images of ANG-2. Immunofluorescence of ANG-2 is represented by green color. ANG-2 was increased in the endothelial cells of angiomas and angiomas + PDL when compared with normal skin.
DISCUSSION
We evaluated the expression of four angiogenic factors at one specific time point (7 days) after PDL treatment of angiomas. Although VEGF is the most widely described and studied angiogenic factor, our data did not show any consistent changes between normal skin compared to angioma nor angioma as compared to angioma + PDL. Perhaps VEGF is not the angiogenic mediator to focus on if the goal is optimization of vascular lesion removal. This is particularly interesting as previous studies demonstrated changes in VEGF after incisional wound injuries [6,7]. Hayashi et al. [6] performed serial immunohistochemistry evaluation of human skin wounds, measuring VEGF expression as a variable of time (group I (0–12 hours); II (1–4 days); III (7–14 days); and IV (17–21 days)). They noted the greatest increase in VEGF-positive cells in group III followed by group IV [6]. Kumar et al. [7] studied VEGF expression in surgical wounds 3 days and 2 years after injury, and noted an increase at both time points.
There were trends observed with the other three factors when comparing normal skin, angiomas, and angiomas + PDL. This strongly suggests that there is a difference in angiogenic factor expression with vascular pathology as well as a change in this dynamic following light-based therapy.
Staining patterns varied somewhat between subjects. Several reasons may account for this variability. Our results are from eight patients; a larger sample size may reveal more consistent trends. Also, although great caution was taken to keep all conditions consistent among samples, there may have been some slight variability in the protocol due to the inherent complex nature of staining protocols. Alternatively or additionally, while the angiomas appeared clinically similar, there may be differences in factor expression in these lesions. It is important to note that such lesion differences may be even more significant in the context of larger more difficult to remove lesions such as port wine stain birthmarks. These lesions will be studied in future research work which may elucidate whether similar angiogenesis factor expression occurs following PDL of all lesions or if expression is lesion specific.
This was a pilot study and at the start of the work, there was no literature to guide hypothesis based testing predicting changes for the studied factors. As such, we report trends observed in this first of a kind evaluation. This work can be used to guide testing in the future, which will more fully evaluate mediator expression following PDL in studies with larger sample sizes and detailed statistical analysis. It is also important to note that we evaluated PDL in one type of lesion (angiomas), at one time point (1 week) and using one set of treatment parameters (7 mm spot, 1.5 millisecond; 9 J/cm2) that were chosen because they are a reasonable setting for treatment of these lesions. Decisions had to be made to make evaluation manageable for a pilot study. However, an infinite number of further evaluations are possible looking at different lesions, different time points, and different parameters. It is entirely possible and perhaps likely that mediator expression will change with each of these variables. For example, PDL treatment of port wine stain birthmarks may result in an increase in VEGF as we recently found in a single case study (unpublished data KMK).
In this manuscript, we take the first step towards designing a targeted, combined laser and anti-angiogenic agent therapy. Novel treatment approaches are needed because, while some cutaneous vascular lesions are easily removed, others persist after many treatments, or recur after removal [8–12]. Combining laser and anti-angiogenic agent therapy may enhance efficacy of cutaneous vascular lesion removal, greatly improving treatment outcomes.
It has been observed that the effect of an anti-angiogenic agent is most impressive when adjuvant methods are used to achieve initial vascular destruction [13]. Given the ability of light based therapies to selectively target tissue vasculature, a therapeutic combination of light-based treatments with anti-angiogenic agents is a natural pairing. Preliminary literature supports the theory that light-based therapies in combination with anti-angiogenic agents can provide an enhanced therapeutic option. Augustin and Offermann reported improved vision after only one treatment in patients with choroidal neovascularisation when verteporfin photodynamic therapy, anti-VEGF therapy and anti-inflammatory therapy were combined [14]. This contrasts to the use of single agent therapies, where multiple interventions or prolonged administration of medications were required. Kosharskyy et al. [15] reported enhanced efficacy of PDT followed by administration of an anti-angiogenic agent, TNP-470 as compared to PDT alone for treatment of prostate cancer in an animal model. They concluded that combining destructive and anti-angiogenic therapies can improve treatment outcomes for cancer and this approach warranted further investigation. A recent randomized prospective study by our group demonstrated enhanced PWS lightening with PDL in combination with imiquimod (an agent with anti-angiogenic properties) compared to PDL alone [16]. Chang et al. [17] similarly evaluated 20 Asian patients with port-wine stains and found that the combined use of PDT with imiquimod cream led to superior blanching responses compared to either treatment alone. Topical rapamycin has been evaluated in animal models and normal human [18]. Other macrolides inhibiting the mTOR pathway have also been evaluated including temsirolimus [19].
In order to develop a successful combined treatment protocol, it will be important to know which angiogenesis mediators are present in cutaneous vascular lesions and which are altered post-laser therapy. Our data did indicate an alteration in angiogenesis mediators after PDL and will foster future work on this topic.
In conclusion, alterations in angiogenesis mediators were noted after PDL. Angiogenesis mediator changes associated with PDL treatment differed from those previously reported for incisional biopsies. This pilot study can guide future work on laser-induced alterations in vascular lesions and such information may ultimately be used to optimize treatment outcomes.
ACKNOWLEDGMENTS
The American Society for Laser Medicine and Surgery provided research funds which contributed to this work (KMK, VL, TC). We would like to acknowledge and deeply thank Danielle R. Coles and Donald R. Ferris for their expertise and technical assistance.
