Abstract
NicVAX®, a nicotine vaccine (3’AmNic-rEPA), has been clinically evaluated to determine if higher antibody concentrations are associated with higher smoking abstinence rates and if doses and frequency of administration are associated with increased antibody response. This randomized, double-blinded, placebo-controlled multicenter clinical trial (N=301 smokers) tested 200 and 400 µg doses administered 4 or 5 times over 6 months compared to placebo. 3’AmNic-rEPA recipients with the highest serum anti-nicotine antibody response (top 30% by AUC) were significantly more likely to attain 8 weeks continuous abstinence from weeks 19 through 26 than the placebo recipients (24.6% vs. 12.0%, p=0.024, OR=2.69, 95% CI, 1.14–6.37). The 5 injection 400 µg dose regimen had the greatest antibody response and had significantly higher abstinence rates than placebo. This study demonstrates proof-of-concept that 3’AmNic-rEPA elicits antibodies to nicotine and is associated with higher continuous abstinence rates, justifying its further development as a treatment for nicotine dependence.
Keywords: 3’AmNic-rEPA, NicVAX, nicotine immunotherapeutic, nicotine vaccine, cigarette, smoking cessation, antibody, P. aeruginosa r-Exoprotein A, aminomethyl nicotine, cotinine, CO
Introduction
Worldwide, smoking prevalence is 1.2 billion and approximately 5 million people die each year of smoking caused illnesses [1]. The global rate of smoking and smoking related deaths is anticipated to increase over the next 20 years unless significant public health measures are instituted. These include effective cessation interventions such as pharmacological treatments, which improve cessation rates by 1.5 to 3 fold over placebo intervention [2, 3]. Approved pharmacotherapies (e.g., nicotine replacements, bupropion SR, varenicline) for smoking cessation act on the central nervous system, each with a different mechanism of action. Other novel medications are being developed including immunotherapeutics targeting nicotine.
Nicotine conjugate vaccines stimulate the immune system to develop nicotine specific antibodies (Abs) using an immunogen comprised of nicotine covalently linked to a larger carrier protein. Conceptually, the mechanism of action is that anti-nicotine antibodies bind nicotine molecules and the resulting complex is too large to cross the blood-brain barrier. With increasing Ab levels more nicotine is captured and sequestered in the blood and prevented from entering the brain, leading to less reinforcing effects from nicotine. Animal studies have demonstrated that passive or active immunization results in approximately 30% to 90% less nicotine entering the brain compared to control rats [4–7] and attenuated locomotor [4, 5] and behavioral [8, 9] responses to nicotine. Furthermore, vaccination reduced nicotine elimination from the body in a study with rats [10, 11], which may also contribute to reduced smoking.
Although human studies are limited, published data evaluating different nicotine vaccines support the general concept that nicotine vaccines can be effective for smoking cessation in some smokers [12, 13]. Unfortunately, these studies either had small sample sizes [12], did not use an intent-to-treat population of smokers [13] or did not perform statistical analysis of the data [14].
The primary aim of the present study was to establish the proof-of-concept that (i) anti-nicotine antibodies are useful as an aid to smoking cessation and (ii) higher serum anti-nicotine antibody concentrations are associated with higher abstinence rates in an intent-to-treat population of smokers. One of the challenges with immunotherapeutics, such as vaccines, is attainment of therapeutic levels of antibody in most people. Therefore, this study tested two different doses of 3’-aminomethylnicotine P. aeruginosa r-exoprotein A - NicVAX (3’AmNic-rEPA) to identify a dose and schedule for further development: 200 and 400 µg across two different schedules (4 or 5 injections) compared to placebo for immunogenicity, efficacy and safety.
Results
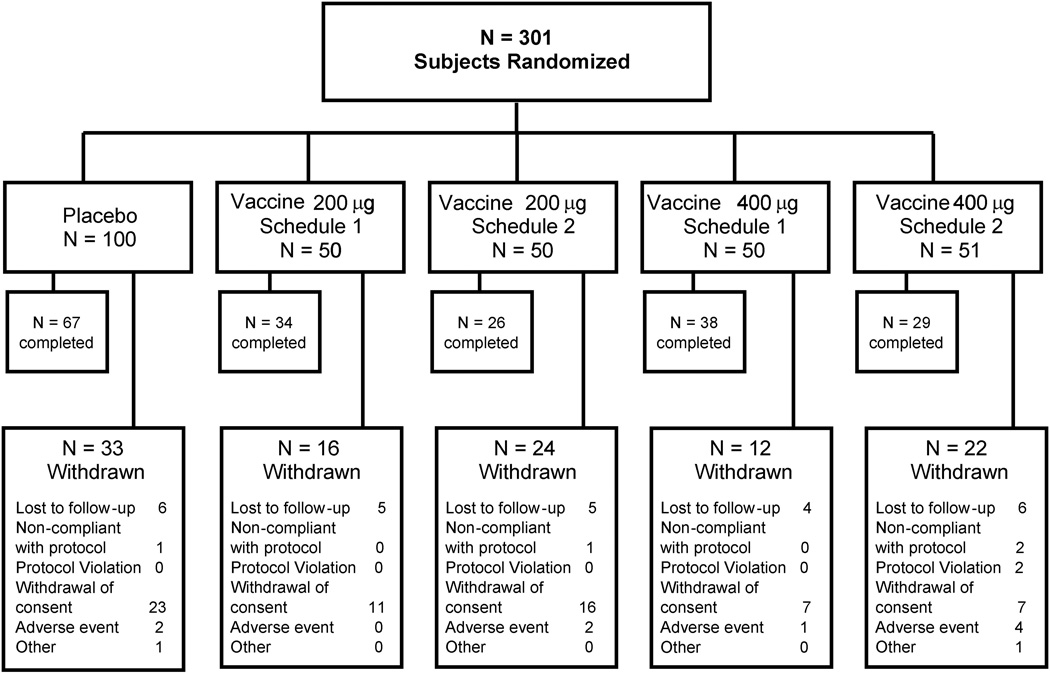
A total of 301 subjects were randomized. Figure 2 shows the disposition and number of subjects within each treatment group. No significant group differences were observed in the demographic or smoking history by treatment or antibody levels (see Table 1).
