Abstract
Polyketides and nonribosomal peptides represent two large families of natural products (NPs) with diverse structures and important functions. They are synthesized by polyketide synthase (PKS) and nonribosomal peptide synthetase (NRPS), respectively. Lysobacter enzymogenes is emerging as a novel biocontrol agent against pathogens of crop plants and a new source of bioactive NPs, such as antibacterial antibiotic WAP-8294A2 and antifungal antibiotic HSAF. Genome survey of strain OH11, a Chinese L. enzymogenes isolate, detected four novel PKS, NRPS or hybrid gene clusters, designed as cluster A to D. We further individually mutated five genes (PKS or NRPS) located in these four gene clusters, and showed that a PKS gene in cluster A and an NRPS gene in cluster D were involved in the antibacterial activity via a WAP-8294A2 dependent way. The data also showed that none of the five genes was associated with antifungal activity and the regulation of HSAF biosynthesis. Our results reveal the unusual regulatory role of these PKS and NRPS genes that were discovered from genome mining in L. enzymogenes.
Keywords: Lysobacter enzymogenes, polyketide synthase, nonribosomal peptide synthetase, WAP-8294A2
Introduction
The genus of Lysobacter belongs to Xanthomodaceae family and is one of the most ubiquitous environmental microorganisms. This genus displays unique characteristics, such as yellow-orange appearance, high genomic G+C content (approximately 70%), flagella-independent surface motility and production of abundant lytic enzymes capable for degrading the cell walls of pathogens (Christensen and Cook, 1978; Zhang and Yuen, 1999; Kobayashi et al., 2005). In this genus, L. enzymogenes, first proposed by Christensen and Cook in 1978, is the best studied species. This species is emerging as a novel biocontrol agent against pathogens of crop plants and a new source of bioactive natural products (NPs), including two antibiotic metabolites HSAF and WAP-8294A2 (Li et al., 2006; Yu et al., 2007; Xie et al., 2012; Zhang et al., 2011). HSAF is a heat-stable and broad-spectrum antifungal compound with a novel mode of action, whereas WAP-8294A2 is a cyclic lipodepsipeptide with antibacterial (anti-Gram-positive) activity, especially exhibiting anti-MRSA (methicillin-resistant Staphylococcus aureus) activity (Li et al., 2008; Yu et al., 2007; Zhang et al., 2011).
Polyketide synthase (PKS) and nonribosomal peptide synthetase (NRPS) are biosynthetic enzymes responsible for the production of a wide range of polyketides and nonribosomal peptides, respectively (Cane, 1997). Both types represent large families of natural products (NPs) which are assembled by using acyl-coenzyme A or amino acid building blocks (Cane, 1997; Silakowski et al., 2001). Polyketides and nonribosomal peptides are structurally diverse and biologically important; a multitude of NPs belonging to these two groups have been widely applied in pharmaceuticals, agrochemicals and veterinary agents (Cane and Walsh, 1999).
We finished the draft genome sequencing of strain OH11, a Chinese L. enzymogenes isolate (Qian et al., 2009; Lou et al., 2011; Qian et al., 2013). Genome survey revealed that L. enzymogenes OH11 contained at least nine clusters containing PKS, NRPS or hybrid genes. Of these, three have previously been characterized to be responsible for the biosynthesis of bioactive secondary metabolites (Lou et al., 2011; Zhang et al., 2011; Wang et al., 2013). The remaining clusters have not been examined for their function(s), especially in antimicrobial activity in L. enzymogenes.
The objective of this study is to investigate the biological functions of four uninvestigated clusters containing PKS, NRPS or hybrid genes in L. enzymogenes. In the present study, we report the involvement of both a PKS gene and an NRPS gene in the regulation of antibacterial activity and antibiotic production in L. enzymogenes. This information will be important for discovery of novel bioactive secondary metabolite(s) from L. enzymogenes.
Material and Methods
Bacterial strains, plasmids and growth condition
The bacterial strains and plasmids used in this study are listed in Table S1. Escherichia coli strain DH5α was used for plasmid constructions and were grown in LB broth at 37 °C. Unless otherwise stated, Lysobacter enzymogenes strains were grown in 10% TSB (Trypic Soy Broth, Sigma) medium at 28 °C. When required, antibiotics were added to the medium as the following final concentrations: kanamycin (Km), 25 µg/mL and gentamicin (Gm), 25 and 150 µg/mL for E. coli and L. enzymogenes, respectively.
