Abstract
Urinary citrate is an important inhibitor of calcium stone formation. Most of citrate reabsorption in the proximal tubule is thought to occur via a dicarboxylate transporter NaDC1 located in the apical membrane. OK cells, an established opossum kidney proximal tubule cell line, transport citrate but the characteristics change with extracellular calcium such that low calcium solutions stimulate total citrate transport as well as increase the apparent affinity for transport. The present studies address several fundamental properties of this novel process: the polarity of the transport process, the location of the calcium-sensitivity and whether NaDC1 is present in OK cells.
OK cells grown on permeable supports exhibited apical > basolateral citrate transport. Apical transport of both citrate and succinate was sensitive to extracellular calcium whereas basolateral transport was not. Apical calcium, rather than basolateral, was the predominant determinant of changes in transport. Also 2,3-dimethylsuccinate, previously identified as an inhibitor of basolateral dicarboxylate transport, inhibited apical citrate uptake.
Although the calcium-sensitive transport process in OK cells is functionally not typical NaDC1, NaDC1 is present in OK cells by Western blot and PCR. By immunolocalization studies, NaDC1 was predominantly located in discrete apical membrane or subapical areas. However by biotinylation, apical NaDC1 decreases in the apical membrane with lowering calcium.
In sum, OK cells express a calcium-sensitive/regulated dicarboxylate process at the apical membrane which responds to variations in apical calcium. Despite the functional differences of this process compared to NaDC1, NaDC1 is present in these cells, but predominantly in subapical vesicles.
INTRODUCTION
Kidney stones are a common and serious medical disorder, causing significant medical costs (47). Urinary citrate is an important inhibitor of calcium stones and low urinary citrate is a common contributor to many stone types (1). Citrate, a tricarboxylate, keeps calcium soluble in the urine; however, the regulation of urinary citrate has received little recent attention and remains poorly understood at the cell and molecular level.
After NaDC1 was cloned, the assumption was that this single apical transporter accounted for all of renal citrate reabsorption and control of urinary excretion. However, some findings indicate that this may not be the case. First, human NaDC1 has a very low affinity for citrate (2), which would limit the complete reabsorption of citrate. Also, our previous studies strongly suggest that a novel calcium-sensitive transport process is present in cultured proximal tubule cells and this transport process does not appear to be NaDC1 (3;4). This transport process corresponds with the clinical observations that urinary citrate increases with urinary calcium in normal individuals (5). In these studies, we demonstrated that OK cells (a widely used proximal tubule cell line derived from the opossum kidney) transport both citrate and succinate (3;6). However, surprisingly the magnitude and properties of this transport appear to vary with extracellular calcium (3). These findings could have important implications for understanding regulation of urinary citrate. In our previous studies, we demonstrated that in OK cells decreasing extracellular calcium increases both succinate and citrate transport and also appears to dramatically increase the affinity of the transport process for various dicarboxylates (4). These studies also determined that NaDC1 expressed in oocytes is not calcium-sensitive. Taken together these studies indicate that OK cells express a novel calcium-sensitive dicarboxylate transporter in addition to NaDC1.
The present studies were designed to address several unanswered issues regarding the calcium-sensitive/regulated dicarboxylate transport process and NaDC1 in OK cells: the polarity (apical versus basolateral membrane) of the calcium-sensitive transport process, the polarity of the calcium effect and whether OK cells express NaDC1 at all. The studies presented here demonstrate that: 1) the calcium-sensitive dicarboxylate transport process in OK cells is present on the apical membrane, 2) this transport is inhibited by 2,3-dimethylsuccinate, usually an inhibitor of basolateral dicarboxylate transport, 3) dicarboxylate transport on the basolateral membrane of OK cells is not consistently calcium-sensitive, 4) apical calcium influences citrate and succinate transport much more than any effect of basolateral calcium, 5) NaDC1 is present in OK cells despite the predominance of the apparently distinct calcium-sensitive/regulated transport process, and 6) apical membrane NaDC1 decreases with lowering extracellular calcium, opposite to the direction of citrate transport. All of these findings support and further define a potentially novel mechanism of citrate transport in the kidney.
METHODS
Uptake studies using OK cells grown on permeable supports
As described previously, OK cells between passages 90 and 100 were maintained in MEM (Minimum Essential Medium Eagle, Sigma) containing 26 mM HCO3− and supplemented with 10% fetal bovine serum (Gibco-Invitrogen), 25 mM HEPES, 11 mM L-glutamine, 100 IU penicillin, and 100 mg/ml streptomycin in a humidified atmosphere of 5% CO2-95% air at 37 °C (11;12;15). In these experiments, cell monolayers were grown on 12 mm permeable supports (Transwell, Corning-Costar), with media changes every 2 days. After reaching confluence, cell monolayers were changed to serum-free media for a minimum of 24 h before study.
Citrate transport was measured by the uptake of radiolabeled citrate into cell monolayers. Just before uptake measurement, the apical and basolateral sides were rinsed free of media and equilibrated for 2 minutes at 37 °C in a buffer containing either normal (1.2 mM) or low calcium (< 60 μM, nominally calcium free). The remaining components of the buffer were as follows (in mM): 109 NaCl, 3 KCl, 2 KH2PO4, 1 MgSO4, 5 alanine, 8.3 glucose, 1 Na acetate, 25 HEPES; osmolality was 290 mmol/kg H2O and pH 7.4. Analysis of uptake buffers determined ionized calcium at 1.14 mM in the normal and < 60 μM in the nominally calcium free solutions.
The uptake experiments were performed at 37 °C. Apical uptakes were begun by adding 0.525 ml of the appropriate uptake buffer (1.2 mM or < 60 μM calcium containing uptake buffer) that contained 0.5 mCi per ml [1,5-14C]-citrate (Sigma-Aldrich) or [1,4-14C]-succinate (Perkin-Elmer) added only to the apical side of individual wells. (For basolateral uptakes, 1.5 ml of uptake buffer is used.) The final concentration of isotope was approximately 0.014 mM for citrate or 0.0106 mM for succinate. The uptake solution also contained 1 μl/ml [3H]-mannitol (Perkin-Elmer, specific activity 15–30 Ci/mmol, 1 mCi/1000 μl) to determine the residual extracellular volume.
After 5 minutes the uptake solution was rapidly removed and the wells were rinsed three times with ice-cold 0.1 M MgCl2. The membrane was cut away from the support and placed in a scintillation vial. The cell monolayer was lysed with 250 μl of 0.1 N NaOH. Each vial was then mixed by vortex and allowed to incubate in the NaOH for 5 minutes followed by the addition of 5 ml of scintillation fluid (ScintiSafe Econo 2, Fisher Scientific). Samples were mixed by vortex then placed in the scintillation counter.
