Abstract
Epithelial differentiation requires a balancing act to commit to epithelial fate while inhibiting both progenitor and mesenchymal traits. In this issue of Developmental Cell, Lee et al.(2014)and Watanabe et al.(2014) uncover a critical role of Ovol proteins in safeguarding this intricate process.
Epithelial cells hold remarkable plasticity: they are able to self-renew, expand, and differentiate during embryonic development, tissue maintenance, and disease pathogenesis. It is also well documented that epithelial cells can lose defining epithelial features and transition into a mesenchymal state by activating the Epithelial-Mesenchymal Transition (EMT) program. Although the upstream inducing signals vary in different tissues, execution of the EMT program is orchestrated by a group of EMT core transcription factors, including the Snail, Twist, and Zeb family proteins(Yang and Weinberg, 2008). This program is essential for many embryonic developmental events during which epithelial cells give rise to migratory mesenchymal descendants. Surprisingly, two studies in the current issue of Developmental Cell reveal that the simmering EMT program in epithelial tissues needs to be actively repressed to ensure normal epithelial differentiation (Lee et al.,2014; Watanabe et al.,2014).
These two studies examined epithelial differentiation in two of the most well-characterized epithelia, the epidermis and the female mammary gland. Developmentally, the multilayered epidermis is derived from a single-layered surface ectoderm with intact epithelial junctions and classical polarized epithelial morphology. During epidermal differentiation, the basal cells delaminate, migrate, and differentiate while maintaining their progenitor cell pool. The crucial epithelial junction protein, E-cadherin, and its cytoplasmic partner β-catenin are indispensible during this process. Additionally, EMT regulators such as Snail1 (Jamora et al., 2004), Snail2 (Shirley et al., 2010), and Twist2 (Šošić et al., 2003) are present at the epidermis and disruption of their expression individually results in abnormalities in epidermis development. The mammary gland, which consists of epithelial luminal cells lined with myoepithelial cells in the structure of a ductal tree, first forms prenatally, then undergoes branching expansion during puberty and pregnancy. The growth of the mammary ductal tree during puberty is achieved by secondary branching from the primary ducts and bifurication of the terminal end buds (TEBs), small acinar-like structures at the tip of the mammary ducts. The differentiating TEBs elongate the mammary ducts, invade the mammary fat pad, and continue to proliferate to form alveolar structures during pregnancy. Similar to the epidermis, the EMT regulator Snail2 (Guo et al., 2012) was also found to play an important role in regulating mammary stem cell states.
In the current issue of Developmental Cell, Lee et al.(2014) and Watanabe et al.(2014) reveal the critical role of two Ovol family transcription factors, Ovol1 and Ovol2, in safeguarding the lineage specificity of epithelial cells against the mesenchymal state in the embryonic epidermis and the mammary gland. Ovol1 and Ovol2 are zinc-finger transcription factors that function downstream of key developmental pathways such as Wnt, EGF, and BMP. Although deletion of Ovol proteins in mice results in various epithelial anomalies(Dai et al., 1998), their cellular functionsin epithelial development are largely unknown.
Lee et al. (2014) found that in the embryonic epidermis, depletion of Ovol1 and Ovol2 triggered expansion of the epidermal basal compartment, where the skin progenitor cells reside. Meanwhile, Watanabe et al. (2014) showed compelling data that upon deletion of Ovol2 in the mammary gland, branching morphogenesis during puberty was impaired, as demonstrated by severe underdevelopment of the mammary ductal trees. Mechanistically, both studies showed that deletion of Ovol proteins led to EMT in the epithelial tissues, characterized by increased number of vimentin-positive cells and overall enrichment of EMT-related gene signatures. Using immortalized mouse mammary cells, Watanabe et al (2014) showed that Ovol2 functions as a master suppressor of almost all known EMT-inducing transcription factorsby directly binding to their promoters. Zeb1 was revealed to be a key downstream target of Ovol in both epithelial systems, as Zeb1 depletion alone was able to rescue the differentiation defects stemming from depletion of Ovol proteins in both the epidermis and the mammary duct. These studies highlight the importance of Ovol proteins in suppressing mesenchymal traits during epithelial differentiation and add new players to the already complex epithelial differentiation program.