Antimicrobial activity assays
The antimicrobial activity assays were performed as described previously (Qian et al., 2012; 2013). The details were provided in Supplementary file.
Detection of protease production in Lysobacter enzymogenes
The protease production assay was performed as described previously (Qian et al., 2013). The details were provided in Supplementary file.
Extraction and analysis of WAP-8294A2 and HSAF
Extraction and HPLC (High-Performance Liquid Chromatography) determination of antifungal factor HSAF and antibacterial compound WAP-8294A2 was performed as described previously (Yu et al., 2007; Lou et al., 2011; Zhang et al., 2011). The details were provided in Supplementary file.
Real-time PCR
The assays of RNA extraction, cDNA synthesis and real-time PCR were performed as described previously (Qian et al., 2013). The details were provided in Supplementary file.
Results and Discussions
Identification of four new clusters containing PKS or NRPS genes in Lysobacter enzymogenes strain OH11
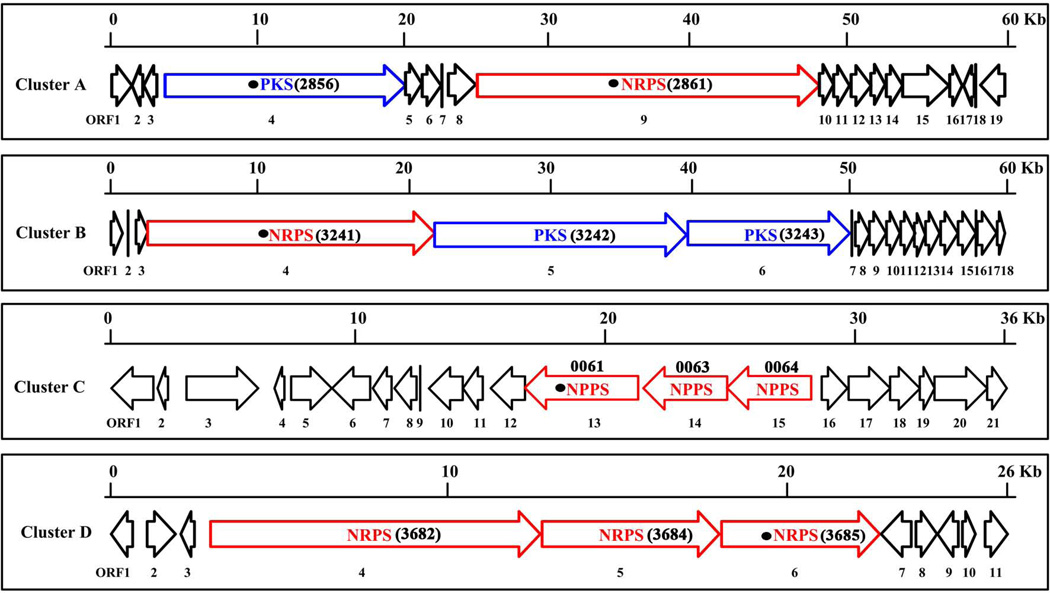
We previously obtained the genome draft of L. enzymogenes OH11 (Lou et al., 2011; Zhang et al., 2011). The mining for PKS and NRPS genes in the genome was through searching for open reading frames (ORF) that encode for large, modular proteins, because of the characteristic organization of bacterial PKS and NRPS (Fischbach and Walsh, 2006). Also because genes for natural product biosynthesis are usually clustered together, our search was on large gene clusters that are physically linked together. This process revealed at least nine gene clusters, including the antifungal antibiotic HSAF cluster (Lou et al., 2011), the antibacterial antibiotic WAP-8294A2 cluster (Zhang et al., 2011), and the Xanthomonadin-like pigment cluster (Wang et al., 2013). Four of the remaining clusters appear particularly interesting because they contain multi-modular, large PKS, NRPS and/or hybrid PKS/NRPS that form clusters with putative tailoring genes and regulatory genes (Figure 1). We designated these four clusters as A, B, C, and D. As shown in Figure 1 and Table S4, cluster A contains one PKS gene (annotated as the gene 2856 in genome) and one NRPS gene (2861), whereas cluster B contains two PKS genes (3242 and 3243) and one NRPS gene (3241). The remaining two (cluster C and D) both contain three typical NRPS genes (0061, 0063 and 0064 in cluster C; 3682, 3684 and 3685 in cluster D).
