Abstract
The treatment of refractory or relapsed non-Hodgkin lymphoma (NHL) remains challenging. In this retrospective study, 88 patients with refractory or relapsed NHL received treosulfan and fludarabine as a reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Of the 88 intensely pre-treated patients, 73 experienced a relapse, with 18 of the 88 patients experiencing an early relapse (ER; <6 months from the last chemotherapy). At the time of allo-HSCT, 26 patients were in complete remission (CR) and 43 in partial remission (PR), 12 patients had progressive disease (PD) and 7 had stable disease (SD). A total of 47 patients received an autologous graft followed by allo-HSCT. Following allo-HSCT, 69 of the 88 patients were in CR and 7 were in PR, resulting in an overall response rate of 86.4% (76/88). A total of 33 patients achieved a CR from PR, as did 6 patients from PD and 5 from SD. Of the 88 patients, 43 (49%) were alive at the end of the follow-up period. The patients who directly underwent allo-HSCT without prior auto-HSCT exhibited a better disease-free survival (DFS; P=0.038) with a tendency (P=0.077) for a better overall survival (OS). The patients with ER exhibited a probability of OS of 0.35±0.12 after 3 and 7 years. Chronic graft-versus-host disease (cGvHD) exerted a positive effect on OS and DFS (for limited cGvHD vs. no cGvHD, P=0.002 and 0.004, respectively). In conclusion, allogeneic stem cell transplantation following conditioning with treosufan and fludarabine constitutes a viable therapeutic option for patients with refractory or relapsed NHL and should be considered early during the course of salvage treatment.
Keywords: treosulfan, non-Hodgkin lymphoma, conditioning, transplantation
Introduction
The treatment of patients with refractory or relapsed aggressive non-Hodgkin lymphoma (NHL) represents a challenge. In addition to polychemotherapy with regimens such as R-DHAP (rituximab, dexamethasone, high-dose cytarabine and cisplatin), R-ICE (rituximab, ifosfamide, carboplatin and etoposide) or Dexa-BEAM (dexamethasone, carmustine, etoposide, cytarabine and melphalan), hematopoietic stem cell transplantation (HSCT) constitutes a therapeutic option. Autologous and allogeneic HSCT (allo-HSCT) have been employed in this setting. The most satisfactory results for autologous HSCT have been obtained in patients with relapsed but chemosensitive diffuse large B-cell lymphoma (1). However, the patient characteristics have changed over the years, as the majority of the patients received antibody-based immunochemotherapies. Moreover, other aggressive histological types, such as peripheral T-cell lymphoma, mantle cell lymphoma and Burkitt lymphoma, generally do not achieve sustained remissions following autologous HSCT (2,3). Under these conditions, the patients may benefit from the graft-versus-lymphoma (GvL) effect following allo-HSCT, despite target structures still requiring proper definition in NHL. By contrast, autologous transplantation lacking this allo-recognition may not be sufficient, particularly for patients with early relapse (ER) or refractory disease (4). As regards allo-HSCT, reduced-intensity conditioning (RIC) has been used for patients with relapsed or refractory NHL, due to the fact that these patients are extensively pretreated and may be older than 60 years (5,6).
The combination of treosulfan and fludarabine as a conditioning regimen has been proven to be feasible and efficient in several types of malignancies, including acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma (7–10). However, despite encouraging data, treosulfan/fludarabine conditioning preceding allo-HSCT has not been systemically investigated in patients with relapsed or refractory NHL.
To the best of our knowledge, this study is the first to present an analysis of 88 patients with refractory or relapsed aggressive NHL who received this conditioning regimen and an allo-HSCT at the transplantation units of the University of Essen, the University of Jena and the University of Rostock. The efficacy of the treosulfan/fludarabine regimen was assessed, as was the time to engraftment, acute and chronic graft-versus-host disease (cGvHD), graft failure, overall survival (OS) and disease-free survival (DFS).
Patients and methods
Patient characteristics
A total of 88 patients with relapsed or refractory NHL were treated at the Stem Cell Transplant Units of the University of Essen (n=45), the University of Jena (n=10) and the University of Rostock (n=33), between 2001 and 2010. The patient characteristics are summarized in Table I.
Table I.
Patient characteristics (n=88).
| Variables | Values |
|---|---|
| Median age at HSCT, years (range) | 50 (21–71) |
| Male/female, n (%) | 52/36 (59/41) |
| Earlier therapies | |
| Prior therapy regimens, n (range) | 2.5 (1–7) |
| Prior auto-HSCT, n | 47 |
| Histology, n (%) | |
| Chronic lymphocytic leukemia | 23 (26.1) |
| Diffuse large B-cell lymphoma | 22 (25.0) |
| Transformed aggressive NHL | 11 (12.5) |
| Mantle cell lymphoma | 8 (9.1) |
| Follicular lymphoma | 7 (8.0) |
| High-grade T-NHL | 4 (4.5) |
| Peripheral T-cell lymphoma-NOS | 4 (4.5) |
| Immunocytoma | 2 (2.3) |
| Primary mediastinal large B-cell lymphoma | 2 (2.3) |
| Anaplastic large-cell lymphoma | 2 (2.3) |
| T-cell prolymphocytic leukemia | 2 (2.3) |
| Burkitt lymphoma | 1 (1.1) |
| Relapsed patients, n (%) | |
| Total relapses | 73 (83.0) |
| Early relapses (<6 months) | 18 (20.5) |
| Remission status directly prior to HSCT, n (%) | 88 |
| CR | 26 (29.5) |
| PR | 43 (48.9) |
| SD | 7 (8.0) |
| PD | 12 (13.6) |
HSCT, hematopoietic stem cell transplantation; NHL, non-Hodgkin lymphoma; T-NHL, T-cell non-Hodgkin lymphoma; NOS, not otherwise specified; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
Notably, the treatment of NHL prior to the transplantation included a mean of 2.5 therapy regimens, with a range of 1–7 pre-therapies. These therapies included R-CHOP (rituximab, cyclophosphamide, daunorubicin, vincristin, prednisolone), R-DHAP, Dexa-BEAM, as well as prior autologous HSCT in 47 of the 88 patients (53.4%). Of the 88 patients, 73 (83.0%) relapsed, with 18 patients (20.5%) relapsing within 6 months after the initial treatment. The remission status was assessed according to the guidelines of the National Cancer Institute-sponsored International Working Group (11).