Uptake was calculated from the measured [14C] radioactivity per well; appropriate windows and crossover calculations were used to distinguish [3H]-mannitol and [14C]. Uptake was further factored for the residual extracellular volume that was not removed by the triplicate rinsing; the residual extracellular volume was calculated from the residual [3H]-mannitol. Experiments that had a residual extracellular volume of > 1% of the initial uptake media were eliminated. In each experiment, [3H]-mannitol and [14C]-citrate and succinate transepithelial fluxes (or leaks) were also measured (apical to basolateral solution or vice versa); these were consistently negligible. In cases with significant leak, individual wells were eliminated from calculations. Since the apical compartment sits above and separate from the basolateral, after five minutes of incubation in uptake buffer a 10 μl sample is taken from the compartment opposite the tracer addition and placed in individual scintillation vials; this sample is counted to check for appearance of isotope in the opposite compartment to assess for leaks due to membrane puncture or non-confluent cells, if this was significantly above background that Transwell was omitted. Because of variability in absolute transport rates, each experiment had a simultaneous control on the same plate of cells. Variability was less when separate experiments were done over a relatively short period of time and using similar cell passage numbers; in these cases, absolute rather than normalized uptake is provided.
PCR Detection and Sequencing of NaDC1
For detection by PCR and sequencing of NaDC1 in OK cells, total RNA isolation was performed using cells grown on 24 mm permeable supports (Transwell, Corning-Costar) using RNeasy mini kit (Qiagen). Cells were collected in 600 μl of Buffer RLT (RNeasy Lysis Buffer, Qiagen) containing 1% of β-mercaptoethanol, cell lysates were homogenized by placing into QIAshredder Spin Column (Qiagen) and processed following the standard spin protocol for isolation of total RNA from animal cells, including the on-column DNase digestion step, using RNase-free DNase Set (Qiagen).
PCR primers were designed using the opossum (Didelfis virginiana) NaDC1 mRNA sequence (accession number AY186579) and the GENERUNNER program for four overlapping fragments of about equal size of the whole gene. The specific primers used are provided in Table 1. The four predicted PCR products covered the whole NaDC1 mRNA sequence with the exception of a few nucleotides before the polyA tail.
Table 1.
PCR primers and predicted product size
| F1 | 5′-TTCTCCTCGGTTCAGCACCG-3′ |
| R1 | 5′-GGTGGCATTGATGTTGGGACAG-3′ |
| Product P1 659 bp (1–659) | |
| F2 | 5′-GATGGGCCACGTCAACATCTC-3′ |
| R2 | 5′-AGCCCTGCTGCTGAGAGAAGAC-3′ |
| Product P2 725 bp (546–1270) | |
| F3 | 5′-TGTGGCCTTCTTCATCAGCATC-3′ |
| R3 | 5′-GATCACCGGCTTCTCTCTGAGC-3′ |
| Product P3 703 bp (1197–1899) | |
| F4 | 5′-GGCCCAGATCAACAGTACCTCC-3′ |
| R4 | 5′-GGTACAGAAAAATTTTTAATGTGGCAAATAC-3′ |
| Product P4 593 bp (1790–2383) | |
RT-PCR was performed using SuperScript III One-step PCR kit with Platinum Taq DNA polymerase (Invitrogen) in a PTC-200 thermocycler (BioRad). PCR cycling conditions were: 55°C 30 min, 94°C 2 min, (94°C 16 sec, 58°C 30 sec, 68°C 1 min) x 40 cycles, 68°C 5min. PCR products were purified using QIAquick Gel Extraction Kit (Qiagen). DNA Sequencing was performed from 70 ng of purified PCR product.
Western Analysis and Biotinylation
Protein samples from OK cells or from rat kidney cortex (~ 20 μg per lane) were separated by SDS-polyacrylamide gel electrophoresis on 10% tricine gels. The samples were transferred to nitrocellulose by electrotransfer. The nitrocellulose membranes were blocked for a minimum of 1 hour in PBS-TM (phosphate buffered saline containing 0.05% Tween 20 and 1% high purity BSA). The primary antibody was diluted 1:1000 in PBS-TM and applied for 1–2 hours or overnight with gentle shaking at 4 °C. The secondary antibody, horseradish peroxidase-linked anti rabbit Ig (Amersham-Pharmacia) was diluted 1:20,000 in PBS-TM and applied for 1 hour. All washes were completed with shaking at room temperature. Antibody binding was visualized by enhanced chemiluminescence using the Supersignal HRP substrate system (Pierce). Molecular weights were estimated by comparison with pre-stained rainbow protein standards (BioRad) in combination with Magic Mark protein standards (Invitrogen).
For biotinylation of apical membrane proteins, cells were rinsed twice at 37 °C with either 1.2 mM or <60 μM calcium solution and incubated for 2 min @37 °C. Cells were then rinsed again and incubated @37 °C for 3 minutes. (3,4,6) This incubation procedure is the same used for uptake experiments. After rinsing twice at 4 °C, ice-cold biotin solution (Sulfo-NHS-SS-Biotin, 0.5 mg/ml) is applied at 4 °C for 30 min. The cells are then quenched, collected, rinsed, and centrifuged at 500xg for 3 minutes. The supernatant is removed and the cells are washed with 5 ml TBS, followed by incubation with lysis buffer for 30 min on ice. The cell lysates are centrifuged at 10,000xg for 5 min at 4 °C. The biotin-labeled proteins are isolated on a NeutrAvidin column and incubated for 60 min @ RT on a rotator. The columns are centrifuged for two minutes at 10,000xg and washed 3 times with PIC containing washing buffer. The biotinylated protein is eluted, heated for 5 min at 95 °C followed by centrifugation for 2 minutes at 10,000xg. Samples are run on a 10% Tricine gel and probed with NaDC1 antibody overnight (Upstate).
Immunofluorescence Localization of NaDC1
For immunostaining of cells on Transwells, cells were fixed, embedded, sectioned, stained, and then mounted. For cells on coverslips, cells were simply permeabilized and stained. All immunofluorescence studies were performed on cells in 1.2 mM calcium.
For the latter, cells were rinsed briefly with 0.5 M Tris buffer pH 7.6 and then incubated in diluted primary antibody for one hour at room temperature. After rinsing with 0.5 M Tris buffer, cells were incubated in secondary antibody (goat anti-rabbit IgG Alexa 488, Molecular Probes, Invitrogen) and diluted in PBS for 1 hour at room temperature. After a final rinse with 0.5 M Tris buffer, cells were mounted and viewed. Photographs were taken using a Nikon Diaphot Epifluorescent microscope with a 100X oil objective that is connected to a CCD-cooled digital camera (Cool Snap, Princeton Instruments) interfaced with an image analysis system (Metamorph, Universal Imaging). Camera shutter control was via computer utilizing the imaging software (Metamorph).
Confocal imaging was performed at the LSU Imaging Core Facility. Cells were grown on 12 and 24 mm permeable supports (Transwell, Corning-Costar) and were fixed in 4% methanol free formaldehyde for 15 minutes and thoroughly washed in PBS. Permeabilization of cells was performed with 0.1% Triton-X for 10 minutes followed by washing and blocking with 5% goat serum diluted in 1% BSA in PBS for 20 minutes. Incubation followed for 90 minutes at room temperature with primary antibodies (NaDC1) diluted in 1% BSA in PBS along with corresponding negative controls subjected to either a blocking or antibody diluent only. Next, cells were washed in PBS and incubated with a goat anti-rabbit Ig (H+L) Alexa Fluor 488 (Invitrogen, Molecular Probes, Eugene OR) diluted to 2 μg/mL in 1% BSA in PBS for 30 minutes and rinsed to complete indirect fluorescence detection. A Radiance 2100 Laser Scanning Confocal Microscope (BioRad, Hercules, CA) equipped with a dual Kr/Ar laser and blue diode was used to capture representative images. Acquisition entailed optimized, simultaneous scans of green and blue fluorescence using the argon laser with a 560DCLP/515-30 PMT and a blue diode with a 500DCLP/442-45 PMT configuration, respectively. Optical sectioning was performed through an oil immersion 60X Plan Fluor objective (NA + 1.320) with an additional 2.0X digital zoom at 0.15 μm per plane, for an average total of 30 planes per sample. Stacks were acquired and rendered with the system’s Radiance 2000 software; however, 3D, xz views and analysis were performed with LaserVox.