Figure 1. Four gene clusters containing PKS-NRPS in Lysobacter enzymogenes OH11.
The PKS and NRPS genes were shown in red and blue respectively in each cluster. Of them, the genes highlighted with black circle were selected for gene deletion. The detailed information for each gene in these clusters (A to D) is provided in Table S4.
It has been reported that some Lysobacter species produce cyclic peptides such as lysobactin (Bonner et al., 1988) and tripropeptins (Hashizume et al., 2001) as well as hybrid polyketide-peptide cephabacins (Sohn et al., 2001). In silico analyses of these four gene clusters suggested that they were not responsible for the biosynthesis of any of these metabolites. However, the organizational features of these clusters clearly suggest that their products have interesting structural features, if the biosynthetic genes are active or activated. More importantly, our data showed that the strains retained activities against certain bacteria (Clavibacter michiganensis, a Gram-positive bacterium) and fungi (Saccharomyces cerevisiae) even after the dihydromaltophilin (HSAF) genes or WAP-8294A-like genes were disrupted (Fig. 2 and Fig. S7). This suggests that the strains probably produced yet-to-be-identified active NPs in addition to those already identified. Thus, we investigated the potential role(s) of these four gene clusters, especially in antimicrobial activity and antibiotic production in L. enzymogenes.
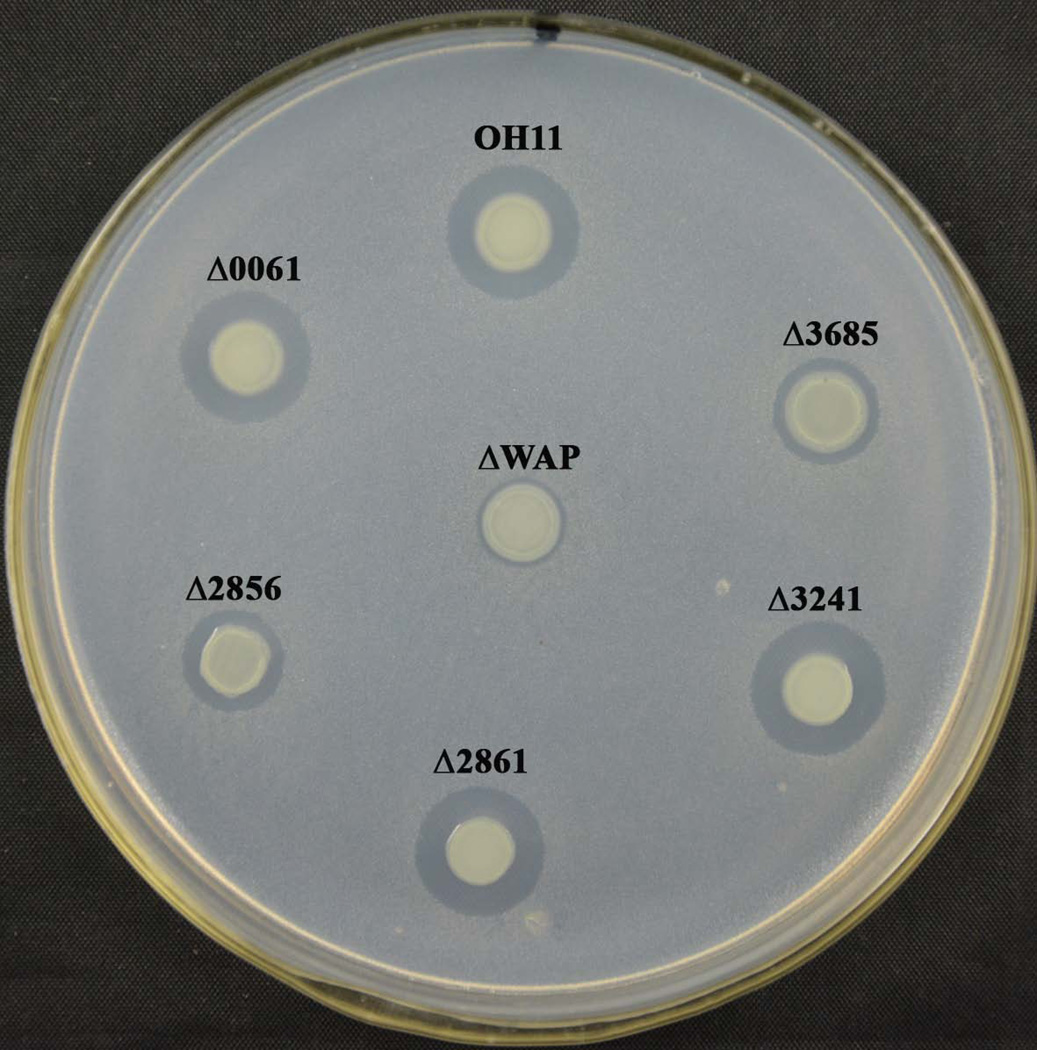
Figure 2. Antibacterial activity of the wild-type OH11 and the mutant strains.