In addition to Ovol proteins, a previous study showed that the transcription factor Elf5 appears to suppress EMT during mammary gland alveologenesis by suppressing Snail2 transcription (Chakrabarti et al., 2012a). Deletion of Elf5 leads to enrichment of vimentin-positive cells and K8/K14 double positive progenitor cellsin the breast (Chakrabarti et al., 2012a; Chakrabarti et al., 2012b). Together with the two studies in this issue of Developmental Cell, these observations suggest an interesting model for epithelial differentiation in which the differentiating epithelial cells require an active gatekeeper, such as the Ovol proteins, to prevent mesenchymal transdifferentiation and maintain their epithelial identity (Figure 1). This model raises the question as to why committed epithelial cell lineage retains the capacity to undergo EMT. One plausible clue is that in both the epidermis and mammary TEBs, migration of the epithelial progenitor cells is critical for their proper differentiation. Is it possible that the EMT program is transiently and/or partially turned on to fulfill such migratory need of the progenitor cells? If so, how do these mesenchymal-like cells relate to the epithelial progenitor cells? These questions may be addressed through cellular and molecular characterization of such cells in vivo. In the mammary gland, deletion of Ovol2 results in EMT in TEBs during puberty. In contrast, deletion of Elf5 only leads to EMT in alveoli during pregnancy. Does this suggest that different EMT suppressors operate at different stages of mammary epithelial differentiation, and if so, is there cooperation between these processes? Further studies are needed to clarifythese newly raised questions.
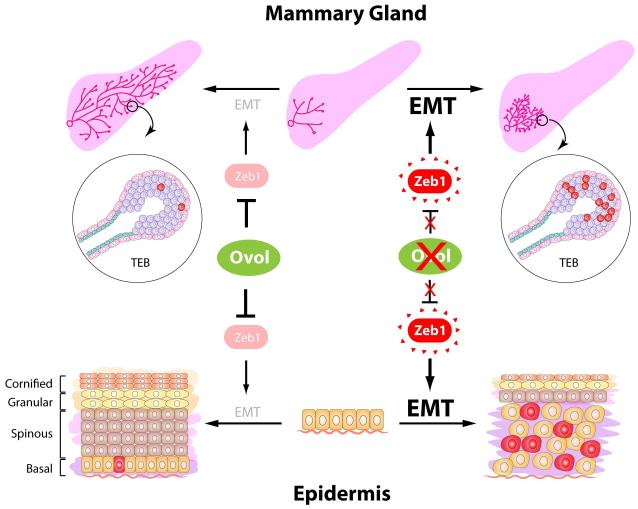
Figure 1. Ovol proteins guard epithelial differentiation by suppressing EMT.
During normal development (left side of the figure) of the mammary gland and epidermis in mice, Ovol transcription factors suppress the expression of Zeb1 and the EMT program to safeguard epithelial identity during differentiation. Deletion of the Ovol proteins (right side of the figure) causes excessive activation of the EMT program mainly through the up-regulation of Zeb1 expression. Increased numbers of vimentin positive cells (labeled in red) were evident in both the mammary TEB and epidermal basal cells. This misactivated EMT program interferes with proper epithelial differentiation and results in defects in mammary duct elongation and expansion of the epidermal basal compartment.
Lastly, it bears noting that the EMT program is critical for breast cancer metastasis. In light of this,Watanabe et al. (2014) touched upon the possible involvement of Ovol2 in breast cancer progression and showed that low Ovol2 expression is significantly correlated with poor survival in patients. Given the dynamic involvement of the EMT/MET program at different steps of metastasis (Tsai and Yang, 2013), further examination of Ovol proteins in mouse tumor models and patient samples will be needed to fully understand their biological significance in metastatic breast cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukačišin M, Romano R-A, Smalley K, Liu S, Yang Q, Ibrahim T, et al. Nat Cell Biol. 2012a;14:1212–1222. doi: 10.1038/ncb2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R, Wei Y, Romano R-A, DeCoste C, Kang Y, Sinha S. STEM CELLS. 2012b;30:1496–1508. doi: 10.1002/stem.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Schonbaum C, Degenstein L, Bai W, Mahowald A, Fuchs E. Genes & Development. 1998;12:3452–3463. doi: 10.1101/gad.12.21.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Keckesova Z, Donaher Joana L., Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zürrer-Härdi U, Bell G, et al. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Lee P, Kocieniewski P, Azhar M, Hosokawa R, Chai Y, Fuchs E. PLoS Biol. 2004;3:e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Villarreal-Ponce A, Fallahi M, Ovadia J, Sun P, Yu Q-C, Ito S, Sinha S, Nie Q, Dai X. Developmental Cell. 2014 doi: 10.1016/j.devcel.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley SH, Hudson LG, He J, Kusewitt DF. Molecular Carcinogenesis. 2010;49:851–861. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šošić D, Richardson JA, Yu K, Ornitz DM, Olson EN. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Yang J. Genes & Development. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Villarreal-Ponce A, Sun P, Salmans LM, Fallahi M., Andersen, B., Dai X. Developmental Cell. 2014 doi: 10.1016/j.devcel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA. Developmental Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]