RESULTS
Citrate Transport in OK Cells Grown on Permeable Support
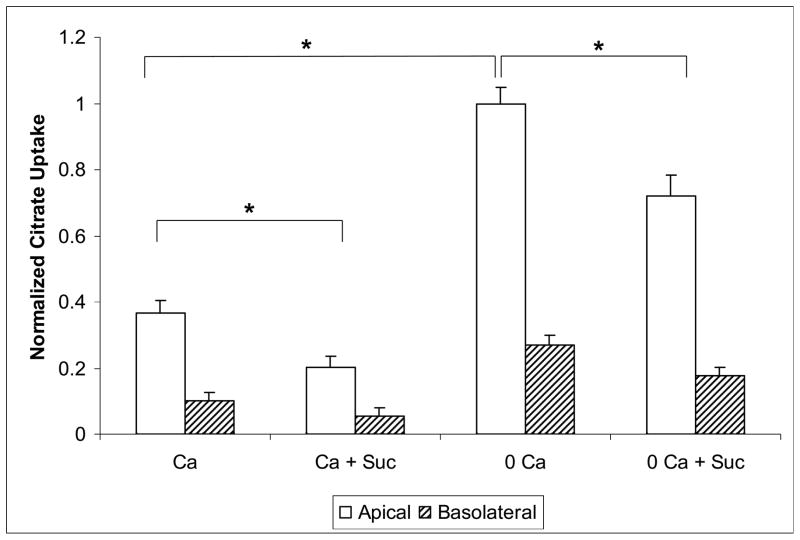
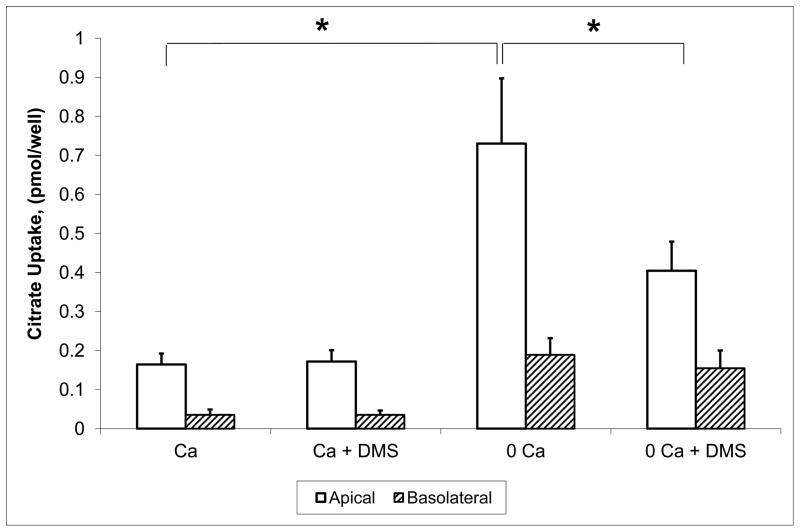
Citrate uptake was measured from the apical and basolateral solution in parallel experiments (performed in adjacent wells). In the initial experiments shown in Figure 1, the composition of the apical and basolateral solutions was identical (including calcium and succinate). The [14C]-citrate isotope was added only to the apical or the basolateral side. Figure 1 shows apical and basolateral citrate uptake with varying levels of calcium and succinate. Succinate was used as a known competitive inhibitor of dicarboxylate citrate transport. With 1.2 mM calcium solutions, apical citrate uptake is significantly higher than basolateral uptake (0.60 ± 0.05 vs 0.14 ± 0.07 pmol/filter). The second set of bars from the left show the result of adding cold succinate as a competitor of dicarboxylate transport. Apical citrate uptake (still in the presence of 1.2 mM calcium) was inhibited by 1 mM succinate (0.31 ± 0.05 vs 0.60 ± 0.05 pmol/filter); basolateral uptake was inhibited (or competed) as well by succinate, but this was not statistically significant. When extracellular calcium was lowered to < 60 μM (shown in the third set of bars), apical citrate transport approximately tripled over that in the presence of 1.2 mM calcium; basolateral uptake increased as well in this set of experiments but not in others as noted below. Mean apical uptake in absolute terms (non-normalized) was 1.7 ± 0.13 pmol/filter and mean basolateral uptake 0.49 ± 0.068 pmol/filter. The far right set of bars in Figure 1 demonstrates that 1 mM succinate significantly inhibited (or competes with) apical citrate uptake and to some extent basolateral citrate uptake. In sum, apical citrate transport is inhibited by succinate consistent with dicarboxylate transport and stimulated by lowering extracellular calcium. Basolateral citrate transport is stimulated to a lesser extent by lowering extracellular calcium in this series of experiments, but not stimulated at all in other experiments as seen below.
Figure 1. Apical and Basolateral Citrate Transport in OK Cells Grown on Permeable Support.
Normalized [14C]-citrate uptake in OK cells in the presence of 1.2 mM extracellular calcium (Ca) or < 60 μM Ca. Because of a ~ 4 fold variation in citrate uptake in various groups of cells (by week), uptake was normalized in a given group of cells to the mean apical uptake in low calcium solution without succinate. Apical uptake is represented by the open bars and basolateral by the hatched bars. Cells grown on permeable supports were exposed for 5 minutes to ~ 0.014 mM [1,5-14C]-citrate at 37 °C. Total extracellular calcium was nominally 1.2 mM in the left two sets of bars and < 60 μM in the right two sets of bars. Experiments in the second and fourth sets of bars have 1 mM succinate (Suc) added as a competitive inhibitor. In all cases, apical uptake was significantly higher than basolateral uptake. * = P < 0.05. Citrate uptake from the apical media is significantly greater in the < 60 μM Ca media than with 1.2 mM Ca. Citrate uptake from the apical solution is significantly less with 1 mM succinate in both the 1.2 mM and < 60 μM calcium solutions. n = at least 12 permeable supports from at least 5 separate groups of cells.