Mutation of 2856 and 3685 impaired the wild-type antibacterial activity against Clavibacter michiganensis, a Gram-positive bacterium causing potato ring rot. ΔWAP is a WAP-8294A2-deficient mutant (Zhang et al., 2011), which was used as a negative control.
Individual mutation revealed a PKS gene and an NRPS gene were involved in the WAP-8294A2-dependent antibacterial activity
Because PKS and NRPS are the enzymes that assemble the main skeletons of the natural products, the disruption of these genes is expected to give the clearest impact on the metabolite profiles and their biological functions. To demonstrate the role(s) of these four gene clusters (A to D) in L. enzymogenes, we in-frame deleted a total of five PKS/NRPS genes within the four clusters, based on their multi-modular features. The five target PKS/NRPS genes includes 2856 and 2861 (a PKS and an NRPS gene in cluster A, respectively), 3241 (an NRPS gene in cluster B), 0061 (an NRPS gene in cluster C) and 3685 (an NRPS gene in cluster D) (Fig. 1 and Table S4). Generation of gene deletion mutants was provided in Supplementary file. The deletion mutant of each of the genes was confirmed by diagnostic PCR (Table S3). Due to the large size of the five genes, it is difficult to conduct a functional complementation of the mutants. Therefore, we compared the phenotypic difference of the wild-type OH11 and each of the in-frame deletion mutants. This strategy had previously been applied to the studies of the hybrid PKS/NRPS or NRPS genes responsible for HSAF (antifungal factor), WAP-8294A2 (anti-Gram-positive bacterial factor) and yellow-pigments in L. enzymogenes (Lou et al., 2011; Zhang et al., 2011; Wang et al., 2013).
Next, we aimed to understand the role(s) of these five genes in antimicrobial activity in strain OH11, because this strain has strong antifungal and antibacterial (anti-Gram-positive) activities (Qian et al., 2009; Zhang et al., 2011). For this purpose, we first compared the antibacterial activity between the wild-type OH11 and each of the mutants. As shown in Figure 2, we found that the mutation of 2856 (a PKS gene) and 3685 (an NRPS gene) clearly impaired the antibacterial activity against Clavibacter michiganensis, a Gram-positive bacterium. Under the same condition, mutation of the other 3 NRPS genes had no effect on this activity. As a control, the WAP-8294A2-disruption mutant almost completely lost this activity (Fig. 2).