Figure 2.
Subject disposition.
Table 1.
Demographics and Smoking History at Baseline
| Placebo | Vaccine 200 µg Schedule 1 |
Vaccine 200 µg Schedule 2 |
Vaccine 400 µg Schedule 1 |
Vaccine 400 µg Schedule 2 |
Total | |
|---|---|---|---|---|---|---|
| N=100 | N=49–50 | N=49–50 | N=50 | N=51 | N=301 | |
| Variables | ||||||
| Age (years) | ||||||
| Mean±SD | 47±11 | 50±10 | 46±11 | 48±11 | 48±12 | 48±11 |
| Gender | ||||||
| n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | N (%)b | |
| Female | 50 (50.0) | 26 (52.0) | 29 (58.0) | 29 (58.0) | 24 (47.1) | 158 (52.5) |
| Male | 50 (50.0) | 24 (48.0) | 21 (42.0) | 21 (42.0) | 27 (52.9) | 143 (47.5) |
| Race | ||||||
| n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | N (%)b | |
| White | 88 (88.0) | 47 (94.0) | 42 (84.0) | 47 (94.0) | 47 (92.0) | 271 (90.0) |
| Other | 12 (12.0) | 3 (6.0) | 8 (16.0) | 3 (6.0) | 4 (8.0) | 30 (10.0) |
| Fagerström Totalc | ||||||
| Mean±SD | 6.1±1.9 | 6.3±2.1 | 5.8±2.0 | 5.8±2.0 | 6.6±1.7 | 6.0±2.0 |
| Median (range) | 6 (1–10) | 7 (1–10) | 6 (1–10) | 6 (1–10) | 6 (3–10) | 6 (1–10) |
| Cigarettes Per Day | ||||||
| Mean±SD | 24.7±8.8 | 24.8±9.1 | 22.6±7.0 | 24.3±9.4 | 25.6±10.5 | 24.0±9.0 |
| Median (range) | 20 (15–50) | 20 (15–50) | 20 (15–40) | 20 (15–60) | 20 (15–70) | 20 (15–70) |
| Previous Quit Attempts | ||||||
| n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | N (%)b | |
| Yes | 96 (96.0) | 48 (96.0) | 47 (94.0) | 48 (96.0) | 49 (96.1) | 288 (95.7) |
Percentages based on total number of subjects within treatment group.
Percentages based on total number of subjects who received treatment.
Fagerström score assesses the severity of nicotine addiction ranging from 0 (minimum) to 10 (maximum).
Compliance
All 301 subjects received injection 1, 96.7%, 84.1%, 72.4% and 61.2% received injections 2, 3, 4 and 5 (Schedule 2 subjects only), respectively. No significant differences were observed across treatment groups for subjects receiving injections 2 through 4 for Schedules 1 and 2. Mean in-study duration was 286 ± 121 days.
Proof-of-concept
Effects of high Ab on abstinence
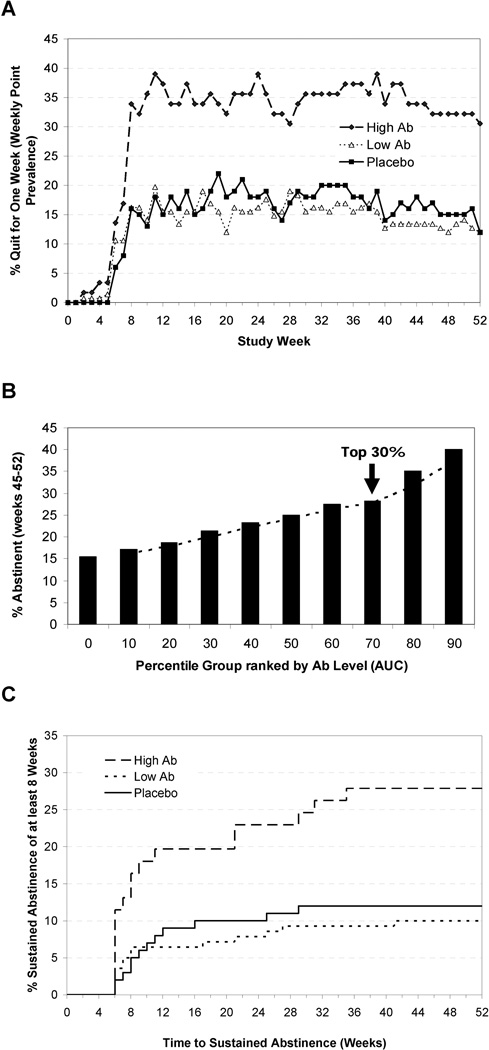
High Ab responders to 3’AmNic-rEPA were defined as the top 30% of responders by AUC (0 to 26 weeks) and the low Ab group as the remaining bottom 70% of responders. 3’AmNic-rEPA recipients in the high Ab group were significantly more likely to attain 8 weeks of continuous abstinence from weeks 19 through 26 than placebo (24.6% vs. 12.0%, p=0.024, OR=2.69, 95% CI, 1.14–6.37). No significant differences were observed between the 3’AmNic-rEPA low Ab group vs. placebo (9.3% vs. 12.0%., p=0.46). As a secondary outcome, continuous abstinence rate (CAR) to 52 weeks were evaluated from weeks 19 to 52 and were significantly higher for the high Ab group vs. placebo (19.7% vs. 10.0%, p=0.044, OR=2.64, 95% CI, 1.03–6.79) with no significant difference between the low Ab group and placebo (7.1% vs. 10.0%, p=0.43). 7-day point prevalence abstinence results show that subjects with high Ab levels were significantly more likely to abstain compared to placebo at 26 weeks (36.1% vs. 16.0%, p=0.0024, OR=3.30, 95% CI, 1.53–7.13) and 52 weeks (31.1% vs. 12.0%, p=0.0021, OR=3.69, 95% CI, 0.42–2.14). No significant differences were observed in the point prevalence abstinence rates between the low Ab group and placebo at 26 and 52 weeks (12.9% vs. 16.0%, p=0.51 and 11.4% vs. 12.0%, p=0.89, respectively). As shown in Figure 3A, abstinence rates remained essentially the same following the target quit date (TQD) for the duration of the study.