This study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board (University of Rostock, Rostock, Germany). All the patients signed an informed consent prior to this study.
Conditioning regimen
Treosulfan (Medac GmbH, Hamburg, Germany) was administered on 3 consecutive days (days 6-4), at a dose of 14 g/m2, or on 5 consecutive days (days 6-2), at a dose of 10 g/m2. Fludarabine (Schering AG, Berlin, Germany) was administered intravenously at a dose of 30 mg/m2 on 5 consecutive days (days 6-2), to a total dose of 150 mg/m2.
GvHD prophylaxis and anti-infective prophylaxis
In case of matched unrelated donors, but not in the case of matched related donors, anti-thymocyte globulin was administered at a dose of 10 mg/kg body weight (BW) (days 4-2). The patients received cyclosporine A at a dosage of 1.5 mg/kg BW every 12 hours. Full dosage of cyclosporine was maintained for 3 months and tapered thereafter. As an additional immunosuppressant, the patients received either methotrexate or mycophenolate mofetil (CellCept; F. Hoffmann-La Roche Ltd., Basel, Switzerland). The patients received a standard prophylaxis for viral, bacterial and fungal infections and for Pneumocystis jirovecii, according to local standards.
Definition of engraftment, GvHD
Leukocyte engraftment was defined as the first of 3 consecutive days, with an absolute neutrophil count of ≥0.5×109/l neutrophils.
Acute GvHD was evaluated in patients surviving for at least 30 days and classified according to the modified Seattle Glucksberg criteria (12). cGvHD was assessed in patients with a follow-up of at least 100 days post-transplantation and scored according to the revised Seattle criteria (13).
Statistical analysis
The disease remission status and response were classified on an intent-to-treat basis. Patients with a survival or follow-up of at least 60 days after the HSCT were included in the response analysis. DFS was defined as the time from HSCT to death or disease progression/relapse. OS was defined as the time from the HSCT to death or the last follow-up.
The SPSS/PC software package, version 15.0 (SPSS Inc., Chicago, IL, USA) was used for processing and statistical analysis of all data. Descriptive statistics were computed for continuous and categorical variables. The computed statistics included mean or median and range of continuous variables, frequencies and percentages of categorical factors. OS and DFS were calculated and graphically presented using the Kaplan-Meier method. Differences between curves were assessed by the Mantel’s log-rank test for censored survival data.
All the P values resulted from two-sided statistical tests and P<0.05 was considered to indicate a statistically significant difference. The calculation of the median follow-up was based on the time from the HSCT to the last follow-up for patients who were alive and from the HSCT to June 1, 2010 as reference data for patients who succumbed to the disease.
Results
Sequence of transplantation and hematopoietic reconstitution
A total of 88 patients with different types of NHL were included in the analysis of this retrospective study (Table I). Of these 88 patients, 39 received only an allogeneic graft and 47 received tandem transplantation with ≥1 autologous grafts, followed by allogeneic transplantation preceded by a conditioning regimen with treosulfan/fludarabine. One of the patients received treosulfan/fludarabine conditioning prior to both autologous and allo-HSCT. Two patients received a second allograft due to graft rejection. Of the 88 patients with allo-HSCT, 22 received a graft from a matched related donor, 19 received a graft from a mismatched unrelated donor and the majority (47/88) received a graft from a matched unrelated donor. Further specification of the mode and sequence of transplantations is provided in Table II on a patient-per-patient basis. The mean number of transplanted CD34+ HSCs/kg BW of the recipient was 6.08 (range, 1.15–16.86). Hematopoietic reconstitution occurred in all but one patient, who experienced a graft failure. The mean duration of neutropenia was 16.7 days (range, 8–36 days).
Table II.
Synopsis of patient characteristics and results.
| Patient no. | Age/gender | Donor type | Diagnosis | No. of chemotherapies prior to auto-/allo-Tx | Remission status prior to auto-/allo-Tx | Chemo-sensivity | Response after auto-/allo-HSCT | aTreo/flud in allo-or auto-Tx | No. of CD34+ cells per kg BW | Duration of neutropenia (days) | Survival (days after HSCT) | DFS | Cause of death | aGvHD (overall grading) | cGvHD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57/M | MUD | MCL | 1 | PR, PR | Yes | CR/CR | Auto/Alloa | 1.49 | 25 | 125 | 125 | Alive | 0 | No |