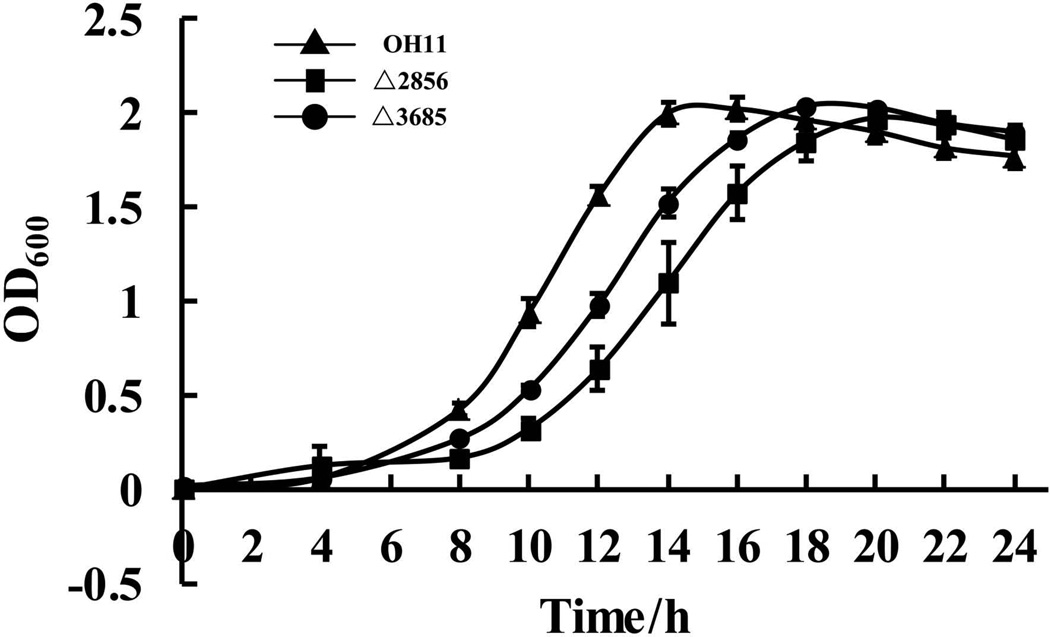
It is possible that the decrease in antibacterial activity of these two mutants was due to growth deficiency under the tested condition. To test this possibility, we compared the growth ability of the wild-type OH11 and these two mutants (Δ2856 and Δ3685) in 1/10 TSB medium, which was used for antibacterial assay. As shown in Figure 3, we found that the 2856 or 3685 mutants exhibited a slightly decreased growth ability compared to that of the wild-type strain in tested medium at logarithmic phase (approximately from 8 to 18 h), whereas both mutants showed a similar level with the wild-type OH11 at the stationary phase (approximately from 18 to 24 h). As WAP-8294A2 was extracted from the supernatant of the wild-type strain and these two mutants at stationary phase, it is likely that the involvement of these two genes (2856 and 3685) in antibacterial activity was not due to the growth deficiency in L. enzymogenes.
Figure 3. Growth curve of the wild-type OH11 and mutant strains in 1/10 TSB broth.
Mutation of 2856 and 3685 slightly impaired growth ability at logarithmic phase (approximately from 8 to 18 h), but not at stationary phase (approximately from 18 to 24 h).
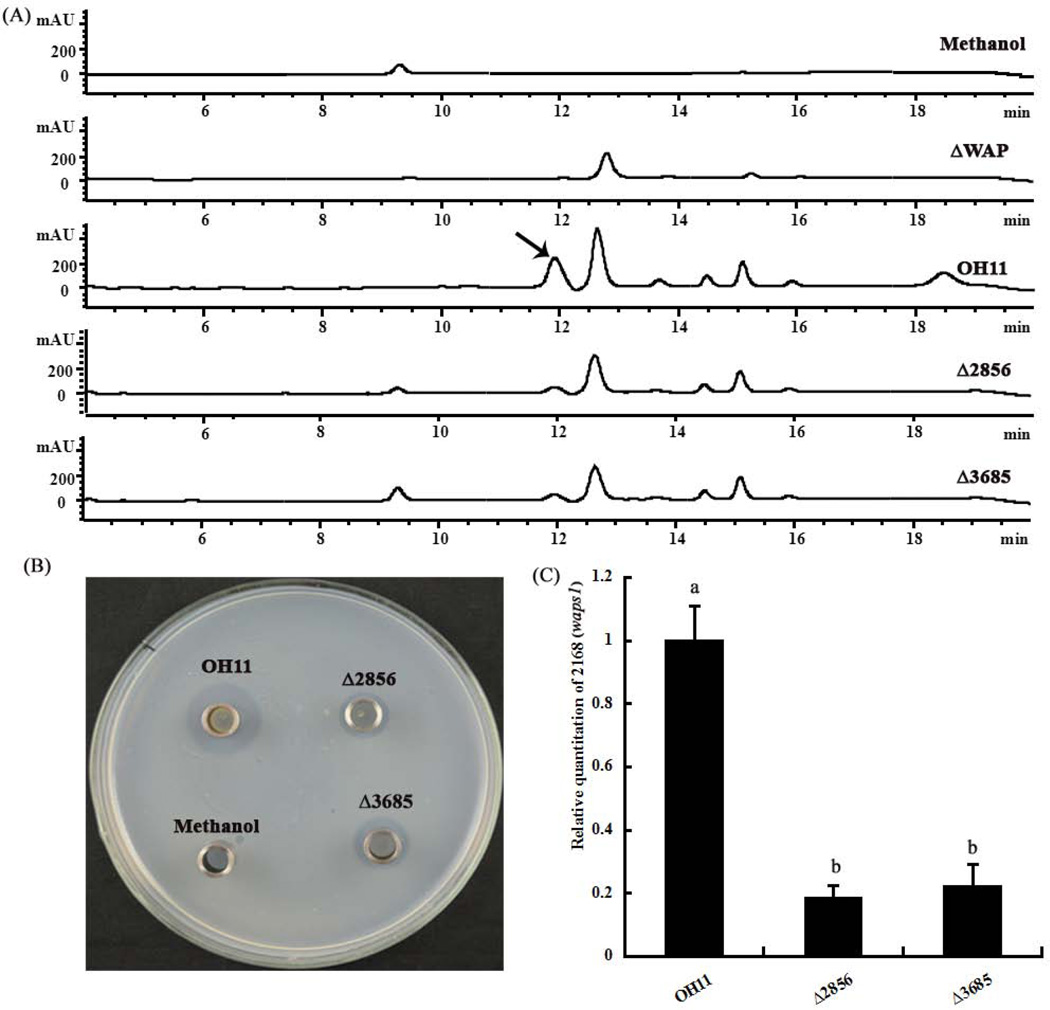
Subsequently, we investigated whether these two genes (2856 and 3685) were involved in the regulation of WAP-8294A2 production, resulting in a reduced antibacterial activity because WAP-8294A2 is a key antibacterial (anti-Gram-positive) factor. To address this possibility, we determined the WAP-8294A2 production level in these two mutants by HPLC method. As shown in Figure 4A, we observed that mutation of 2856 or 3685 impaired the WAP-8294A2 production level. The WAP-8294A2 extracts used for HPLC analysis were further selected for testing antibacterial activity. We found that these extracts from both mutants exhibited dramatically reduced antagonistic activity against C. michiganensis (a Gram-positive bacterium) compared to the wild-type OH11 (Fig. 4B). This finding was consistent with the HPLC result. Next, we compared the transcriptional level of waps1, a key NRPS gene responsible for WAP-8294A2 biosynthesis between the wild-type OH11 and these two mutants. We found that mutation of 2856 or 3685 significantly