Effect of Graded Changes in Extracellular Calcium on Citrate Uptake
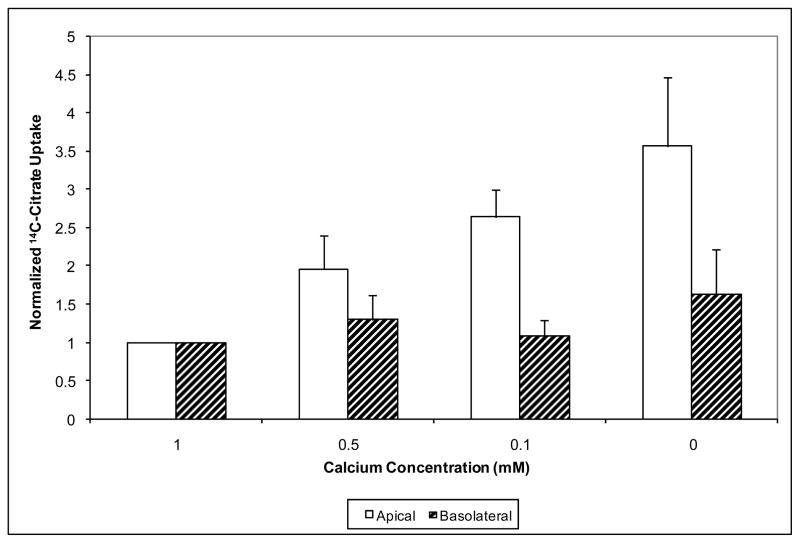
In our previous studies (3), graded levels of calcium (from 0 added to 1.2 mM) altered citrate transport. This supported the concept that the increase in citrate transport with lowering calcium may be a physiologic effect rather than just a phenomenon that could be observed only at extremely low levels of calcium. Therefore similar experiments, illustrated in Figure 2, were repeated in OK cells grown on permeable support and the calcium concentration is identical in both the apical and basolateral solutions. Here, as the extracellular calcium levels are lowered (moving from the left to the right sets of bars) apical citrate uptake significantly increases consistent with the findings in Figure 1. Basolateral citrate uptake in these experiments did not change significantly. This again indicates that extracellular calcium concentration is affecting primarily the apical transport system for citrate and not the basolateral transport system.
Figure 2. Apical and Basolateral Citrate Transport with Varying Extracellular Calcium.
This figure shows the effect of graded changes of extracellular calcium on apical citrate uptake. Calcium concentrations ranged from 1.0 mM (far left) to < 60 μM extracellular calcium (far right). Apical uptake is represented by the open bars and basolateral by the hatched bars. Uptake from each compartment was normalized to the uptake in 1 mM calcium. The apical and basolateral sides are changed to the same calcium containing solution simultaneously. As calcium concentration is lowered, only citrate uptake from the apical compartment increased significantly in a graded fashion with changes in extracellular calcium. * P < 0.0001 for trend, n = 7–8 permeable supports.
Effects of Apical or Basolateral Calcium Changes on Citrate Uptake
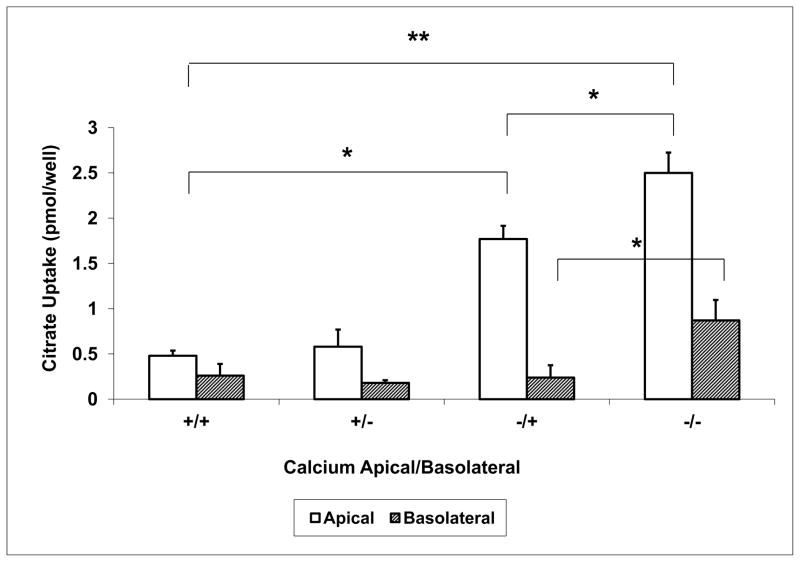
In the experiments shown in Figures 1 and 2, the calcium concentration was simultaneously changed in the apical and basolateral solutions. To investigate whether the effect of extracellular calcium on citrate transport is due to varying calcium concentrations at the apical or the basolateral side, extracellular calcium was changed separately in the apical and basolateral solutions, Figure 3. Comparing the second set of bars to the first, basolateral calcium removal has minimal effects on citrate uptake from either side of the permeable support. In the third set of bars lowering extracellular calcium in the apical solution only resulted in an increase in citrate uptake from the apical side (0.48 ± 0.06 vs 1.77 ± 0.15 pmol/filter); no change in citrate uptake is observed at the basolateral side. Removing calcium from both sides resulted in a further increase of citrate uptake on the apical side. However, some stimulation of basolateral citrate uptake is also observed in this series when low calcium is present in both the apical and basolateral solutions. Thus, the changes in basolateral citrate uptake when calcium is removed from both apical and basolateral solutions are similar to the series in Figure 1 but contrast with the series in Figure 2. Because the focus of the present studies was to examine and confirm the apical location of the calcium-sensitive transport, and this had been confirmed, experiments to exclude small leakage of either calcium or citrate to account for the variable basolateral uptake results were not pursued. Although increased permeability of the paracellular pathway could theoretically account for some of the effects when calcium was lowered in both the apical and basolateral solutions, no increase in mannitol permeability was noted.
Figure 3. Effect of Separate Changes in Apical or Basolateral Ca2+ on Citrate Transport.
Calcium was changed separately on the apical and basolateral sides to determine whether apical and/or basolateral calcium independently altered citrate transport. A “+” sign in figure 3 represents 1.2 mM calcium and a “−”sign represents < 60 μM calcium. Apical and basolateral solutions are noted as “apical/basolateral”; for example, +/− is apical 1.2 mM and basolateral < 60 μM extracellular calcium. Apical uptake is represented by the open bars and basolateral by the hatched bars. Low apical calcium significantly stimulated apical citrate uptake; this is further enhanced by simultaneous low basolateral calcium. * P < 0.05, ** P < 0.001, n = 7–8 permeable supports.