| 2 | 43/M | MRD | DLBCL | 1 | ER, SD | No | CR/CR | Autoa/Allo | 3.02 | 10 | 147 | 138 | Relapse | 0 | No |
| 3 | 60/M | MUD | DLBCL | 1 | ER, CR | Yes | CR | Alloa | 6.54 | 16 | 196 | 44 | Alive | 0 | No |
| 4 | 47/M | MUD | CLL | 3 | PD | No | CR | Alloa | 5.06 | 15 | 386 | 210 | Alive | 2 | No |
| 5 | 49/M | MUD | CLL | 2 | R, PR | Yes | CR | Alloa | 7.02 | 16 | 420 | 420 | Alive | 1 | No |
| 6 | 21/M | MUD | Hm T-NHL | 1 | ER, PR | Yes | PR/CR | Auto/Alloa | 5.33 | 10 | 134 | 134 | PD, sepsis | 2 | No |
| 7 | 49/F | MRD | DLBCL | 1 | ER, PR | Yes | PD | Auto/Alloa | 4.02 | 28 | 120 | 120 | PD | 0 | No |
| 8 | 57/M | MUD | CLL | 1 | PD | No | CR | Alloa | 3.60 | 12 | 921 | 768 | Alive | 2 | Limited |
| 9 | 60/M | MUD | MCL | 1 | R, CR | Yes | CR | Auto/Alloa | 3.38 | 16 | 301 | 101 | PD, sepsis | 0 | Limited |
| 10 | 53/M | MUD | Trans hm NHL | 1 | ER, PR | No | PR | Autoa/Allo | 1.15 | 12 | 152 | 0 | PD | 3 | No |
| 11 | 51/M | MUD | MCL | 1 | ER, CR | Yes | CR | Autoa/Alloa | 5.51 | 10 | 1165 | 1165 | Alive | 2 | Extensive |
| 12 | 54/M | MMUD | IC | 3 | ER, PD | Yes | CR | Alloa | 7.06 | 15 | 305 | 305 | Pneumonia, MOF | 0 | Limited |
| 13 | 51/M | MRD | BL | 1 | ER, CR | Yes | PD | Auto/Alloa | 4.26 | 17 | 69 | 0 | PD | 0 | No |
| 14 | 55/M | MRD | CLL | 1 | PR | Yes | CR | Alloa | 3.20 | 16 | 2005 | 2005 | NSCLC | 1 | No |
| 15 | 44/f | MRD | DLBCL | 1 | ER, SD | No | CR | Auto/Alloa | 5.13 | 20 | 1989 | 1989 | Alive | 0 | No |
| 16 | 53/M | MUD | FL | 2 | RD, PR | Yes | CR | Alloa | 2.05 | 24 | 2042 | 2042 | Alive | 0 | No |
| 17 | 34/M | MRD | DLBCL | 1 | PD | No | PR | Auto/Alloa | 2.14 | 15 | 93 | 0 | Sepsis→MOF | 3 | No |
| 18 | 38/F | MUD | DLBCL | 1 | R, PD | No | PD | Auto/Alloa | 2.71 | 31 | 104 | 0 | PD | 0 | No |
| 19 | 59/M | MUD | DLBCL | 1 | R, CR | Yes | CR | Auto/Alloa | 3.17 | 21 | 83 | 83 | Sepsis→MOF | 3 | No |
| 20 | 36/M | MRD | PTCL-NOS | 1 | PR | Yes | N.E. | Auto/Alloa | 8.17 | 17 | 44 | 44 | Sepsis, ARDS | 0 | No |
| 21 | 36/M | MRD | FL | 2 | ER, SD | No | CR | Auto/Alloa | 1.50 | 15 | 3071 | 395 | Alive | 1 | Extensive |
| 22 | 37/F | MRD | FL | 2 | R, CR | Yes | CR | Auto/Alloa | 3.02 | 13 | 1934 | 1934 | AV III°→CPR | 2 | Limited |
| 23 | 61/M | MUD | CLL | 2 | SD | No | CR | Alloa | 3.40 | 13 | 273 | 273 | ICH | 4 | No |
| 24 | 43/F | MRD | FL | 2 | R, PR | Yes | CR | Alloa | 1.79 | 11 | 2940 | 2787 | Alive | 0 | Limited |
| 25 | 48/F | MUD | CLL | 1 | R, PR | Yes | PR | Allo/Alloa | 2.56 | 13 | 3016 | 3016 | Alive | 0 | No |
| 26 | 40/M | MUD | FL | 1 | RD, SD | No | CR | Alloa | 3.10 | 13 | 436 | 436 | cGvHD | 4 | Extensive |
| 27 | 57/M | MUD | CLL | 4 | PR | Yes | CR | Alloa | 2.40 | 9 | 1615 | 1615 | cGvHD | 1 | Limited |
| 28 | 43/M | MRD | PTCL-NOS | 1 | ER, CR | Yes | CR | Alloa | 3.10 | 10 | 88 | 88 | Sepsis, GvHD, MOF | 3 | No |
| 29 | 52/F | MUD | FL | 1 | R, CR | Yes | CR | Alloa | 1.43 | 10 | 3331 | 2082 | Alive | 3 | No |
| 30 | 50/F | MUD | FL | 1 | R, PR | Yes | CR | Alloa | 15.90 | 8 | 3436 | 1764 | Alive | 0 | Limited |
| 31 | 52/M | MUD | DLBCL | 2 | CR | Yes | CR | Auto/Alloa | 6.68 | 23 | 83 | 83 | Alive | 0 | No |
| 32 | 49/F | MUD | Trans hm NHL | 2 | CR | Yes | CR | Auto/Alloa | 5.88 | 28 | 75 | 75 | Alive | 0 | No |
| 33 | 35/F | MRD | Hm T-NHL | 2 | PR | No | CR | Alloa | 2.28 | 13 | 61 | 61 | Alive | 0 | No |
| 34 | 52/M | MRD | MCL | 1 | CR | Yes | CR | Alloa | 7.40 | 12 | 1373 | 1373 | Alive | 3 | Limited |
| 35 | 49/F | MMUD | DLBCL | 3 | ER, RD | No | CR | Alloa | 9.00 | 10 | 239 | 239 | Sepsis | 2 | Extensive |
| 36 | 35/F | MMUD | DLBCL | 2 | ER, PR | Yes | CR | Alloa | 5.79 | 26 | 249 | 128 | Sepsis, PD | 0 | No |
| 37 | 63/M | MUD | MCL | 4 | ER, PR | Yes | CR | Alloa | 5.90 | 8 | 394 | 394 | GvHD | 2 | Extensive |
| 38 | 56/F | MUD | Hm T-NHL | 2 | R, PR | Yes | N.E. | Alloa | 5.00 | 34 | 46 | 46 | MOF | 2 | No |
| 39 | 41/F | MUD | DLBCL | 3 | R, PR | Yes | CR | Alloa | 4.20 | 31 | 754 | 754 | Alive | 0 | No |
| 40 | 42/F | MUD | DLBCL | 3 | ER, RD | No | N.E. | Auto/Alloa | 6.13 | NA | 26 | 26 | MOF | 0 | No |
| 41 | 27/F | MUD | BL | 5 | ER, PR | Yes | CR | Auto/Alloa | 7.13 | 16 | 636 | 636 | Alive | 0 | Limited |
| 42 | 51/M | MRD | DLBCL | 3 | ER, RD | No | CR | Auto/Alloa | 7.25 | 22 | 548 | 395 | Alive | 0 | Limited |