Figure 3.
For the intent-to-treat population: (A) 7-day point prevalence abstinence rates for high antibody (top 30% AUC), low antibody (bottom 70% AUC) groups and placebo over the course of 52 weeks; (B) Percent abstinent for week 45–52 (8 week continuous abstinence) by AUC. AUC is displayed in 10th percentile point increments; (C) Time to 8-week of sustained abstinence prior to week 46 and continuous abstinence maintained through week 52, stratified by group with high antibody (top 30% AUC), low antibody (bottom 70% AUC) and placebo (dropouts censored at week 52).
To further validate the proof-of-concept, the relationship between abstinence during the final eight weeks of the study and anti-nicotine Ab concentrations (AUC) is shown in Figure 3B for all subjects receiving the vaccine. Continuous abstinence rates from week 45–52 are displayed for each 10th percentile increase in AUC. The proportion of abstinent subjects increased with increasing AUC percentile, and the ordered ranking maintained.
Effects of high Ab on time to continuous abstinence
An exploratory analysis examined the rate and time to continuous abstinence through study end (Figure 3C). Most smokers quit soon after the TQD, with a clear divergence of the high Ab group from the placebo and low Ab group. Among the 18 high Ab continuous abstainers, 12 initiated abstinence prior to the primary endpoint while 6 initiated their abstinence after the start of the primary endpoint. Furthermore, 3 of the 15 subjects who abstained during the primary endpoint relapsed by the end of the study. Cox proportional hazards analysis demonstrated superiority of the high Ab group compared to placebo (p=0.0069, hazard ratio of 2.76).
Evaluation of long-term abstinence
As most subjects achieved abstinence shortly after their TQD, additional analyses were undertaken to evaluate prolonged abstinence to 6 and 12 months allowing for a 2 week grace period after the TQD [15]. (Prolonged abstinence is defined as not a single puff during the period from 2 weeks after the TQD for 20 weeks and 44 weeks, respectively).
Prolonged abstinence rates to 6 months were significantly higher in the high Ab group vs. placebo (19.7% vs. 6.0%, p=0.0060, OR=4.41, 95% CI, 1.53–12.71) with no significant differences between the placebo and low Ab groups (7.9% vs. 6.0%, p=0.60). Subjects with high Ab were also significantly more likely to be abstinent for 12 months compared to placebo (18.0% vs. 6.0%, p=0.014; OR of 3.84; 95% CI, 1.32–11.20). The low Ab group did not differ significantly from placebo (7.1% vs. 6.0%, p=0.67).
Cigarette smoking in non-quitters
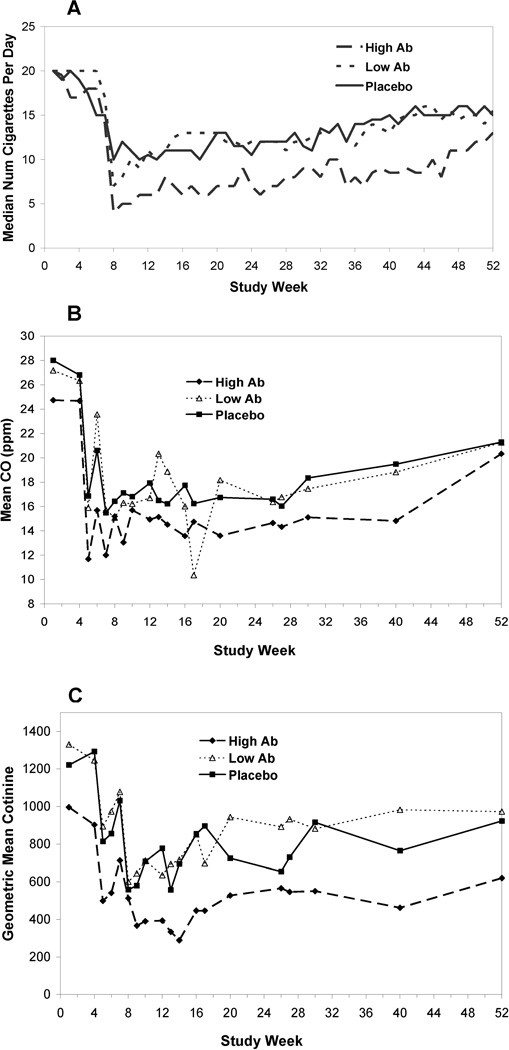
Statistically significant differences were observed in reduction of daily cigarette consumption and cotinine between non-abstainers (weeks 19–52) with high Ab levels and non-abstainers in the placebo group (p=0.0015 and 0.019, respectively; see Figures 4A and 4C. The difference in the median reduction in cigarette consumption, following the TQD, between the high Ab non-abstainers and placebo non-abstainers was on average 4.6 cigarettes per day. Similarly, cotinine geometric mean concentrations (GMCs) were 19.0% lower on average following the target quit date in the high Ab non-abstainers as compared to placebo non-abstainers. Median cigarettes per day and cotinine GMC for placebo and low Ab group are nearly identical over the study period. Differences in mean CO were not observed across all three groups (see Figure 4B).
Figure 4.
(A) Median number of cigarettes per day, (B) CO levels and (C) geometric mean cotinine levels among those subjects not abstaining from cigarettes across those subjects with high antibody (top 30% AUC), low antibody (bottom 70% AUC) and placebo. Time on the x-axis is adjusted to align the target quit date between Schedule 1 and Schedule 2.