impaired the waps1 transcription (Fig. 4C). This indicated that the contribution of 2856 or 3685 to WAP-8294A2 biosynthesis was, at least partially through decreasing the transcript of the NRPS gene, waps1. Nevertheless, these results indicated that these two genes (2856 and 3685) were involved in regulating the antibacterial activity, partially via a WAP-8294A2 dependent way.
Figure 4. Mutation of 2856 and 3685 impaired the production level of antibacterial antibiotic WAP-8294A2 in Lysobacter enzymogenes.
A: HPLC determination of WAP-8294A2 production level. B: Antibacterial activity of WAP-8294A2 extracts (used for HPLC analysis) against Clavibacter michiganensis. C: Determination of transcriptional level of waps1 of the wild-type OH11 and the two mutants. Methanol, the solvent for dissolving WAP-8294A2, was used as a negative control in part A and B. The gene waps1 (annotated as 2168 in the genome of OH11) is a key gene responsible for WAP-8294A2 biosynthesis (Zhang et al., 2011). The arrow indicated the peak of WAP-8294A2. For each part, triple replicates for each treatment were used, and the experiment was performed three times. In part C, vertical bars represent standard errors. Different letters above bars indicate a significant difference in gene expression between the wild-type strain OH11 and the tested mutants (P<0.05; t-test).
It is important to note that the inhibition of C. michiganensis by both mutants (Δ2856 and Δ3685) were quantitatively different (Fig. 2) from those of wild-type OH11. While the production of WAP-8294A2 in these two mutants was almost completely gone (Fig. 4A), their crude extracts still showed inhibition against target bacteria. As the cluster containing 2856 or 3685 gene was completely different from that responsible for WAP-8294A2 biosynthesis (Zhang et al., 2011), these observations suggest that unknown antibacterial compound(s) other than WAP-8294A2 may also be produced in the mutants. This raises at least two possibilities: (i) the two genes (2856 and 3685) may be involved in synthesis of metabolites that could function as regulators for WAP-8294A2 production; (ii) WAP-8294A2 is not the sole antibacterial factor for inhibition of tested bacterium (C. michiganensis). Unidentified and WAP-8294A2-independent antibacterial factor(s) may be produced in these two mutants (Δ2856 and Δ3685).