| 43 | 50/M | MRD | Trans hm NHL | 3 | R, PR | Yes | CR | Alloa | 7.80 | 12 | 205 | 205 | Alive | 3 | No |
| 44 | 62/F | MMUD | CLL | 1 | R, CR | Yes | CR | Allo/Alloa | 5.38 | 18 | 2161 | 2161 | Alive | 0 | Limited |
| 45 | 47/M | MMUD | CLL | 3 | R, PD | Yes | CR | Alloa | 3.48 | 15 | 1640 | 1640 | Alive | 3 | No |
| 46 | 60/F | MMUD | CLL | 2 | PD | Yes | PR | Auto/Alloa | 3.23 | 18 | 684 | 684 | Alive | 2 | Extensive |
| 47 | 47/M | MUD | CLL | 3 | R, PR | Yes | CR | Auto/Allo/Alloa | 6.68 | 16 | 1387 | 333 | Alive | 1 | No |
| 48 | 58/M | MUD | CLL | 2 | R, PR | Yes | CR | Auto/Alloa | 9.86 | 15 | 1311 | 1311 | Alive | 1 | Limited |
| 49 | 64/F | MUD | CLL | 2 | R, PD | No | CR | Alloa | 11.50 | 19 | 1142 | 1142 | Alive | 2 | Limited |
| 50 | 45/M | MMUD | CLL | 2 | R, PR | Yes | CR | Alloa | 4.50 | 16 | 64 | 64 | Sepsis, MOF | 4 | No |
| 51 | 46/M | MUD | CLL | 2 | R, CR | Yes | CR | Alloa | 5.75 | 16 | 813 | 813 | Alive | 2 | Extensive |
| 52 | 64/M | MMUD | CLL | 2 | R, PR | Yes | CR (MRD+) | Alloa | 5.33 | 18 | 567 | 567 | Alive | 3 | Extensive |
| 53 | 56/F | MUD | CLL | 2 | R, PD | No | SD | Alloa | 7.30 | 21 | 106 | 106 | Sepsis, MOF | 4 | Extensive |
| 54 | 57/M | MUD | CLL | 2 | R, PR | Yes | CR | Alloa | 1.99 | 15 | 512 | 512 | Alive | 0 | Limited |
| 55 | 55/M | MRD | Trans hm NHL | 4 | R, PR | Yes | CR | Alloa | 5.90 | 17 | 312 | 312 | Sepsis | 1 | No |
| 56 | 53/F | MMUD | CLL | 2 | R, PD | No | PR | Alloa | 9.01 | 18 | 178 | 178 | Pneumonia, MOF | 1 | Limited |
| 57 | 59/M | MUD | CLL | 3 | R, CR | Yes | CR | Auto/Alloa | 2.90 | 18 | 73 | 73 | GvHD | 4 | No |
| 58 | 57/M | MRD | CLL | 1 | R, PR | Yes | CR | Alloa | 6.86 | 15 | 169 | 169 | Sepsis, pneumonia | 0 | Extensive |
| 59 | 52/M | MMUD | CLL | 1 | ER, CR | Yes | CR | Alloa | 16.86 | 18 | 288 | 288 | Alive | 3 | No |
| 60 | 43/M | MUD | Trans hm NHL | 3 | R, CR | Yes | CR | Auto/Alloa | 6.95 | 16 | 735 | 181 | Relapse | 0 | No |
| 61 | 26/M | MRD | DLBCL | 7 | R, PR | Yes | SD | 3× Auto/Alloa | 5.80 | 18 | 61 | 30 | Relapse | 2 | No |
| 62 | 37/F | MMUD | Hm T-NHL | 4 | R, SD | Yes | N.E. | Auto/Alloa | 5.86 | 17 | 48 | 48 | Sepsis | 0 | No |
| 63 | 29/F | MUD | PMLBL | 2 | R, PR | Yes | CR | Auto/Alloa | 7.48 | 16 | 452 | 452 | GvHD, sepsis | 1 | Extensive |
| 64 | 46/M | MUD | DLBCL | 6 | R, PR | Yes | N.E. | 2× Auto/Alloa | 10.64 | N.A. | 2 | 2 | Sepsis | 0 | No |
| 65 | 45/M | MRD | MCL | 4 | R, PR | Yes | CR | Auto/Alloa | 2.80 | 19 | 1406 | 551 | Pneumonia | 1 | No |
| 66 | 25/F | MMUD | DLBCL | 4 | R, PR | Yes | PR | Auto/Allo/Alloa | 7.10 | 14 | 206 | 24 | Relapse | 0 | No |
| 67 | 36/F | MMUD | ALCL, ALK+ | 4 | R, CR | Yes | CR | Auto/Alloa | 3.30 | 17 | 1955 | 1955 | Alive | 1 | Limited |
| 68 | 54/M | MRD | DLBCL | 2 | R, PR | Yes | CR | 4× Auto/Alloa | 6.60 | 17 | 1899 | 1899 | Alive | 2 | Limited |
| 69 | 46/M | MMUD | PMLBL | 3 | R, PR | Yes | CR | 3× Auto/Alloa | 7.60 | 15 | 166 | 166 | Pneumonia | 4 | No |
| 70 | 48/F | MRD | ALCL, ALK+ | 3 | R, CR | Yes | CR | Auto/Alloa | 11.90 | 11 | 225 | 171 | Relapse | 0 | No |
| 71 | 62/F | MMUD | DLBCL | 7 | R, PR | Yes | CR | Auto/Alloa | 9.29 | 12 | 102 | 102 | Sepsis, MOF | 1 | No |
| 72 | 45/M | MMUD | CLL | 3 | R, PR | Yes | CR | Auto/Allo/Alloa | 13.00 | 15 | 1366 | 1366 | Alive | 3 | Limited |
| 73 | 42/F | MUD | Trans hm NHL | 3 | R, PR | Yes | PR | Auto/Alloa | 10.90 | 17 | 268 | 100 | Relapse | 0 | Limited |
| 74 | 53/F | MMUD | Trans hm NHL | 3 | R, PR | Yes | CR | Alloa | 9.96 | 22 | 918 | 918 | Alive | 0 | No |
| 75 | 57/F | MUD | MCL | 4 | R, CR | Yes | CR | 2× Auto/Alloa | 12.56 | 21 | 120 | 120 | Pneumonia | 2 | No |
| 76 | 55/F | MRD | PTCL-NOS | 3 | R, CR | Yes | CR | Auto/Alloa | 6.30 | 22 | 877 | 877 | Alive | 1 | Limited |
| 77 | 71/M | MUD | MCL | 7 | R, PR | Yes | N.E. | Alloa | 5.56 | 14 | 48 | 48 | Sepsis, MOF | 2 | No |
| 78 | 59/M | MUD | Trans hm NHL | 5 | R, PR | Yes | CR | Alloa | 4.07 | 18 | 797 | 797 | Alive | 2 | Limited |
| 79 | 70/M | MUD | Trans hm NHL | 4 | R, SD | No | N.E. | Alloa | 5.94 | 14 | 58 | 58 | Sepsis | 3 | No |
| 80 | 55/M | MMUD | DLBCL | 3 | R, CR | Yes | CR | Auto/Alloa | 7.60 | 36 | 463 | 463 | Alive | 0 | No |
| 81 | 57/M | MUD | Trans hm NHL | 6 | R, PR | Yes | CR | 4× Auto/Alloa | 2.60 | 15 | 365 | 365 | Alive | 3 | Extensive |
| 82 | 55/F | MUD | DLBCL | 4 | R, CR | Yes | CR | Auto/Alloa | 16.50 | 17 | 68 | 68 | HF | 0 | No |