15 out of the 301 subjects smoked more than a 2-fold higher number of cigarettes following the TQD as compared to baseline with no significant difference between the placebo (n=4/100) and 3’AmNic-rEPA (n=11/201) groups. The highest smoking levels observed post-TQD were 5-fold baseline in the placebo and 4-fold baseline in the 3’AmNic-rEPA groups. Elevated smoking levels for 11 of these 15 subjects returned to below 2-fold baseline by study end. Subjects with remaining elevated levels were similar between the 3’AmNic-rEPA group (n=3/201) and placebo (n=1/100). Individual subjects with CO levels elevated by 2-fold or higher than baseline were also assessed and results were similar with no significant difference between the placebo (n=5/100) and 3’AmNic-rEPA (n=13/201) groups.
Withdrawal symptoms
Significant differences were not observed in overall withdrawal severity between placebo and the high and low Ab groups (p >0.22).
Effects of dose and schedule
Immunogenicity and Efficacy by Study Group
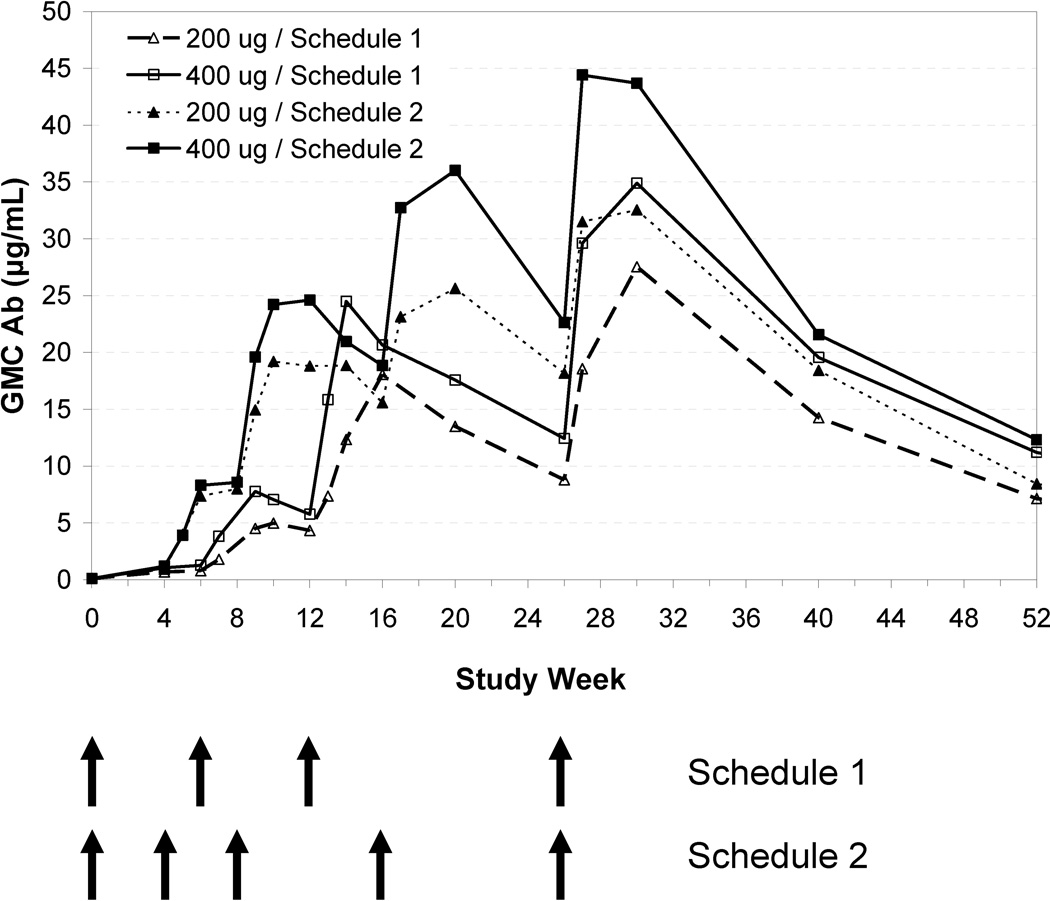
Figure 5 depicts immune response by study group from baseline to week 52. Anti-nicotine Ab GMCs increased across all active treatment groups after each vaccination, with each subsequent dose resulting in a higher Ab response than the previous dose. Schedule 2 showed a higher initial increase in Ab concentrations. The 400 µg/Schedule 2 group demonstrated the highest Ab concentrations. However, no significant differences (p >0.05) were observed in AUC, Cmax and Cavg across the treatment groups to 26 or 52 weeks likely because the study was not powered to detect such difference.
Figure 5.
Geometric mean antibody concentrations (µg/mL) by treatment group.
An intent-to-treat analysis demonstrated that the Schedule 2, 400 µg dose group had significantly higher prolonged abstinence to 6 months as compared to placebo (17.6% vs. 6.0%; p=0.015; OR of 4.14; 95% CI, 1.32–13.02), although not significant for the Schedule 2, 200 µg dose (14.0% vs. 6.0%, p=0.054; OR=3.23; 95% CI, 0.98–10.67) or between placebo and Schedule 1 for each of the doses (p >0.84). The Schedule 2, 400 µg dose group also had the highest rates of prolonged abstinence to 12-months, significantly higher than placebo (15.7% vs. 6.0%, p=0.038; OR=3.44; 95% CI, 1.07–11.04), but not significant for the Schedule 2, 200 µg dose (14.0% vs. 6.0% for 200 (p=0.056; OR=3.21; 95% CI, 0.97–10.63) or between placebo and Schedule 1 for each dose (p >0.88).