Succinate Transport: Effects of Apical or Basolateral Calcium Changes
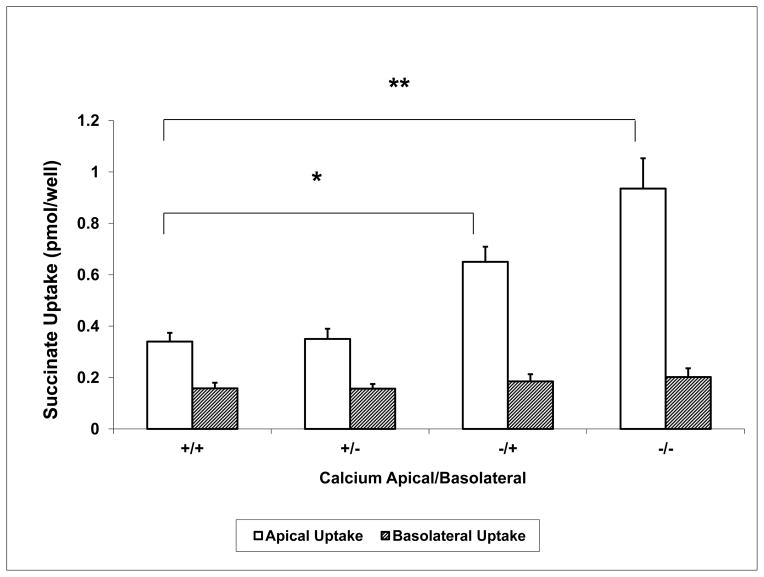
Succinate is the dicarboxylate which is most widely used as a model substrate for dicarboxylate transport. In comparison to citrate, succinate complexes with calcium significantly less (~ 100 fold less complexation) (7), and is predominantly divalent at physiologic pH. Therefore, succinate uptake should not be influenced by calcium complexation or by transport of more than one ionic species (such as citrate−2 and citrate−3 for citrate). [14C]-Succinate uptake was measured similar to experiments in Figure 3 using the normal 1.2 mM calcium or the < 60 μM calcium solutions in the apical and basolateral compartments; these experiments were performed in the absence of citrate. Results are shown in Figure 4. The left two bars show succinate uptake higher at the apical than the basolateral side (0.34 ± 0.03 vs 0.16 ± 0.02 pmol/filter). There is no change in succinate uptake with the lowering of basolateral calcium, shown in the second set of bars. However, in the third set of bars apical succinate uptake approximately doubles (to 0.65 ± 0.06 pmol/filter) with no change in the basolateral uptake when extracellular calcium is lowered only at the apical side. In the fourth set of bars a further increase in succinate uptake is observed on the apical side when the < 60 μM calcium solution is applied simultaneously to both the apical and basolateral solutions. Again there is no change in the level of basolateral succinate uptake. In sum, changes in extracellular calcium affect only apical succinate transport, and apical rather than basolateral extracellular calcium is the predominant modulator of succinate transport. These effects are similar to those for citrate, but the interpretation is unaffected by calcium complexation of the transported substrate.
Figure 4. Succinate Transport in Cells Grown on Permeable Supports with +/− Ca2+ in Apical or Basolateral Solutions.
Similar experiments as in Figure 3 except [14C]-succinate uptake is measured. Removal of calcium from the apical side only significantly increases succinate uptake. Apical uptake is represented by the open bars and basolateral by the hatched bars. A “+” sign represents 1.2 mM calcium and a “−” sign represents < 60 μM calcium. Apical and basolateral solutions are noted as “apical/basolateral”; for example, +/− is apical 1.2 mM and basolateral < 60 μM extracellular calcium. Cells grown on permeable supports are exposed for 5 minutes to ~ 0.014 mM [1,4-14C]-succinate at 37 °C. * P < 0.05, ** P < 0.001, n = 12 permeable supports.
Effect of 2,3-Dimethylsuccinate on Citrate Uptake
To address the nature of the apical calcium-sensitive citrate transport process, we studied the effect of 2,3-dimethylsuccinate (DMS) which has been found to inhibit basolateral dicarboxylate transport, but does not inhibit NaDC1 (8;9). Basolateral dicarboxylate transport has previously been reported as a high affinity process and our prior findings (4) indicated that the calcium-sensitive OK cell transport also has high citrate and succinate affinity. Results are shown in Figure 5. Citrate uptake from the apical and basolateral membrane is shown in normal or low calcium and in the absence and presence of 1 mM DMS. Clearly the reduction of extracellular calcium stimulates apical citrate transport (and in this case, moderately basolateral transport). More importantly DMS only inhibits the apical calcium-sensitive transport. This directly suggests the possibility that a basolateral type transporter such as NaDC3 could be involved in apical transport.
Figure 5. The Effect of 2,3-Dimethylsuccinate (DMS) on Apical and Basolateral Citrate Transport.
Apical citrate uptake is represented by the open bars and basolateral by the hatched bars. Experiments were performed as in Figure 1, except that experiments in the second and fourth sets of bars have 1 mM 2,3-dimethylsuccinate added as an inhibitor. In all cases, apical uptake was significantly higher than basolateral uptake. DMS significantly inhibited apical citrate uptake in the < 60 μM calcium solutions but not in the normal calcium solutions; basolateral citrate uptake was not altered by DMS. n = at least 12 permeable supports from 3 separate groups of cells.
Evidence of NaDC1 mRNA and protein in OK cells
NaDC1 has been clearly demonstrated in the related OKP cell line, one of 3 clonal sublines derived from the parental OK cell line by Cole et al (14). These subclones were found to have morphological and functional characteristics distinct from that of the parental cell line (9). Importantly OKP cells exhibited no functional activity until NaDC1 was over-expressed (10). Although our previous results with citrate transport in OK cells were different from reported NaDC1 properties, we next used a variety of methods to determine whether NaDC1 was present in OK cells.
PCR detection and sequencing of NaDC1
PCR was used both to detect NaDC1 mRNA and to screen for possible sequence variations from reported isoforms, particularly that from OKP cells, which might account for the unusual functional properties (e.g., calcium-sensitivity and relatively high dicarboxylate affinity found in OK cells). Each of the PCR primer pairs produced single bands at the expected molecular weight on 1% TBE-agarose gels. The PCR product bands were excised, purified and sequenced. The sequences were 100% identical to that reported from OKP cells (10). These results demonstrate that NaDC1 mRNA is present in OK cells, and suggest that alternative isoforms (at least closely related ones such as alternative splice products) are not present.
NaDC1 protein detection and localization in OK Cells
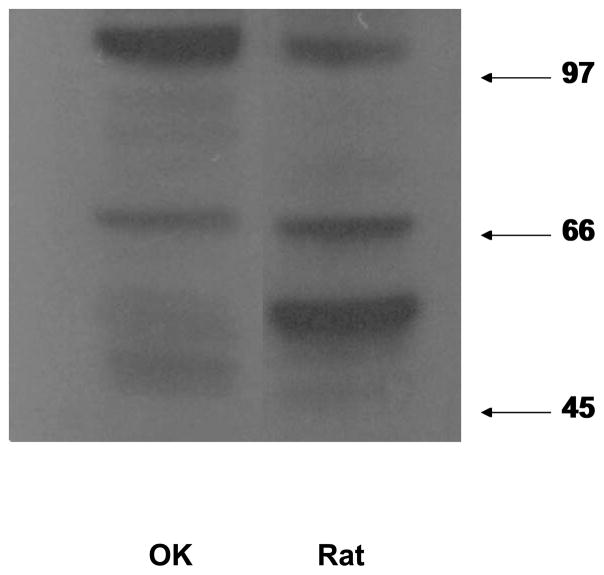
Chicken and rabbit antibodies directed against the second cytoplasmic loop of NaDC1 were obtained from Dr. Ana Pajor (University of California, San Diego) and from Upstate-Millipore to assess NaDC1 presence and localization in OK cells. First, Western analysis was utilized to determine whether NaDC1 protein is expressed in OK cells. Figure 6, a representative western blot, demonstrates that NaDC1 is present in OK cells and is compared to rat kidney cortex (run on the same gel).
Figure 6. Western blot of whole cell lysates prepared from OK cells (OK) and rat kidney cortex (Rat).
The mature form of NaDC1 protein at ~66 kDa is seen in both lanes. This is in agreement with the predicted mass of the primary transcript based on the sequence of NaDC1, 65.6 kDa (27). Lanes are from the same gel with an intervening lane removed.