| 83 | 62/F | MUD | DLBCL | 3 | R, PR | Yes | CR | Auto/Alloa | 5.00 | 16 | 317 | 317 | Alive | 1 | No |
| 84 | 42/F | MUD | DLBCL | 2 | R, CR | Yes | CR | Auto/Alloa | 9.32 | 13 | 264 | 264 | Alive | 0 | No |
| 85 | 57/M | MMUD | Trans hm NHL | 4 | R, PR | Yes | CR | Auto/Alloa | 7.10 | 14 | 82 | 82 | Renal failure, sepsis | 3 | No |
| 86 | 68/M | MUD | PTCL-NOS | 3 | R, CR | Yes | CR | Auto/Alloa | 5.98 | 15 | 245 | 245 | Alive | 1 | No |
| 87 | 58/F | MUD | T-PLL | 3 | R, CR | Yes | CR | Alloa | 6.89 | 15 | 947 | 947 | Alive | 2 | Limited |
| 88 | 52/F | MUD | T-PLL | 2 | R, CR | Yes | CR | Alloa | 11.05 | 14 | 185 | 136 | Relapse | 0 | Extensive |
Age is provided in years. M, male; F, female; Tx, transplant; Treo/flud, treosulfan/fludarabine; BW, body weight; MUD, matched unrelated donor; MRD, matched related donor; MMUD, mismatched unrelated donor; DLBCL, diffuse large B-cell lymphoma; MCL, mantle cell lymphoma; ALCL, anaplastic large-cell lymphoma; ALK, anaplastic lymphoma kinase; PMLB, primary mediastinal large B-cell lymphoma; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; Hm T-NHL, highly malignant T-cell NHL; Trans hm NHL, transformed highly-malignant NHL; T-PLL, T-cell prolymphocytic leukemia; FL, follicular lymphoma; BL, Burkitt lymphoma; IC, immunocytoma; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; R, relapse; ER, early relapse; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; N.E., not evaluable; N.A., not applicable; HSCT, hematopoietic stem cell transplantation; DFS, disease-free survival; GvHD, graft-versus-host disease; aGvHD, acute GvHD; cGvHD, chronic GvHD; MOF, multi-organ failure; NSCLC, non-small-cell lung carcinoma; RD, refractory disease; ARDS, acute respiratory distress syndrome; AV III°, atrioventricular block; CPR, cardiopulmonary resuscitation; ICH, intracranial hemorrhage; HF, heart failure.
Response to treatment and survival
The results patient-per-patient are presented in Table II. In general, the majority of the patients maintained or developed a complete remission (CR). In 69 of the 88 patients, a CR was observed post-transplantation, 7 patients achieved or maintained a partial remission (PR) and 3 patients developed progressive disease (PD). Prior to allo-HSCT, 69 of the 88 patients (78.4%) were in CR and PR, but only 29 of these were in CR. Notably, 5 of the 7 patients with stable disease (SD) prior to allo-HSCT achieved a CR after allo-HSCT. Of the 12 patients with PD prior to allo-HSCT, 9 achieved a remission (6 CR and 3 PR). Of note, 1 patient with PD achieved a CR following administration of a donor lymphocyte infusion. Seven patients died within 60 days of the transplant and were therefore not evaluable for response to treatment (Table III).
Table III.
Summary of results after allo-HSCT.
| Outcome | Patient no. |
|---|---|
| Response to treatment (n=88) | |
| CR | 69 |
| PR | 7 |
| PDa | 3 |
| SD | 2 |
| NEb | 7 |
| Causes of death (n=45) | |
| Disease progression | 4 |
| Disease progression and infectious complications | 3 |
| Infection, sepsis, MOF without progression | 22 |
| GvHD | 4 |
| Relapse | 7 |
| Other causes of death | |
| Intracranial bleeding | 1 (d +273 in CR) |
| NSCLC | 1 (d +2,005 in CR) |
| AV III°, CPR | 1 (d +1,943 in CR) |
| Heart failure | 1 (d +68 in CR) |
| Renal failure | 1 (d +82 in CR) |
One patient developed a CR following a donor lymphocyte infusion;
not evaluable due to early death before day 60.
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; CR, complete remission; PR, partial remission; PD, progressive disease; SD, stable disease; MOF, multi-organ failure; GvHD, graft-versus-host disease; NSCLC, non-small-cell lung cancer; AV III°, atrioventricular block; CPR, cardiopulmonary resuscitation; d, day.
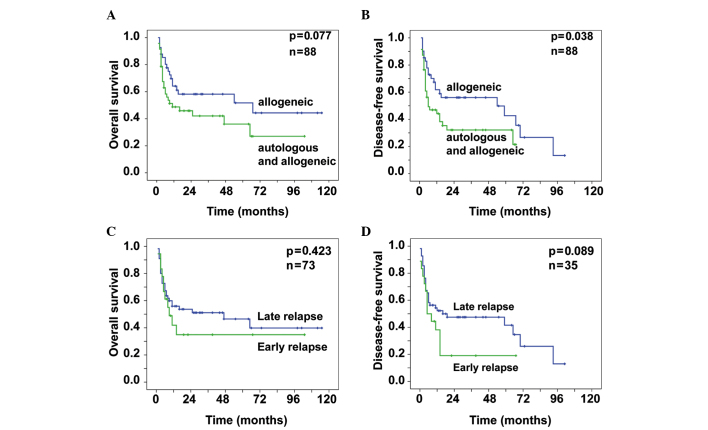
The patients who directly underwent allo-HSCT without preceding auto-HSCT had an OS probability of 0.58±0.08 after 3 years and 0.44±0.11 after 7 years (Fig. 1A). For these patients, the probability of DFS was 0.56±0.08 after 3 years and 0.27±0.11 after 7 years (Fig. 1B). For the patients who received allo-HSCT following an autologous graft, the OS probability was 0.42±0.08 after 3 years. After 7 years, 0.27±0.10 of these patients remained alive (Fig. 1A) and the probability of DFS was 0.32±0.08 after 3 years (Fig. 1B). The difference in the DFS in favor of the patients who directly received an allogeneic graft was significant (P=0.038), with a similar tendency for OS (P=0.077).
