Abstract
This study was conducted to investigate whether fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is useful for predicting the distance of intrahepatic metastases and microvascular invasion from the main tumor and the pattern of postoperative recurrence. A total of 89 consecutive patients who underwent 18F-FDG PET/CT prior to liver resection for hepatocellular carcinoma (HCC) between April, 2006 and December, 2011 were enrolled in this study. The distance between the microsatellite lesion and the main nodule (microsatellite distance) was analyzed and measured pathologically. The correlation between maximal standardized uptake values (SUVmax) and microsatellite distance was analyzed and the independent risk factors for microsatellite distance >1 cm were assessed. The postoperative recurrence patterns were divided into no recurrence, intrahepatic recurrence and extrahepatic recurrence. SUVmax and the distribution of microsatellite lesions were compared among these groups. The postoperative recurrence patterns were also analyzed according to the SUVmax and the microsatellite lesion pattern. SUVmax was found to be significantly correlated with the distance from the microsatellite lesion to the main nodule (r=0.57, P<0.0001). On the multivariate analysis of microsatellite distance >1 cm, the only significant factor was SUVmax [P=0.002; hazard ratio=1.60; 95% confidence interval (CI): 1.23–2.26]. The optimal cutoff value of SUVmax for microsatellite distance >1 cm was 8.8. The mean SUVmax and the microsatellite distance were highest in patients with postoperative extrahepatic metastases (8.6 and 9,160 μm, respectively). In conclusion, the SUVmax of 18F-FDG PET/CT reflects microsatellite distance and the patterns of postoperative recurrence in HCC. Therefore, 18F-FDG PET/CT may be a useful imaging modality for determining the resection margin and the treatment protocol for HCC.
Keywords: fluorine-18 fluorodeoxyglucose, positron emission tomography and computed tomography, hepatocellular carcinoma, microsatellites, neoplasm recurrence, local
Introduction
Although there are various treatment protocols for hepatocellular carcinoma (HCC), including liver resection and radiofrequency ablation (RFA), >598,000 patients succumbed to this disease worldwide in 2012 (1). Intrahepatic metastasis and microvascular invasion (microsatellite lesions) are associated with recurrence rate and prognosis (2–4). The survival time of HCC patients with microsatellite lesions remains unsatisfactory (5).
The mechanism of distant metastasis and recurrence is often considered to be via the systemic circulation (6). Previously, Sasaki et al (7) reported that the distance of the microsatellite lesion from the main tumor (microsatellite distance) was associated with prognosis. However, microsatellite distance cannot be evaluated by computed tomography (CT) or magnetic resonance imaging (MRI). If there remain microsatellite lesion after treatment, recurrence is inevitable. When the strategy for HCC treatment is being established, it is important to evaluate the microsatellite distance. The prediction of preoperative microsatellite distance is of particular importance for treatment selection in HCC.
Fluorine-18 fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) is effective for detecting and staging a variety of malignant diseases. 18F-FDG PET/CT is based on the fact that a number of malignant tumors take up glucose. However, 18F-FDG PET/CT was not found to be effective in detecting HCC, particularly well-differentiated HCC, although FDG accumulation was found to reflect malignant potential (8,9). Patients with tumors exhibiting high FDG accumulation are associated with a poor prognosis after therapy (10,11). However, 18F-FDG PET/CT was found to be effective for detecting extrahepatic metastases (12).
The aim of the present study was to investigate whether 18F-FDG PET/CT is a useful preoperative imaging modality for predicting the distance of the microsatellite lesion from the main tumor and the postoperative recurrence pattern.
Patients and methods
Patients and follow-up
This was a retrospective study. All the study protocols were approved by the Institutional Ethics Committee of the Ehime Prefectural Central Hospital, Ehime University Graduate School of Medicine (Ehime University Hospital) (approval number: 1106005 and UMIN ID number: 000008652) and all the patients provided written informed consent. In this study, patients with initial HCC who underwent hepatic resection (systematic segmentectomy) at Ehime University Hospital and Ehime Prefectural Central Hospital between April, 2006 and July, 2011 were enrolled. Preoperatively, the patients underwent abdominal ultrasonography (US), CT, MRI, or angiography. 18F-FDG PET/CT was performed within 1 month prior to hepatic resection. Preoperative transcatheter arterial chemoembolization was not performed. The diagnosis of HCC was confirmed by pathological findings in all the cases.
Adjuvant therapy was not administered in this study. During follow-up, abdominal US or contrast-enhanced CT (CECT) was performed every 3 months. The diagnosis of recurrence was based on imaging findings. Extrahepatic metastasis was diagnosed with a whole-body imaging study, including CECT or 18F-FDG PET/CT. The period for disease recurrence was defined as the number of days from hepatic resection to detection of disease recurrence.