Despite the prior evidence that NaDC1 does not account for the calcium-sensitive citrate transport (12), further experiments were done using biotinylation of surface proteins to determine if NaDC1 at the surface membrane increases in response to lowering calcium. Results are shown in Figure 7 and demonstrate that less NaDC1 protein is labeled at the surface membrane in conditions of low extracellular calcium despite the citrate transport being higher (shown in Figures 1–3). Although these changes in NaDC1 at the surface membrane may be important, since the direction of changes is opposite to the transport effects, this aspect was not further pursued for the purposes here.
Figure 7.
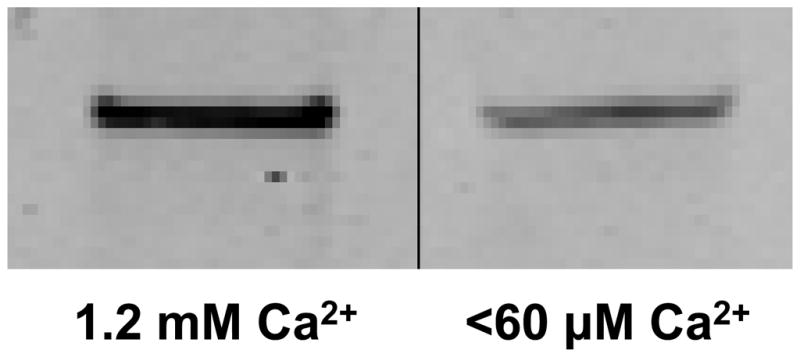
Biotinylation of NaDC1 on the apical membrane of cells incubated in normal or low calcium solutions. In contrast to the transport results, less NaDC1 is labeled in the low calcium solutions.
Further studies were performed to localize NaDC1. Figure 8a shows a low power cross-sectional view of the immunolocalization of NaDC1 in the apical membrane of OK cells grown on permeable support. Figure 8b shows cells grown on coverslips and viewed at a higher magnification. Punctate staining appearing to be discreet vesicles predominantly in the subapical region was seen. In each of these immunolocalization studies, sections without the primary antibody revealed no staining. Further imaging studies were obtained using confocal microscopy. Shown in Figure 8c is a cross sectional view of the z-axis reconstruction from similar images of an OK cell grown on a coverslip; the red is a pseudo color indicator of fluorescent intensity. Thus NaDC1 appears to be present at or near the apical and/or subapical region and predominantly in discreet punctuate areas, possibly membrane vesicles or within microvilli.
Figure 8. Immunolocalization of NaDC1 in OK Cells.
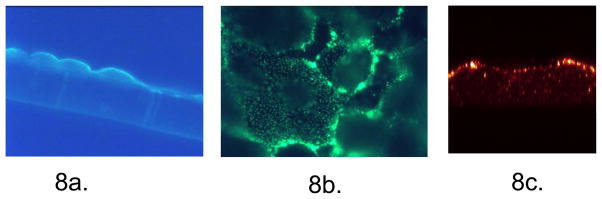
Figure 8a. Cross-sectional view of NaDC1 in OK cells grown on permeable support. To assess NaDC1 localization, OK cells were grown on permeable support. Shown here is the cross sectional view of the immunolocalization of NaDC1 antibody to the apical membrane of OK cells. Sections without the primary antibody revealed no staining. Figure 8b. Transmission view of punctuate appearance of NaDC1 in cells grown on solid support. OK cells shown here are grown on coverslips and are viewed at a higher magnification (100X fluorescent objective, Nikon). Subapical vesicles represented by punctate staining were observed when focusing up and down through the cells. Cells without the primary antibody revealed no staining. Figure 8c. Confocal cross sectional z-axis reconstruction. Similar images of an OK cell grown on a coverslip; the red color is a pseudo color indicator of fluorescent intensity. Here NaDC1 is located at the apical surface as well as in discrete punctate areas.
DISCUSSION
A calcium-sensitive citrate and dicarboxylate transport process in the OK proximal cell line was established in our previous studies (3;4). This is a novel process, defined particularly by the calcium-sensitivity and relatively high affinity for dicarboxylates, both properties not found with the previously established apical dicarboxylate transport process. The present studies demonstrate that the calcium-regulated citrate transport process in OK cells is located on the apical membrane and is sensitive to apical calcium. In addition, the current studies demonstrate that NaDC1 is present in OK cells despite the lack of clear functional activity (discussed below), perhaps due to inadequate surface membrane expression.
In the present studies, apical transport of citrate was clearly a dicarboxylate transport process (rather than transport of the more abundant tricarboxylate citrate) since succinate was both transported and also inhibited citrate transport. This was true in both the normal and in the low extracellular calcium solutions. In contrast, in our prior studies with cells grown on plastic, citrate transport was minimally affected by succinate in normal calcium solutions (4;6). In our first studies utilizing OK cells grown on plastic (6), in normal (1.2 mM) calcium solutions, citrate transport appeared to be via a tricarboxylate transporter similar to that reported in basolateral membrane vesicles (11;12). The cells grown on permeable support are likely more differentiated and express the calcium-sensitive dicarboxylate transport process more robustly. In cells grown on plastic this transport process may be so poorly expressed that the functional dicarboxylate transport is small compared to the observed tricarboxylate transport process (not competed by succinate) in normal calcium solutions (6).
In our previous studies (3;4), we addressed the issue of complexation of citrate by calcium and magnesium. Calcium in high concentrations can certainly complex citrate and influence citrate transport (13); as citrate complexes calcium, less divalent citrate is available for transport. However, complexation cannot account for most of the findings found in OK cells, since succinate has significantly less complexation with calcium and yet changes in extracellular calcium had similar effects as with citrate. In addition, citrate transport in cells expressing NaDC1 do not exhibit the same calcium sensitivity (4).
NaDC1 is not calcium sensitive, as demonstrated and discussed in our previous paper (4). However, NaDC1 mRNA is present in OK cells as previously shown by Aruga et al (10) in OKP cells and herein. Also the present study reveals the presence of NaDC1 near the apical membrane of OK cells utilizing antibodies directed against NaDC1. However, this does not establish that the citrate transport process shown in our studies is NaDC1. Aruga et al. cloned an opossum ortholog of NaDC1 from OKP cells1, but they were unable to measure citrate isotope uptake in these cells until they transfected oNaDC1 back into the OKP cell line (10). In addition to these lines of evidence that NaDC1 does not account for the observed citrate transport in OK cells, NaDC1 in general has a much lower affinity (higher Km) for citrate and succinate than the apical transport process in OK cells (9;15;16); the Km for total citrate is > 0.6 mM in all species studied (9), but the Km for total citrate in OK cells is < 0.1 mM (4). To determine whether a closely related ortholog in OK cells (as distinct from OKP cells) might result in a transporter with higher substrate affinity and calcium sensitivity, we sequenced the apparent NaDC1 mRNA from the OK cells but found only oNaDC1, the exact sequence that Aruga et al found. Therefore, a transporter distinct from oNaDC1 is likely present and functional in the apical membrane of the OK cell line.