Safety
Table 2 lists the number of subjects experiencing local and systemic reactogenicity. Reactogenicity events were aggregated over all injections. Overall, ache and tenderness were the most commonly reported local events, with at least one report by nearly all subjects (86% to 98%) in each treatment group. Myalgia, malaise, and headache were the most commonly reported systemic events (64% to 88% of subjects). Swelling, heat, burning, erythema, and nausea were reported by about half the subjects. Fever and vomiting were less common (4% to 16%).
Table 2.
Comparison of the Presence of Reactogenicity Events by Treatment Group
| Number (Percent) of Subjects by Treatment | ||||||
|---|---|---|---|---|---|---|
| Placebo | 200 µg Schedule 1 |
200 µg Schedule 2 |
400 µg Schedule 1 |
400 µg Schedule 2 |
p-value | |
| (N=100) | (N=50) | (N=50) | (N=50) | (N=51) | ||
| Local | ||||||
| Ache | 96 (96.0) | 48 (96.0) | 43 (86.0) | 47 (94.0) | 49 (96.1) | 0.129 |
| Burning | 42 (42.0) | 23 (46.0) | 22 (44.0) | 21 (42.0) | 23 (45.1) | 0.988 |
| Erythema | 39 (39.0) | 29 (58.0) | 23 (46.0) | 27 (54.0) | 22 (43.1) | 0.180 |
| Heat | 42 (42.0) | 23 (46.0) | 25 (50.0) | 27 (54.0) | 22 (43.1) | 0.661 |
| Swelling/Induration | 60 (60.0) | 33 (66.0) | 32 (64.0) | 33 (66.0) | 29 (56.9) | 0.827 |
| Tenderness | 95 (95.0) | 49 (98.0) | 44 (88.0) | 48 (96.0) | 50 (98.0) | 0.126 |
| Systemic | ||||||
| Fever | 10 (10.0) | 5 (10.0) | 2 (4.0) | 8 (16.0) | 6 (11.8) | 0.403 |
| General Discomfort/Malaise | 79 (79.0) | 38 (76.0) | 38 (76.0) | 42 (84.0) | 42 (82.4) | 0.803 |
| Headache | 67 (67.0) | 35 (70.0) | 32 (64.0) | 35 (70.0) | 36 (70.6) | 0.945 |
| Myalgia | 86 (86.0) | 39 (78.0) | 41 (82.0) | 46 (92.0) | 45 (88.2) | 0.315 |
| Nausea | 44 (44.0) | 17 (34.0) | 25 (50.0) | 21 (42.0) | 25 (49.0) | 0.501 |
| Vomiting | 6 (6.0) | 3 (6.0) | 2 (4.0) | 2 (4.0) | 8 (15.7) | 0.111 |
A total of 1184 treatment-emergent adverse events (AEs), predominantly rated mild or moderate, were reported by 266 of 301 subjects; 87.1% of 3’AmNic-rEPA recipients and 91.0% of placebo recipients. On average, 3.7 and 4.3 events were observed per person in the vaccinated and placebo groups, respectively, including subjects with no events. The distribution of 161 physician-determined treatment-related AEs, according to severity and relationship to treatment, was similar for the 3’AmNic-rEPA and placebo arms. Seven 3’AmNic-rEPA recipients (3.5%) and 2 subjects in the placebo arm (2.0%) withdrew from the study due to adverse events.
18 Serious AEs were reported: 8 events in the 3’AmNic-rEPA treatment groups among 7 subjects (3.5% of the 3’AmNic-rEPA recipients) and 10 events in the placebo group among 5 subjects (5.0% of the placebo recipients). Only one of these SAEs (anaphylactic reaction in a 3’AmNic-rEPA 400 µg/Schedule 2 recipient) was considered by the Investigator to be treatment related. This subject, who had a history of urticaria to penicillin and seasonal allergies, experienced difficulty breathing, throat tightness, facial erythema and urticaria 70 minutes after the initial vaccination. The subject was treated with a single injection of subcutaneous epinephrine and diphenhydramine, which resolved the symptoms. Herpes Zoster was reported in 6 subjects; one occurring within 3–5 hours after the first vaccination which would be impossible to link to vaccination. Of the remaining cases, 4 (2%) occurred in 3’AmNic-rEPA recipients; while 1 occurred in placebo (1%). In contrast, the related herpes virus, simplex infection was reported in 4 3’AmNic-rEPA recipients (1%) and 3 placebo recipients (2%).
Discussion
Results demonstrated the proof-of-concept that smokers who achieved higher anti-nicotine Ab concentrations were more likely to quit and remain abstinent from smoking. The high Ab group demonstrated the highest abstinence rates independent of the time period of ascertainment. Similarly, in a separate study conducted by Cornuz and coworkers [13] testing a different nicotine vaccine (nicotine derivative conjugated to a virus-like particle derived from bacteriophage Qβ), post hoc analysis showed subjects stratified to the highest Ab group had a significantly higher quit rate than placebo. However, unlike the current study, which used the intent-to-treat (ITT) population to establish proof of concept, the reported finding by Cornuz et al. [13] was observed after eliminating about a third of the subjects who used nicotine replacement therapies during the course of the study or who had incomplete Ab titer values.
In the present 3’AmNic study, subjects in the high Ab group had observed odds ratios of 4.4 (95% CI 1.5–12.7) and 3.9 (95% CI 1.3–11.2) for prolonged abstinence rates to 6 and 12 months versus placebo. Although no direct comparisons can be made, these odds ratios are not unlike ones observed in the meta-analyses conducted for the U.S. Clinical Practice Guideline where the reported odds-ratios ranged from 1.5 (95% CI 1.2–1.7) for nicotine gum to 3.1 (95% CI 2.5–3.8) for varenicline at 6 months post-quit [3]. If current study findings are confirmed in larger studies, imunotherapeutics are likely to emerge as an important aid to smoking cessation.