Finally, we also determined the production level of protease in wild-type OH11 and these two mutants. It is possible that the products of these two genes (2856 and 3685) could alter the protease production and thus alter the antibacterial activity,. We observed that mutation of 2856 or 3685 in the wild-type background had no effect on the production of protease (Fig. S1), suggesting the involvement of these two genes (2856 and 3685) in antibacterial activity was unlikely due to protease production. Taken together, our results indicated that these two genes (2856 and 3685) were involved in antibacterial activity via a WAP-8294A2 dependent (at least partially), but protease independent way in L. enzymogenes.
None of the five selected PKS or NRPS genes was involved in antifungal activity
In addition to antibacterial activity, we also investigated the role(s) of the selected 5 genes in antifungal activity in L. enzymogenes. As shown in Figure S2, we found that individual mutation of the five genes did not affect the antifungal activity against two plant pathogens in 1/10 TSA plates. In this medium, high-level HSAF production was determined in each of the mutants (Fig. S3). This caused an interfered effect on evaluating the roles of each selected gene on antifungal activity, because HASF is broad-spectrum antifungal factor (Li et al., 2008). Thus, to reduce the overwhelming effect of HSAF in antifungal activity test in each mutant in the HSAF-production medium (1/10 TSA), we perform the antifungal assay in full-strength TSA medium, because the wild type L. enzymogenes does not produce HSAF in this medium (Fig. S4). As shown in Figure S5, each mutant also displayed the wild-type antifungal activity in full-strength TSA medium. These results indicated that these 5 genes were not involved in the antifungal activity in L. enzymogenes. To confirm this result, we selected HSAF-insensitive yeast (Saccharomyces cerevisiae) (Li et al., 2008) as a target for further antifungal assay. We found purified HSAF was ineffective in anti-yeast activity, but exhibited strong antifungal activity against 2 tested fungal pathogens (Fig. S6). Next, we compared the anti-yeast activity between the wild-type OH11 and each of the selected mutants in 1/10 TSA plates. As shown in Figure S7, we clearly observed that all five mutants exhibited the wild-type ability in anti-yeast activity. Collectively, these results indicated that these five genes were not involved in the antifungal activity against selected fungal pathogens or yeast in L. enzymogenes.
Concluding remarks
This study reported the presence of four new clusters containing PKS-NRPS genes, which are probably responsible for uncharacterized natural product biosynthesis in L. enzymogenes OH11. Mutation and phenotypic analysis revealed both a PKS (2856) and an NRPS (3685) gene were involved in antibacterial activity through regulation of the antibacterial antibiotic WAP-8294A2 production level. In future studies, we aim to: (i) identify the compound(s) synthesized by 2856 and 3685 and verify their potential role in antimicrobial activity regulation, (ii) understand the regulatory mechanism of 2856 and 3685 in WAP-8294A2 biosynthesis in L. enzymogenes. These future works will pave the way for discovery of novel bioactive secondary metabolites from Lysobacter.
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation of China (31371981), Program for New Century Excellent Talents in University of Ministry of Education of China (NCET-13-0863), Fundamental Research Funds for the Central Universities (No. KYZ201205), Zhongshan Scholars of Nanjing Agricultural University. Research in the Du lab is supported in part by NIH R01AI097260 and Nebraska Research Initiatives.
References
- Bonner DP, O’Sullivan J, Tanaka SK, Clark JM, Whitney RR. Lysobactin, a novel antibacterial agent produced by Lysobacter sp. II. biological properties. J Antibiot. 1988;41:1745–1751. doi: 10.7164/antibiotics.41.1745. [DOI] [PubMed] [Google Scholar]
- Cane DE, Walsh CT. The parallel and convergent universes of polyketide synthases and nonribosomal peptide synthetases. Chem Biol. 1999;6:319–325. doi: 10.1016/s1074-5521(00)80001-0. [DOI] [PubMed] [Google Scholar]
- Cane DE. Polyketide and nonribosomal polypeptide biosynthesis. Chem Rev. 1997;97:2463–2706. doi: 10.1021/cr970097g. [DOI] [PubMed] [Google Scholar]
- Christensen P, Cook FD. Lysobacter, a new genus of nonfruiting, gliding bacteria with high base ratio. Int J Syst Bacteriol. 1978;28:367–393. [Google Scholar]
- Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery and mechanisms. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Iqarashi M, Hattori S, Hori M, Hamada M, Takeuchi Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. taxonomy, isolation and biological activities. J Antibiot. 2001;54:1054–1059. doi: 10.7164/antibiotics.54.1054. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi DY, Reedy RM, Palumbo JD, Zhou JM, Yuen GY. A clp gene homologue belonging to the Crp gene family globally regulates lytic enzyme production, antimicrobial activity, and biological control activity expressed by Lysobacter enzymogenes Strain C3. Appl Environ Microbiol. 2005;71:261–269. doi: 10.1128/AEM.71.1.261-269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jochum CC, Yu F, Zaleta-Rivera K, Du L, Harris SD, Yuen GY. An antibiotic complex from Lysobacter enzymogenes strain C3: antimicrobial activity and role in plant disease control. Phytopathology. 2008;98:695–701. doi: 10.1094/PHYTO-98-6-0695. [DOI] [PubMed] [Google Scholar]
- Li SJ, Du LC, Yuen G, Harris SD. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol Biol Cell. 2006;17:1218–1227. doi: 10.1091/mbc.E05-06-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou LL, Qian GL, Xie YX, Hang JL, Chen HT, Zaleta-Rivera K, Li YY, Shen YM, Dussault PH, Liu FQ, Du LC. Biosynthesis of HSAF, a tetramic acid-containing macrolactam from Lysobacter enzymogenes. J Am Chem Soc. 2011;133:643–645. doi: 10.1021/ja105732c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian GL, Hu BS, Jiang YH, Liu FQ. Identification and characterization of Lysobacter enzymogenes as a biological control agent against some fungal pathogens. Agr Sci China. 2009;8:68–75. [Google Scholar]
- Qian GL, Wang YL, Liu YR, Xu FF, He YW, Du L, Venturi V, Fan JQ, Hu BS, Liu FQ. Lysobacter enzymogenes uses two distinct cell-cell signaling systems for differential regulation of secondary-metabolite biosynthesis and colony morphology. Appl Environ Microbiol. 2013;79:6604–6616. doi: 10.1128/AEM.01841-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian GL, Wang YS, Qian DY, Fan JQ, Hu BS, Liu FQ. Selection of available suicide vectors for gene mutagenesis using chiA (a chitinase encoding gene) as a new reporter and primary functional analysis of chiA in Lysobacter enzymogenes strain OH11. World J Microb Biot. 2012;28:549–557. doi: 10.1007/s11274-011-0846-8. [DOI] [PubMed] [Google Scholar]
- Silakowski B, Nordsiek G, Kunze B, Blöcker H, MllÜer R. Novel features in a combined polyketide synthase/non-ribosomal peptide synthetase: the myxalamid biosynthetic gene cluster of the myxobacterium Stigmatella aurantiaca Sga15. Chem Biol. 2001;8:59–69. doi: 10.1016/s1074-5521(00)00056-9. [DOI] [PubMed] [Google Scholar]
- Sohn YS, Nam DH, Ryu DD. Biosynthetic pathway of cephabacins in Lysobacter lactamgenus: molecular and biochemical characterization of the upstream region of the gene cluster for engineering of novel antibiotics. Metab Eng. 2001;3:380–392. doi: 10.1006/mben.2001.0200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qian GL, Li YY, Wright S, Shen YM, Liu FQ, Du LC. Biosynthetic mechanism for sunscreens of the biocontrol agent Lysobacter enzymogenes. PLoS One. 2013;8:e66633. doi: 10.1371/journal.pone.0066633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YX, Wright S, Shen YM, Du LC. Bioactive natural products from Lysobacter. Nat Prod Rep. 2012;29:1277–1287. doi: 10.1039/c2np20064c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FG, Zaleta-Rivera K, Zhu XC, Huffman J, Millet JC, Harris SD, Yuen G, Li XC, Du LC. Structure and biosynthesis of heat-stable antifungal factor (HSAF), a broad-spectrum antimycotic with a novel mode of action. Antimicrob Agents Ch. 2007;51:64–72. doi: 10.1128/AAC.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Li YY, Qian GL, Wang Y, Chen HT, Li YZ, Liu FQ, Shen YM, Du LC. Identification and characterization of the anti-methicillin-resistant Staphylococcus aureus WAP-8294A2 biosynthetic gene cluster from Lysobacter enzymogenes OH11. Antimicrob Agents Ch. 2011;55:5581–5589. doi: 10.1128/AAC.05370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yuen GY. Biological control of Bipolaris sorokiniana on tall fescue by Stenotrophomonas maltophilia Strain C3. Phytopathology. 1999;89:817–822. doi: 10.1094/PHYTO.1999.89.9.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.