Although a distinct calcium-sensitive dicarboxylate transporter might be unique to OK cells, we speculate that two dicarboxylate transporters (NaDC1 and the calcium-sensitive process described here) could be present in the apical membrane of the proximal tubule in vivo in a variety of species. Plasma citrate concentrations are only 0.1 mM, much lower than the Km for citrate of NaDC1. A higher affinity apical transporter might allow for more citrate reabsorption in the proximal tubule. Confirmation of the presence of a separate calcium-sensitive transporter in the native kidney will require molecular isolation and identification of the responsible transporter. The possibility of other significant citrate transport processes is potentially extremely important in understanding regulation of citrate excretion and hence the prevention of calcium stones.
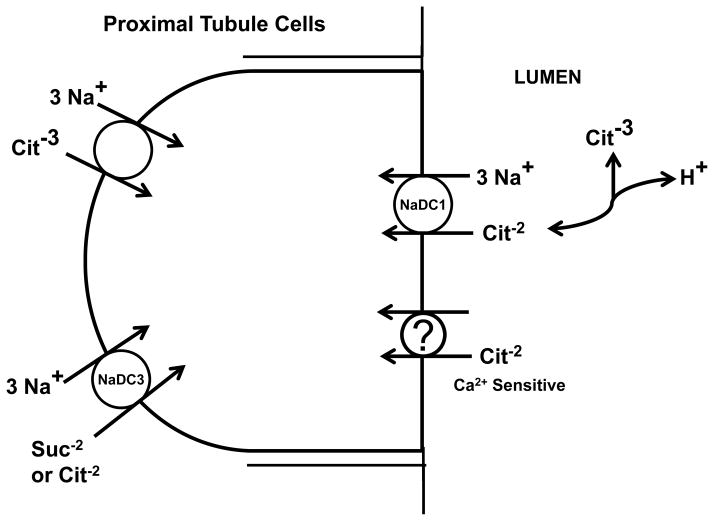
Here we show that both citrate and succinate are also transported from the basolateral aspect as well, although less transport was observed than from the apical solutions. Basolateral transport also demonstrates some qualitative differences in the response to changes in extracellular calcium. For instance, basolateral citrate uptake did not consistently increase with the removal of either apical or basolateral calcium alone; only the removal of calcium in both apical and basolateral solution resulted in an increase in basolateral citrate uptake (Figure 3). This contrasts with the significant increase in apical citrate uptake with the reduction of only apical calcium. Also basolateral uptake of succinate did not change with changes in calcium in either apical, basolateral, or both solutions (Figure 4). Succinate uptake is not complicated by complexation with calcium as discussed above. Based on basolateral membrane vesicle studies and our first studies with OK cells (6;11;12), the basolateral membrane likely has both dicarboxylate transport and a separate tricarboxylate transport process responsible for citrate transport. The dicarboxylate transport on the basolateral aspect has been suggested to be NaDC3 (or SDCT2 for sodium dicarboxylate cotransporter 2) cloned from human, rat, mouse, and winter flounder (17–21) (Figure 9). NaDC3 is a high affinity, sodium dependent dicarboxylate cotransporter and is expressed in placental brush border membrane, liver, brain and in the proximal tubule of the kidney (9). NaDC3 can be distinguished from NaDC1 by its higher substrate affinity and specificity, and by the pH dependence of succinate transport (9). For NaDC3, the Km for succinate is approximately 2–140 μM compared to a broad but generally higher range of Kms for NaDC1 orthologs (> 0.6 mM)(9). Some members of the organic anion transporter family (OAT) located on the basolateral membrane (OAT1 and OAT3) may also be involved in dicarboxylate transport in exchange for organic anions (22). Therefore, although the apical membrane clearly has calcium-sensitive/regulated dicarboxylate transport demonstrated here, dicarboxylate transport on the basolateral membrane may be complicated by other transporters which are clearly not calcium sensitive. The calcium-sensitivity of NaDC3 has not been tested to our knowledge. Even if NaDC3 is calcium-sensitive, other transporters could obscure any effect of calcium.
Figure 9.
Model illustrating that proximal tubule cells may express both NaDC1 and a separate calcium-sensitive dicarboxylate transporter on the apical membrane. On the basolateral membrane, at least two transporters, NaDC3 and a tricarboxylate transporter, likely transport citrate; additional transporters are likely involved in succinate transport
Figure 9 illustrates that we postulate that proximal tubule cells may express both NaDC1 and a separate calcium-sensitive dicarboxylate transporter on the apical membrane. Figure 5 shows only a decrease in calcium-sensitive citrate transport at the apical membrane with 2,3-DMS and no basolateral 2,3-DMS sensitivity with either solution. This suggests NaDC3 or a related protein as a candidate for the apical calcium-sensitive dicarboxylate transport process, since NaDC1 is unaffected by 2,3-DMS whereas 2,3-DMS does interact with high affinity with NaDC3 (17;23).
With calcium signaling predominantly at the apical membrane, one can speculate that some apical sensor or process may be involved in the effects of calcium on citrate and succinate uptake. Possibilities include a novel transporter that is sensitive to calcium, a true sensor such as CaSR, the calcium sensing receptor, or another calcium-sensitive protein or process coupled to transport. Such possibilities include annexins, TRP (Transient Receptor Potential) channels, or a mechanism associated with primary cilia as mechanosensors (24); both CaSR and cilia have been found on the apical membrane of OK cells (14;25). Intracellular calcium has been identified for decades as a key regulator of a number of cell processes. In recent years, extracellular calcium via CaSR and other mechanisms such as TRP channels has also proven to be an important regulator of a number of cellular processes in addition to calcium homeostasis; such processes include urine concentration for CaSR and cell growth and differentiation for certain TRP channels (26). Teleologically, for regulation of citrate excretion and complexation of calcium, the location of regulation by calcium would be most advantageous at the apical membrane to prevent stones. The benefits of a calcium–sensitive citrate transport process in the lumen of the proximal tubule would be most relevant in conditions of increased proximal tubule lumen calcium (e.g. high filtered load of calcium or decreased proximal tubule calcium reabsorption).
The limitations of the study include the use of only cultured cells from a single species and that we have not yet identified the transporter responsible for these findings. Further studies are on-going to address these limitations; preliminary results from other proximal tubule cell lines from other species also demonstrate calcium-sensitivity. There is no indication that cell de-differentiation is responsible for the findings as other transport processes are intact in these cells. The possibility of other citrate transport processes in humans is bolstered by the observation that human NaDC1 has a very low affinity for citrate (2), which would limit the complete reabsorption of citrate.
In summary, the present studies extend our understanding of the apical membrane calcium-sensitive dicarboxylate transport process that we previously described in the OK cell line (3;4;6). These proximal tubule cells express apical > basolateral citrate and succinate transport, and increases in levels of apical calcium result in decreases in both apical succinate and citrate transport. Basolateral dicarboxylate transport was not consistently altered by extracellular calcium levels. Speculatively, this calcium-regulated process may be involved in some fashion in the increase in urinary citrate which occurs in conditions of increased urinary calcium. Thus this apical calcium-sensitive/regulated process may account for the increases in urinary citrate that occur with increases in urinary calcium. More broadly, identification of citrate reabsorptive processes in addition to NaDC1 would represent a change in our understanding of regulation of citrate excretion, perhaps providing alternative routes to increase urinary citrate and lessen calcium stone formation.