No significant compensatory smoking in response to the presence of anti-nicotine antibodies, as determined by the number of cigarettes smoked per day, carbon monoxide levels or cotinine levels, was observed in this study. This result is consistent with observations from other studies [12, 13]. In the current study, subjects in the high Ab group who did not abstain smoked significantly lower number of cigarettes (median reduction of ~5 cigarettes/day) and experienced lower cotinine levels (~20%) than placebo subjects along with no differences observed between the low Ab and placebo groups. However, a small number of individual subjects (N=15/301) smoked more than 2-fold the number of cigarettes at baseline with no significant differences between the active treatment and placebo groups.
A major challenge for immunotherapeutics is to stimulate high Ab levels in the vast majority of smokers trying to quit. Vaccine dose and frequency of vaccination have an impact on the Ab levels attained. The 5 injection/400 µg dose was associated with the highest Ab response, although not statistically significant, possibly due to the small sample size. Importantly, this dose and schedule demonstrated statistically significant improved 44-week CAR compared to placebo. Because the 4 injection/400 µg dose was not associated with higher abstinence rates, this result demonstrates that consideration of both dose and schedule of injection are critical. In an independent, follow-up immunogenicity study to examine if peak Ab levels could be elevated, a total of 74 subjects received 6 injections of 400 µg 3’AmNic-rEPA at week 0, 4, 8, 12, 16, and 26. More than 80% of subjects receiving the 6-dose immunization regimen exceeded a target level of Ab (25 µg/ml) by week 14. In contrast, only 50% of subjects receiving 5 injections of the 400 µg dose achieved this level by week 14 in the current study and, only 7% of subjects attained this level by the target quit date. This finding suggests that more frequent injections and a later quit date may increase treatment success.
In general, 3’AmNic-rEPA was well-tolerated. The frequencies of local and systemic vaccine reactogenicity events were similar in the vaccine and placebo groups and similar to licensed adult vaccines containing Alum adjuvant [16]. The slight increase in cases of herpes zoster observed in this study may be spurious, but continued monitoring is necessary to determine if a causal relationship exists. The occurrence of a single anaphylactic reaction would suggest a need for continued monitoring and follow-up, even considering the subjects’ history of prior drug allergy to penicillin.1
In summary, results from this study support the concept that high anti-nicotine Ab levels are associated with higher rates of abstinence. These findings suggest that vaccines attaining high Ab levels by the target quit date may be more effective. Other future strategies may include examining additional ways to increase Ab levels across all individuals. Nonetheless, this study demonstrates that 3’AmNic-rEPA has significant potential as a smoking cessation, and perhaps a relapse prevention aid.
Methods
Study Population
Smokers were recruited via advertisement across nine geographically diverse U.S. sites. Interested subjects were screened over the telephone and more extensively screened at the first Screening Visit. Subject informed consent was obtained prior to screening. Subjects were 18 years of age or older, smoked ≥15 cigarettes/day, had exhaled CO ≥10 ppm; wanted to quit smoking, and were in good general physical and mental health. Exclusion criteria included recent use of any medications or drugs that might interfere with immune response, interact with the vaccine, and pharmacotherapies or other treatments for smoking cessation. For females, a negative urine pregnancy test at enrollment and active use of acceptable birth control or documentation of surgical sterility was required.
Study Design
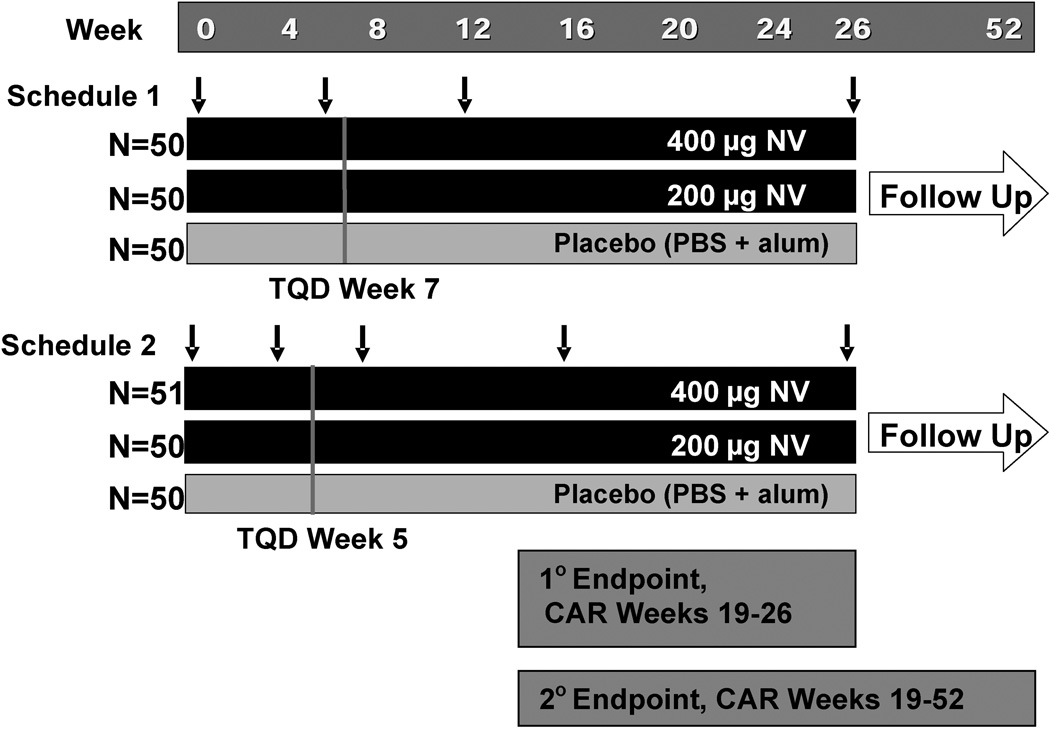
This Phase 2 study was a randomized double-blind, placebo-controlled, parallel-arm trial design (see Figure 1). Four treatment groups varied the dose and/or schedule of intramuscular vaccination: 200 or 400 µg of 3’AmNic-rEPA or placebo according to Schedule 1 (weeks 0, 6, 12, and 26) or Schedule 2 (weeks 0, 4, 8, 16, and 26). Subjects (N=150) were first randomized within Schedule 1 groups in a 1:1:1 ratio (200 µg: 400 µg: placebo) and then 151 subjects randomized within Schedule 2 groups in the same ratio. The TQD was 1 week after the second injection (end of week 7 for Schedule 1 and end of week 5 for Schedule 2). If the subjects relapsed (seven consecutive days of smoking) after the quit date, a second quit date coinciding with a future clinic visit was allowed between time of relapse and week 18. Cessation counseling (based on the USDHHS Clinical Practice Guidelines [17]) for the first quit attempt involved 5 standardized face-to-face sessions (≤10 minutes) and for the second quit attempt, a face-to-face session plus 2 post-quit telephone counseling sessions.