Footnotes
Conflict of interest: Dr. Kristen Kelly received a grant from the ASLMS to partially fund this research.
REFERENCES
- 1.Vallee J, Kelly K, Rohrer T, Arndt K, Dover J. Lasers in the treatment of vascular lesions. In: Kaminer M, Arndt K, Dover J, Rohrer T, Zachary C, editors. Atlas of cosmetic surgery. Saunders; Philadelphia, PA: 2009. pp. 135–152. [Google Scholar]
- 2.Anderson RR, Parrish JA. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 3.Laquer V, Hoang V, Nguyen A, Kelly KM. Angiogenesis in cutaneous disease: Part II. J Am Acad Dermatol. 2009;61(6):945–958. doi: 10.1016/j.jaad.2009.05.053. quiz 959–960. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen A, Hoang V, Laquer V, Kelly KM. Angiogenesis in cutaneous disease: Part I. J Am Acad Dermatol. 2009;61(6):921–942. doi: 10.1016/j.jaad.2009.05.052. quiz 943–924. [DOI] [PubMed] [Google Scholar]
- 5.Solban N, Selbo PK, Sinha AK, Chang SK, Hasan T. Mechanistic investigation and implications of photodynamic therapy induction of vascular endothelial growth factor in prostate cancer. Cancer Res. 2006;66(11):5633–5640. doi: 10.1158/0008-5472.CAN-06-0604. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T. Forensic application of VEGF expression to skin wound age determination. Int J Legal Med. 2004;118(6):320–325. doi: 10.1007/s00414-004-0468-x. [DOI] [PubMed] [Google Scholar]
- 7.Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg. 2009;96(12):1484–1491. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- 8.Kelly KM, Choi B, McFarlane S, Motosue A, Jung B, Khan MH, Ramirez-San-Juan JC, Nelson JS. Description and analysis of treatments for port-wine stain birthmarks. Arch Facial Plast Surg. 2005;7(5):287–294. doi: 10.1001/archfaci.7.5.287. [DOI] [PubMed] [Google Scholar]
- 9.Kono T, Sakurai H, Groff WF, Chan HH, Takeuchi M, Yamaki T, Soejima K, Nozaki M. Comparison study of a traditional pulsed dye laser versus a long-pulsed dye laser in the treatment of early childhood hemangiomas. Lasers Surg Med. 2006;38(2):112–115. doi: 10.1002/lsm.20257. [DOI] [PubMed] [Google Scholar]
- 10.Smit JM, Bauland CG, Wijnberg DS, Spauwen PH. Pulsed dye laser treatment, a review of indications and outcome based on published trials. Br J Plast Surg. 2005;58(7):981–987. doi: 10.1016/j.bjps.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 11.Papadavid E, Markey A, Bellaney G, Walker NP. Carbon dioxide and pulsed dye laser treatment of angiofibromas in 29 patients with tuberous sclerosis. Br J Dermatol. 2002;147(2):337–342. doi: 10.1046/j.1365-2133.2002.04968.x. [DOI] [PubMed] [Google Scholar]
- 12.Paller AS. Responses to anti-angiogenic therapies. J Investig Dermatol Symp Proc. 2000;5(1):83–86. doi: 10.1046/j.1087-0024.2000.00011.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall J. The role of bevacizumab as first-line therapy for colon cancer. Semin Oncol. 2005;32(6 Suppl 9):S43–S47. doi: 10.1053/j.seminoncol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Augustin AJ, Offermann I. Combination therapy for choroidal neovascularisation. Drugs Aging. 2007;24(12):979–990. doi: 10.2165/00002512-200724120-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kosharskyy B, Solban N, Chang SK, Rizvi I, Chang Y, Hasan T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Res. 2006;66(22):10953–10958. doi: 10.1158/0008-5472.CAN-06-1793. [DOI] [PubMed] [Google Scholar]
- 16.Tremaine A, Ortiz A, Armstrong J, Huang YC, Elkeeb L, Choi B, Kelly KM. Abstracts of the American Society for Laser Medicine and Surgery Twenty-Ninth Annual Conference; Apr 1–5, 2009. [DOI] [PubMed] [Google Scholar]; Lasers Surg Med. 2009;21(41 Suppl):22–23. Combined therapy for enhanced microvascular destruction in port wine stains: Pulsed dye laser photothermolysis and imiquimod. [Google Scholar]
- 17.Chang CJ, Hsiao YC, Mihm MC, Jr., Nelson JS. Pilot study examining the combined use of pulsed dye laser and topical imiquimod versus laser alone for treatment of port wine stain birthmarks. Lasers Surg Med. 2008;40(9):605–610. doi: 10.1002/lsm.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia W, Sun V, Tran N, Choi B, Liu SW, Mihm MC, Jr., Phung TL, Nelson JS. Long-term blood vessel removal with combined laser and topical rapamycin antiangiogenic therapy: Implications for effective port wine stain treatment. Lasers Surg Med. 2010;42(2):105–112. doi: 10.1002/lsm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia W, Sun V, Liu T, Choi B, Nelson JS. Abstracts of the American Society for Laser Medicine and Surgery Thirty-First Annual Conference.Apr 1–3, 2011. [Google Scholar]; Lasers Surg Med. 2011;23(43 Suppl):910. Comparison of antiangiogenic agents for inhibiting reperfusion of photocoagulated blood vessels in an animal model. [Google Scholar]