Figure 1.
A total of 88 patients with relapsed or refractory non-Hodgkin lymphoma underwent conditioning with treosulfan and fludarabine. (A) Differences in the overall survival (OS) and (B) differences in the disease-free survival (DFS) between patients who received allogeneic hematopoietic stem cell transplantation (allo-HSCT) with a preceding autologous HSCT and those who directly received allo-HSCT. Prior to the transplantation, 73 of these patients experienced an early or late relapse (<6 or ≥6 months from the last chemotherapy, respectively). Differences in the (C) OS and (D) DFS, respectively, between the patient groups with early and late relapse.
Of the 88 patients, 45 succumbed to the disease. Of these 45 patients, 14 died due to progression or relapse of the underlying disease, 4 experienced progression following transplantation and 3 developed infectious complications. A total of 26 patients succumbed to transplantation-associated complications, 22 of whom developed infectious complications, followed by sepsis and multi-organ failure. Four patients died from acute GvHD. Five patients died while in CR, 3 of which during long-term follow-up, due to disease- or treatment-independent reasons: 1 patient died from intracranial bleeding on day +273; 1 patient developed non-small-cell lung cancer and died on day +2,005 following transplantation; 1 patient died on day +1,934 due to a cardiac arrest, despite cardiopulmonary resuscitation; 1 patient died on day +68 due to heart failure; and 1 patient died due to renal failure on day +82.
Relapse
Patients with ER, i.e., relapse within 6 months following the completion of chemotherapy, had a worse outcome compared with patients who exhibited a later relapse. Notably, there was no significant difference in the probability of OS (P=0.423). After 3 and 7 years, 0.51±0.07 and 0.40±0.09 of the relapsed patients, respectively, remained alive. The probability of OS of the patients who relapsed within the first 6 months was 0.35±0.12 after 3 and 7 years (Fig. 1C).
The difference in the DFS displayed a tendency in favor of patients with late relapse (P=0.089). The probability of DFS was 0.48±0.07 and 0.26±0.10 after 3 and 7 years, respectively, for those patients. Patients with ER had a probability of DFS of 0.19±0.10 after 3 years (Fig. 1D).
GvHD
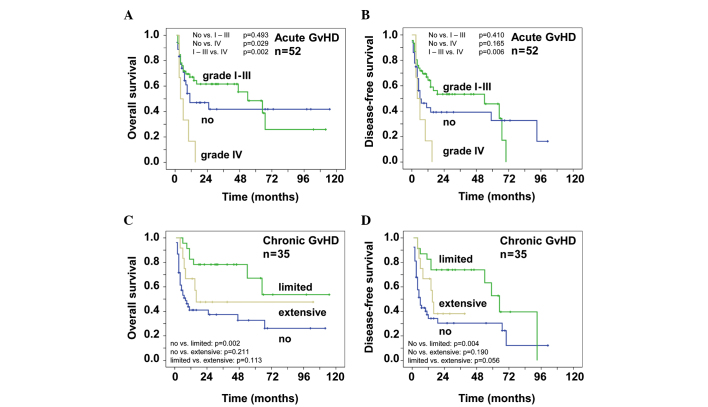
Of the 88 patients included in this analysis, 52 patients experienced acute GvHD. Fifteen patients developed acute GvHD grade I, 17 patients grade II, 14 patients grade III and 6 patients grade IV. Table IV specifies the organ manifestations of acute GvHD. GvHD of the gut was not histologically proven. Fig. 2A shows the OS of patients with acute GvHD. Patients with grade IV acute GvHD succumbed to the disease within the first 15 months. The probability of OS for patients without acute GvHD reached an early plateau: 0.42±0.09 after 3 and 7 years. Patients with grade I–III acute GvHD had a probability of OS of 0.62±0.07 after 3 years and 0.26±0.13 after 7 years. The difference between grade IV acute GvHD vs. no GvHD and grade I–III acute GvHD was highly significant (P=0.029 and P=0.002, respectively). Similar results were observed for the probability of DFS (Fig. 2B). After 3 years 0.53±0.08 of the patients with grade I–III acute GvHD were disease-free. The patients that did not develop acute GvHD had a probability of DFS of 0.39±0.09 and 0.33±0.09 after 3 and 7 years, respectively.
Table IV.
Acute GvHD (n=52).
| Grade | Skin | Gut | Liver | Overall assessment |
|---|---|---|---|---|
| I | 9 | 12 | 4 | 15 |
| II | 20 | 6 | 4 | 17 |
| III | 14 | 6 | 2 | 14 |
| IV | 1 | 5 | 3 | 6 |
| All | 44 | 29 | 13 | 52 |
GvHD, graft-versus-host disease.
Figure 2.
A total of 52 of the 88 transplanted patients with refractory or relapsed non-Hodgkin lymphoma experienced an acute graft-versus-host disease (GvHD). (A) Overall survival (OS) and (B) disease-free survival (DFS) for the different grades of GvHD. A significant survival benefit was observed for grade I–III vs. grade IV. A total of 35 patients developed a chronic GvHD. (C) OS and (D) DFS for patients with limited, extensive or no GvHD. A significant survival benefit was observed for those with no vs. those with limited disease.