Figure 1.
Study design. Arrows denote timing of vaccinations for Schedule 1 (Week 0, 6, 12 and 26) and Schedule 2 (Week 0, 4, 8, 16, and 26). Primary endpoint (percent of subjects abstinent Week 19–26) and secondary endpoint (percent of subjects abstinent Week 19–52 are shown. NV (3’AmNic-rEPA); PBS (Phosphate buffered saline); Alum (aluminum hydroxide adjuvant); TQD (Target quit date).
Subjects were followed for 52 weeks after randomization and the first injection on Day 0 for a total of 21 visits. During the injection day, subjects remained at the study site for 30 to 60 minutes for observation and attended a visit 24 hours after each injection to assess side effects. Otherwise, visits ranged from weekly to bi-weekly and were less frequent after later injections.
Subjects who failed to quit smoking on their TQDs were encouraged to remain in the study and continue to attempt to achieve abstinence. Subjects who terminated from the study were not replaced and presumed to be smokers.
Institutional Review Board approval was obtained from all institutions involved in the study. A Data and Safety Monitoring Board (DSMB) met four times during the study.
Investigational Product
The active investigational product was purified 3’-aminomethylnicotine conjugated to P. aeruginosa r-exoprotein A. For the 200 and 400 µg/mL dose, each single-use syringe contained 3’-aminomethylnicotine conjugated to 200 or 400 µg rEPA, respectively, adsorbed to 1.2 mg aluminum hydroxide adjuvant (Alhydrogel 85) in 1 mL phosphate buffered saline (0.15 M NaCl, 0.002 M NaPO4, pH 7.2, 0.01% polysorbate 80; PBS). For the placebo dose, PBS with 1.2 mg Alhydrogel 85 was included in a 1 mL single-use syringe.
Measures
Cigarette use was recorded daily on an electronic diary for 182 days, and then weekly for the remainder of the study. Exhaled CO and urine cotinine were measured at each study visit, except for visits within 24 hours of vaccination. Questionnaires were collected via electronic diary: Fagerström Test for Nicotine Dependence [18] (administered days 0, 210, 364), Minnesota Nicotine Withdrawal Scale [MNWS, 19] (administered weekly until Month 6) and data on other tobacco usage.
Sera were collected for immunogenicity measurements at 16–17 time points (schedule-dependent) from baseline to week 52. Anti-nicotine antibody concentrations were measured using ELISA (Enzyme-linked Immunosorbent Assay) [12]. Subjects recorded standard local and systemic reactogenicity events for 7 days after each injection. All reactogenicity events were followed until resolution or study completion. Treatment emergent AEs were recorded until 4 weeks after the last dose, with the exception of SAEs, which were collected through week 52. Subjects were also periodically monitored at clinic visits for vital signs, weight, hematology, chemistry and urinalysis.
Statistical Analysis
The Intent-to-Treat (ITT) population was used for evaluation of efficacy, safety and immunogenicity. The ITT population was defined as all subjects who were randomized to treatment.
The primary endpoint was continuous smoking abstinence for a total of 8 weeks measured from the beginning of week 19 to the end of week 26 (determined from subject diaries confirmed by exhaled CO ≤8 ppm). The analysis for proof-of-concept stratified the active-treatment recipients into high (top 30% AUC from weeks 0 to 26) and low (bottom 70% AUC from weeks 0 to 26) Ab responders, regardless of treatment group. An a priori decision was made to establish the antibody cutoff between 50% and 25%. Top 30% by AUC group was selected as the largest group of high antibody responders between the 25% and 50% cutoffs that demonstrated statistical significance as compared to placebo. Smoking outcomes were compared between subjects with high Ab and pooled placebo recipients using logistic regression.
Secondary aims of this study were to assess a) 7-day point prevalence abstinence at various times, CARs during 52 weeks and time to sustained abstinence defined as attaining 8 weeks of continuous abstinence at anytime prior to week 46 and maintaining continuous abstinence through 52 weeks; b) impact on compensatory smoking amongst non-abstainers; c) withdrawal symptoms and d) immunogenicity, efficacy and safety of administration of either 4 or 5 of the 200 and 400 µg doses.
Secondary smoking cessation analyses used logistic regression for binary outcomes and Cox proportional hazards regression models and log-rank tests for time-to-sustained abstinence analyses. Mixed-effects repeated-measures analyses of the number of cigarettes smoked, CO and cotinine adjusted for baseline were utilized in assessing compensatory smoking among non-abstainers or in assessing withdrawal symptoms.
Anti-nicotine Ab responses were summarized as GMC with 95% confidence intervals. Safety was assessed throughout this study primarily using reactogenicity and adverse events (AEs). Reactogenicity data for 7 days after each injection were tabulated, and the proportions of subjects with any post-vaccination reactogenicity, aggregated over all injections, among the five treatment groups were compared using the Generalized Cochran-Mantel-Haenszel test.