Acknowledgments
Supported by a grant from the Institutional Award program of the National Center for Research Resources (P20RR017659), NIH DK54952, and a MREP (KSHS) and Merit Review grant (LLH) from the Department of Veterans Affairs.
Footnotes
OKP cells are a subclone of the original cell line of OK cells (14).
Conflict of Interest
The authors have no conflict of interest to report.
Reference List
- 1.Pak CY. Citrate and renal calculi. Miner Electrolyte Metab. 1987;13(4):257–266. [PubMed] [Google Scholar]
- 2.Pajor AM, Sun N. Functional differences between rabbit and human Na(+)-dicarboxylate cotransporters, NaDC-1 and hNaDC-1. American Journal of Physiology. 1996;271(5 Pt 2):F1093–F1099. doi: 10.1152/ajprenal.1996.271.5.F1093. [DOI] [PubMed] [Google Scholar]
- 3.Hering-Smith KS, Gambala CT, Hamm LL. Citrate and succinate transport in proximal tubule cells. American Journal of Physiology - Renal Fluid & Electrolyte Physiology. 2000;278(3):F492–F498. doi: 10.1152/ajprenal.2000.278.3.F492. [DOI] [PubMed] [Google Scholar]
- 4.Hering-Smith KS, Schiro FR, Pajor AM, Hamm LL. Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol. 2011;300(2):F425–F432. doi: 10.1152/ajprenal.00036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor EN, Curhan GC. Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4(12):1980–1987. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law D, Hering-Smith KS, Hamm LL. Citrate transport in proximal cell line. American Journal of Physiology. 1992;263(1 Pt 1):C220–C225. doi: 10.1152/ajpcell.1992.263.1.C220. [DOI] [PubMed] [Google Scholar]
- 7.Smith RM, Martell AE. Critical Stability Constants. New York: Plenum Press; 1989. [Google Scholar]
- 8.Burckhardt BC, Burckhardt G. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol. 2003;146:95–158. doi: 10.1007/s10254-002-0003-8. [DOI] [PubMed] [Google Scholar]
- 9.Pajor AM. Molecular properties of sodium/dicarboxylate cotransporters. Journal of Membrane Biology. 2000;175(1):1–8. doi: 10.1007/s002320001049. [DOI] [PubMed] [Google Scholar]
- 10.Aruga S, Pajor AM, Nakamura K, Liu L, Moe OW, Preisig PA, et al. OKP cells express the Na-dicarboxylate cotransporter NaDC-1. Am J Physiol Cell Physiol. 2004;287(1):C64–C72. doi: 10.1152/ajpcell.00061.2003. [DOI] [PubMed] [Google Scholar]
- 11.Wright SH, Wunz TM. Succinate and citrate transport in renal basolateral and brush-border membranes. Am J Physiol. 1987;253(3 Pt 2):F432–F439. doi: 10.1152/ajprenal.1987.253.3.F432. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen KE, Kragh-Hansen U, Roigaard-Petersen H, Sheikh MI. Citrate uptake by basolateral and luminal membrane vesicles from rabbit kidney cortex. Am J Physiol. 1983;244(6):F686–F695. doi: 10.1152/ajprenal.1983.244.6.F686. [DOI] [PubMed] [Google Scholar]
- 13.Barac-Nieto M. Effects of pH, calcium, and succinate on sodium citrate cotransport in renal microvilli. Am J Physiol. 1984;247(2 Pt 2):F282–F290. doi: 10.1152/ajprenal.1984.247.2.F282. [DOI] [PubMed] [Google Scholar]
- 14.Cole JA, Forte LR, Krause WJ, Thorne PK. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol. 1989;256(4 Pt 2):F672–F679. doi: 10.1152/ajprenal.1989.256.4.F672. [DOI] [PubMed] [Google Scholar]
- 15.Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y, et al. Cloning, functional characterization, and localization of a rat renal Na+-dicarboxylate transporter. Am J Physiol. 1998;275(2 Pt 2):F298–F305. doi: 10.1152/ajprenal.1998.275.2.F298. [DOI] [PubMed] [Google Scholar]
- 16.Chen XZ, Shayakul C, Berger UV, Tian W, Hediger MA. Characterization of a rat Na+-dicarboxylate cotransporter. J Biol Chem. 1998;273(33):20972–20981. doi: 10.1074/jbc.273.33.20972. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Tsukaguchi H, Chen XZ, Berger UV, Hediger MA. Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J Clin Invest. 1999;103(8):1159–1168. doi: 10.1172/JCI5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kekuda R, Wang H, Huang W, Pajor AM, Leibach FH, Devoe LD, et al. Primary structure and functional characteristics of a mammalian sodium-coupled high affinity dicarboxylate transporter. J Biol Chem. 1999;274(6):3422–3429. doi: 10.1074/jbc.274.6.3422. [DOI] [PubMed] [Google Scholar]
- 19.Steffgen J, Tolan D, Beery E, Burckhardt G, Muller GA. Demonstration of a Na(+)-dicarboxylate cotransporter in bovine adrenocortical cells. Pflugers Arch. 1999;438(6):860–864. doi: 10.1007/s004249900148. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Fei YJ, Kekuda R, Yang-Feng TL, Devoe LD, Leibach FH, et al. Structure, function, and genomic organization of human Na(+)-dependent high-affinity dicarboxylate transporter. Am J Physiol Cell Physiol. 2000;278(5):C1019–C1030. doi: 10.1152/ajpcell.2000.278.5.C1019. [DOI] [PubMed] [Google Scholar]
- 21.Pajor AM, Gangula R, Yao X. Cloning and functional characterization of a high-affinity Na(+)/dicarboxylate cotransporter from mouse brain. American Journal of Physiology - Cell Physiology. 2001;280(5):C1215–C1223. doi: 10.1152/ajpcell.2001.280.5.C1215. [DOI] [PubMed] [Google Scholar]
- 22.Anzai N, Jutabha P, Kanai Y, Endou H. Integrated physiology of proximal tubular organic anion transport. Curr Opin Nephrol Hypertens. 2005;14(5):472–479. doi: 10.1097/01.mnh.0000170751.56527.7e. [DOI] [PubMed] [Google Scholar]
- 23.Burckhardt BC, Lorenz J, Kobbe C, Burckhardt G. Substrate specificity of the human renal sodium dicarboxylate cotransporter, hNaDC-3, under voltage-clamp conditions. Am J Physiol Renal Physiol. 2005;288(4):F792–F799. doi: 10.1152/ajprenal.00360.2004. [DOI] [PubMed] [Google Scholar]
- 24.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 25.Ward DT, McLarnon SJ, Riccardi D. Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J Am Soc Nephrol. 2002;13(6):1481–1489. doi: 10.1097/01.asn.0000015623.73739.b8. [DOI] [PubMed] [Google Scholar]
- 26.Bozic M, Valdivielso JM. Calcium signaling in renal tubular cells. Adv Exp Med Biol. 2012;740:933–44. doi: 10.1007/978-94-007-2888-2_42. [DOI] [PubMed] [Google Scholar]
- 27.Pajor AM. Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem. 1995;270(11):5779–5785. doi: 10.1074/jbc.270.11.5779. [DOI] [PubMed] [Google Scholar]