The occurrence of cGvHD correlated with the survival of the NHL patients included in this study. A total of 35 patients developed cGvHD, 13 of whom developed extensive and 22 patients limited disease, mainly involving the skin and mucosae. Fig. 2C shows a better OS in patients with cGvHD. The probability of OS of patients with limited and no cGvHD was 0.78±0.09 and 0.37±0.08, respectively, after 3 years. After 7 years, the probability of OS was 0.54±0.16 for patients with limited GvHD and 0.26±0.09 for patients without cGvHD. The patients with extensive cGvHD reached a plateau early, so that the probability of OS was 0.48±0.15 after 3 and 7 years. There was a highly significant difference between limited and no cGvHD (P=0.002) and a tendency for a better OS in patients with limited or no vs. extensive GvHD (P=0.113 or 0.211, respectively). These effects are also shown in Fig. 2D that demonstrates the time of DFS. After 3 years, 0.74±0.09 of the patients with limited cGvHD were disease-free. The data of patients with limited cGvHD were highly significant when compared with those of patients with no cGvHD (P=0.004), whereas limited vs. extensive cGvHD showed a tendency for improved survival (P=0.056). The probability of DFS was 0.30±0.07 after 3 years and 0.12±0.10 after 7 years for patients that did not develop cGvHD. After 3 years, the probability of OS was 0.38±0.15 for patients with extensive chronic CGvHD.
Discussion
The treatment of patients with refractory or relapsed NHL remains challenging, as only few salvage chemotherapy protocols are currently available. El Gnaoui et al (14) reported the outcome of 46 patients treated with a salvage therapy containing rituximab, gemcitabine and oxaliplatine. The overall response rate was 83% and the 2-year event-free survival (EFS) and OS were 43 and 65% respectively (14). The majority of these patients had chemotherapy-sensitive disease and a remission of ≥1 year; however, only 57% had received rituximab prior to salvage therapy (14). In a recently published study, Gisselbrecht et al (4) established the International Prognostic Index, the duration of remission (<12 vs. >12 months) and the pre-treatment with rituximab as risk factors for the outcome following autologous HSCT. In that study, 396 patients were randomly assigned to receive either R-ICE or R-DHAP as induction therapy, following high-dose BEAM and autologous HSCT. With regard to the response rate, there was no difference in the 3-year OS (49%) and the 3-year EFS (31%) between the treatment protocols. Martin et al (15) described a significantly worse relapse rate (RR), OS and progression-free survival (PFS) in patients with relapsed NHL after rituximab-containing first-line therapy. Since the majority of patients currently receive a rituximab-based therapy, this is of particular interest, as the group of rituximab-naïve patients experiencing a relapse of high-grade lymphoma may constitute a minority in the future.
In contrast to autologous HSCT, allo-HSCT constitutes the only curative therapy option for patients with aggressive NHL (16–18), mainly due to the GvL effect. The use of RIC extended the option of allo-HSCT to elderly patients and patients who had previously received high-dose chemotherapy and autologous HSCT (19–22). Several studies demonstrated a more potent GvL effect after RIC rather than after myeloablative condition regimens (23–26). This may be due to the lower toxicity towards T cells, which are mainly responsible for the GvL effect.
Treosulfan as an alkylating agent has exhibited limited organ toxicities, even when administered at the maximum dose of 47 g/m2 (27,28). Compared to its prodrug, busulfan, treosulfan may be less toxic, particularly for the skin, mucosae, liver, kidney and heart, which are the organ systems usually targeted in transplantation-associated mortality (TAM) following conventional conditioning. Fludarabine, a nucleoside analogue which has already been included in a variety of RIC regimens, is characterized by its effectiveness against lymphoid diseases and its favorable toxicity profile (29–31). Therefore, we employed the reduced-intensity regimen with treosulfan and fludarabine in 88 patients with relapsed or refractory lymphoma.
In our present study, for patients who received autologous and allo-HSCT, the OS and the DFS were inferior compared to those in patients who only underwent allo-HSCT (Fig. 1A and B). This may be due to the fact that patients with more agressive NHLs achieved a PR only after salvage chemotherapy and were first subjected to autologous HSCT for further reduction of the tumor burden. Furthermore, autologous HSCT preceding the allograft may have caused organ toxicities without eradicating the aggressive disease. Therefore, provided that the patient is eligible, allo-HSCT should be considered and performed early during the course of the disease. We demonstrated that GvHD, in particular limited cGvHD, improved the patient outcome (Fig. 2). However, we did not observe a difference in the outcome of patients with early or late disease relapse (Fig. 1C and D). This finding may indicate that allo-HSCT should be considered even for patients with ER, particulary if they have responded to salvage chemotherapy. Of the 88 patients, 26 (29.5%) succumbed to GvHD and/or infectious complications, i.e., TAM was within the expected range.
A previous study by Hamadani et al (6) was conducted on a cohort of 46 patients with relapsed chemorefractory aggressive NHL. In contrast to our cohort, those patients were treated with a myeloablative regimen (84% of the patients received busulfan and cyclophosphamide). The median follow-up was 5 years. The 5-year OS, PFS and RR were 38, 34 and 35%, respectively. The data of our cohort demonstrated an OS and a DFS of 43 and 37%, respectively. The rate of acute and chronic GvHD was 43 and 75% in the study by Hamadani et al (6) vs. 59 and 40% in our study.
The Lymphoma Working Party of the European Bone Marrow Transplantation Association reported the outcome of 188 lymphoma patients who underwent allo-HSCT after RIC. Twenty-one of these patients had chemotherapy-resistant disease. The sensitivity to chemotherapy was the most important factor in PFS (32). In addition, a previous study by Bishop et al (5) demonstrated the correlation of pre-transplantation and early post-transplantation response assessment with the outcome after RIC allo-HSCT for NHL. Fig. 1C and D demonstrates that there was no significant difference in our study between patients who relapsed within the first 6 months and those who relapsed at any time. This may be due to the fact that there were fewer patients with ER (Table I). In the present study we also observed that patients with good sensitivity to chemotherapy (70/88) exhibited a better survival compared to patients without response to chemotherapy (Table II).
Our findings suggest that RIC with treosulfan/fludarabine and allo-HSCT is feasible and effective in NHL patients, even those with ER and at the stage of SD or PD. The reduction of the tumor load to a minimum appears to be crucial. The occurrence of GvHD is favourable for the outcome of the patients, suggesting a potent GvL effect. This therapeutic option should therefore be considered early during the course of the disease and integrated into the long-run concept of lymphoma therapy.
Acknowledgements
Michael Schmitt, Inken Hilgendorf, Michael Koenigsmann, Jochen Casper and Dietrich W. Beelen received travel grants to conferences from Medac GmbH. Mathias Freund received research funding and honoraria from Medac GmbH.