When a subject dropped out of the study, they were assigned presumed to be smokers. Otherwise, all missing diary data related to cigarette use were imputed utilizing the Last Observation Carried Forward principle. No imputation was carried out for CO level. The missing serology data were imputed by first defining a set of injection windows for each schedule. The missing serology was imputed by using the next available measured serology in its corresponding window; if the next value was not available the value of the nearest previous time point in that window was used. The AUC for anti-nicotine Ab was calculated based on imputed data. For Ab values one week after the target quit date, the value at week 9 was used for Schedule 1 as sera were not collected at week 8.
Acknowledgement
This study was supported in part by National Institutes of Health Public Health Service award 1R01DA017894-01A1 and Nabi Biopharmaceuticals and registered as Identifier NCT00318383 and NCT00598325 at www.ClinicalTrials.gov. We thank the study coordinators, the subjects and the Nabi Team for their substantial contributions to the success of this study. We thank Joni Jensen for her help with the treatment materials. We thank National Institute on Drug Abuse (NIDA) for continued support and NIDA scientists Dr. Ivan Montoya and Dr. Jamie Biswas and for their contributions to this protocol and Dr. Paul Pentel for reviewing the manuscript.
Dr. Cheryl Oncken has received grant funding from Pfizer. Dr. Stephen Rennard has participated as a speaker in scientific meetings and courses under the sponsorship of AstraZeneca, GlaxoSmithKline and Pfizer; consulted with several pharmaceutical companies with relevance to the topics noted in the present manuscript (Almiral, Altana, Amersham, Array Biopharma, AstraZeneca, Aventis, Boehringer Ingelheim, Critical Therapeutics, GlaxoSmithKline, Globomax, Intermune, Merck, Novartis, Ono, Otsuka, Roche, Sanofi, Scios, Wyeth); serves on advisory boards for Altana and Pfizer; has been sponsored by GlaxoSmithKline for several clinical trials and has received laboratory support; conducted clinical trials for Roche, Pfizer, Sanofi and Novartis; has conducted both clinical trials and basic studies under the sponsorship of Centocor; has conducted basic studies under the sponsorship of AstraZeneca.
Footnotes
In subsequent completed and on-going clinical studies comparing 3’-AmNic-rEPA and placebo, over 1800 subjects have received 1–6 vaccinations with 400 µg 3’AmNic-rEPA or placebo. Only two additional cases of herpes zoster have been observed. Moreover, no additional anaphylactic/anaphylactoid-type SAEs have been reported to date.
Conflict of interest/Disclosure
Ms. Akhavain, and Drs. Fahim, Kessler, Kalnik and Niknian are employees of Nabi Biopharmaceuticals and have received salary support, stock and options. All other authors were investigators on the clinical trial funded by NIDA and by Nabi Biopharmaceuticals and some served on an advisory panel. Other disclosures include the following: Dr. Douglas Jorenby has received research support from Pfizer. Dr. David Gonzales has received grant/research support from Pfizer, Addex Pharmaceuticals, Sanofi-Aventis and GlaxoSmithKline; consulting fees and honoraria from Pfizer, GlaxoSmithKline, and Evotech NeuroSciences; speakers fees from Pfizer; and owning five shares of Pfizer stock. Dr. Nancy Rigotti has received research grant support from Pfizer and is an unpaid consultant to Pfizer and Free & Clear. Dr. Elbert Glover has served as a speaker, consultant, grantee, provided advice or is on the advisory board/panel for Pfizer and served as a speaker for Nabi Biopharmaceuticals.
This paper was presented in part at the American Heart Association Scientific Sessions 2007, Orlando, Florida, November 7, 2007.
References
- 1.Mackay J, Ericksen M, Shafey O. The Tobacco Atlas. 2nd ed. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet. 2008;371:2027–2038. doi: 10.1016/S0140-6736(08)60871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiore MC, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 4.Pentel PR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol. Biochem. Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 5.Carrera MR, et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg. Med. Chem. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Cerny EH, et al. Preclinical development of a vaccine 'against smoking'. Onkologie. 2002;25:406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- 7.de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine alters the distribution of nicotine but not the metabolism to cotinine in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 2004;370:299–304. doi: 10.1007/s00210-004-0960-3. [DOI] [PubMed] [Google Scholar]
- 8.LeSage MG, et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology. 2006;184:409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- 9.Malin DH, et al. Passive immunization against nicotine attenuates nicotine discrimination. Life Sci. 2002;70:2793–2798. doi: 10.1016/s0024-3205(02)01523-0. [DOI] [PubMed] [Google Scholar]
- 10.Keyler DE, et al. Reduced nicotine distribution from mother to fetal brain in rats vaccinated against nicotine: time course and influence of nicotine dosing regimen. Biochem. Pharmacol. 2005;69:1385–1395. doi: 10.1016/j.bcp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Keyler DE, Hieda Y, St Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob. Res. 1999;1:241–249. doi: 10.1080/14622299050011361. [DOI] [PubMed] [Google Scholar]
- 12.Hatsukami DK, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin. Pharmacol. Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Cornuz J, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St. Clair Roberts J, Akers CCR, Vanhinsbergh L, McKenna KA, Wood DM, Jack L. Longitudinal safety and immunogenicity data of TA-NIC, a novel nicotine vaccine. Ninth Annual Meeting of the Society for Research on Nicotine and Tobacco; Society for Research on Nicotine and Tobacco; 2003 February 19–22; New Orleans, LA. 2003. [Google Scholar]
- 15.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 16.Committee on Infectious Diseases. Prevention of pertussis among adolescents: recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. Pediatrics. 2006;117:965–978. doi: 10.1542/peds.2005-3038. [DOI] [PubMed] [Google Scholar]
- 17.Fiore MC, et al. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. Jun, Treating tobacco use and dependence. 2000. [Google Scholar]
- 18.Heatherton TF, Koslowski LT, Frecker RC, Fagerström K-O. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Hughes JR, Hatsukami DK. The nicotine withdrawal syndrome: a brief review and update. International Journal of Smoking Cessation. 1992;1:21–26. [Google Scholar]