References
- 1.Verdonck LF, van Putten WL, Hagenbeek A, et al. Comparison of CHOP chemotherapy with autologous bone marrow transplantation for slowly responding patients with aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1995;332:1045–1051. doi: 10.1056/NEJM199504203321601. [DOI] [PubMed] [Google Scholar]
- 2.Chen AI, McMillan A, Negrin RS, et al. Long-term results of autologous hematopoietic cell transplantation for peripheral T cell lymphoma: the Stanford experience. Biol Blood Marrow Transplant. 2008;14:741–747. doi: 10.1016/j.bbmt.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamadani M, Benson DM, Jr, Lin TS, et al. High-dose therapy and autologous stem cell transplantation for follicular lymphoma undergoing transformation to diffuse large B-cell lymphoma. Eur J Haematol. 2008;81:425–431. doi: 10.1111/j.1600-0609.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 4.Koenigsmann M, Casper J, Kahl C, et al. Risk-adapted, treosulfan-based therapy with auto- and allo-SCT for relapsed/refractory aggressive NHL: a prospective phase-II trial. Bone Marrow Transplant. 2014;49:410–415. doi: 10.1038/bmt.2013.199. [DOI] [PubMed] [Google Scholar]
- 5.Bishop MR, Dean RM, Steinberg SM, et al. Correlation of pretransplant and early post-transplant response assessment with outcomes after reduced-intensity allogeneic hematopoietic stem cell transplantation for non-Hodgkin’s lymphoma. Cancer. 2010;116:852–862. doi: 10.1002/cncr.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamadani M, Benson DM, Jr, Hofmeister CC, et al. Allogeneic stem cell transplantation for patients with relapsed chemorefractory aggressive non-Hodgkin lymphomas. Biol Blood Marrow Transplant. 2009;15:547–553. doi: 10.1016/j.bbmt.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casper J, Knauf W, Kiefer T, et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood. 2004;103:725–731. doi: 10.1182/blood-2002-11-3615. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Hieber M, Blau IW, Trenschel R, et al. Reduced-toxicity conditioning with fludarabine and treosulfan prior to allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant. 2007;39:389–396. doi: 10.1038/sj.bmt.1705605. [DOI] [PubMed] [Google Scholar]
- 9.Casper J, Wolff D, Knauf W, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol. 2010;28:3344–3351. doi: 10.1200/JCO.2009.23.3429. [DOI] [PubMed] [Google Scholar]
- 10.Hilgendorf I, Wolff D, Gromke T, et al. Retrospective analysis of treosulfan-based conditioning in comparison with standard conditioning in patients with myelodysplastic syndrome. Bone Marrow Transplant. 2011;46:502–509. doi: 10.1038/bmt.2010.153. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Cutler C, Antin JH. Manifestations and treatment of acute graft-versus-host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoetic Cell Transplantation. 4th edition. Willey-Blackwell; London: 2009. pp. 1287–1303. [Google Scholar]
- 13.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 14.El Gnaoui T, Dupuis J, Belhadj K, et al. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Ann Oncol. 2007;18:1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 15.Martin A, Conde E, Arnan M, et al. GEL/TAMO Cooperative Group. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 16.Le Gouill S, Milpied N, Buzyn A, et al. Société Française de Greffe de Moëlle et de Thérapie Cellulaire: Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societé Française de Greffe de Moëlle et de Thérapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 17.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietrich S, Tielesch B, Rieger M, et al. Patterns and outcome of relapse after autologous stem cell transplantation for mantle cell lymphoma. Cancer. 2011;117:1901–1910. doi: 10.1002/cncr.25756. [DOI] [PubMed] [Google Scholar]
- 19.Chopra R, Goldstone AH, Pearce R, et al. Autologous versus allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma: a case-controlled analysis of the European Bone Marrow Transplant Group Registry data. J Clin Oncol. 1992;10:1690–1695. doi: 10.1200/JCO.1992.10.11.1690. [DOI] [PubMed] [Google Scholar]
- 20.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. European Bone Marrow Transplantation (EBMT) Lymphoma Registry: An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 21.Bierman PJ, Sweetenham JW, Loberiza FR, et al. Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation: Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation - The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–3753. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 22.McSweeney PA, Niederweiser D, Shizuru JA, et al. Hemapoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 23.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. doi: 10.1182/blood.v98.13.3595. [DOI] [PubMed] [Google Scholar]
- 24.Escalon MP, Champlin RE, Saliba RM, et al. Nonmyeloablative allogeneic hematopoietic transplantation: a promising salvage therapy for patients with non-Hodgkin’s lymphoma whose disease has failed a prior autologous transplantation. J Clin Oncol. 2004;22:2419–2423. doi: 10.1200/JCO.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 25.Rezvani AR, Storer B, Maris M, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:211–217. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 26.Glass B, Nickelsen M, Dreger P, et al. Reduced-intensity conditioning prior to allogeneic transplantation of hematopoietic stem cells: the need for T cells early after transplantation to induce a graft-versus-lymphoma effect. Bone Marrow Transplant. 2004;34:391–397. doi: 10.1038/sj.bmt.1704600. [DOI] [PubMed] [Google Scholar]
- 27.Beelen DW, Trenschel R, Casper J, et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic hematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant. 2005;35:233–241. doi: 10.1038/sj.bmt.1704784. [DOI] [PubMed] [Google Scholar]
- 28.Scheulen ME, Hilger RA, Oberhoff C, et al. Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res. 2000;6:4209–4216. [PubMed] [Google Scholar]
- 29.Banna GL, Aversa S, Sileni VC, et al. Nonmyeloablative allogeneic stem cell transplantation (NST) after truly nonmyeloablative and reduced intensity conditioning regimens. Crit Rev Oncol Hematol. 2004;51:171–189. doi: 10.1016/j.critrevonc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Schetelig J, Bornhauser M, Kiehl M, et al. Reduced-intensity conditioning with busulfan and fludarabine with or without antithymocyte globulin in HLA-identical sibling tranplantation - a retrospective analysis. Bone Marrow Transplant. 2004;33:483–490. doi: 10.1038/sj.bmt.1704384. [DOI] [PubMed] [Google Scholar]
- 31.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 32.Robinson SP, Goldstone AH, Mackinnon S, et al. Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation: Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]