18F-FDG PET/CT imaging protocol
The patients were fasted for a minimum of 6 h prior to the injection of 222–370 MBq 18F-FDG. Images were acquired at 60 min post-injection. Blood glucose levels were measured prior to the injection and none of the patients were withdrawn from the study due to high blood glucose levels; no additional drugs for glucose control were administered.
18F-FDG PET/CT scans were obtained using a multislice PET/CT camera (Discovery STE; GE Healthcare, Milwaukee, WI, USA). The PET scanner contains bismuth germanate detectors and reconstructs 35 axial images at 4.25-mm intervals, with a field of view of 15.6 cm. Spatial resolution of this system for 3-dimensional (3D) mode in full-width at half-maximum is 5.12 mm at 1-cm offset from the center (13). CT was performed on the same scanner without contrast administration. The CT scan data were collected with 160–280 mAs (adjusted to the patient’s body weight) and a gantry rotation speed of 0.8 s. All the CT scans were obtained using 3.75-mm axial sections for attenuation correction and diagnosis. Whole-body acquisitions (head to mid-thigh) were performed in 3D mode with the use of 6 or 7 bed positions and emission images were acquired for 3 min per bed position. PET, CT and fused PET/CT images were reconstructed and reviewed on Advantage Workstations version 4.5 (GE Healthcare). The PET/CT and CT diagnostic results were compared.
Interpretation and analysis of PET/CT images
The evaluation of the 18F-FDG PET/CT images was performed by a combined team of experienced nuclear medicine physicians and radiologists, in consensus, who were blinded to clinical and laboratory data and the final clinical diagnosis. For each PET dataset, the tumor with the most intense 18F-FDG uptake of all foci was carefully identified for the maximal count. A volumetric region of interest encompassing the entire tumor was drawn to ensure correct identification of the maximal count. The maximal standardized uptake value (SUVmax) was calculated. In a region of interest, only the lesions that exhibited the most substantial 18F-FDG uptake were selected as the target lesions for evaluating response to therapy. Semi-quantitative indices of the uptake of 18F-FDG were calculated for each vascular segment. A volumetric region of interest encompassing the entire tumor was drawn to ensure correct identification of the maximal counts and its SUVmax.
Pathological evaluation
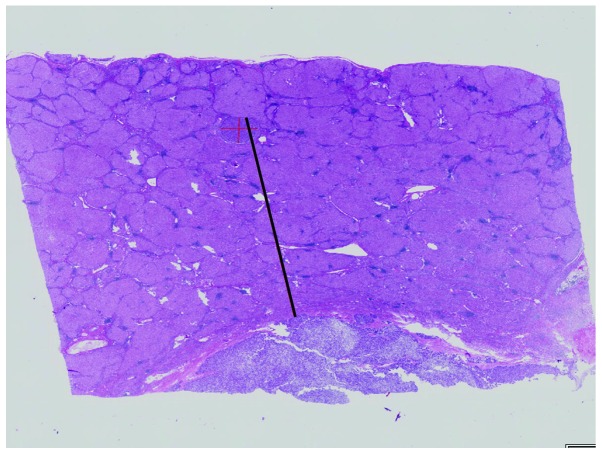
The resected specimens were divided at 10-mm intervals and fixed in 20% formalin. The specimens were embedded in paraffin, cut into 4-μm slices and stained with hematoxylin and eosin. The histological assessment was performed by two experienced pathologists (K.F. and Y.S.). The histological grade of the tumor was evaluated according to the classification of the Liver Cancer Study Group of Japan (14). Satellite lesions in the resected specimens were first assessed macroscopically and, if not identified, microscopic assessment was performed. Satellite lesions were identified as microscopic portal vein invasion or intrahepatic metastasis. To identify satellite lesions, we applied the criteria defined by the Liver Cancer Study Group of Japan: Tumors surrounding the main tumor with multiple satellite nodules or small solitary tumors located near the main tumor that are histologically similar or less differentiated than the main tumor (14). The distance between the satellite lesion and the main tumor was measured using the Virtual Slides system (Olympus Engineering Co., Ltd., Tokyo, Japan) (Fig. 1).
Figure 1.
Hematoxylin and eosin stain (magnification, ×100). Photograph of a microsatellite lesion (smaller tumor) and the main tumor (hepatocellular carcinoma). The distance of the microsatellite lesion from the main tumor was measured (black line) using the Virtual Slide system (Olympus Engineering).
Data analysis
The clinicopathological parameters were compared between the groups using the Student’s t-test and the Chi-square test, as appropriate. The significance of the differences was assessed by non-parametric testing. The correlations between the microsatellite distance and other parameters were assessed by the Pearson’s product-moment correlation coefficient. Receiver operating characteristic (ROC) curves were constructed and the area under the ROC curve (AUC) was calculated by the trapezoidal rule. Optimal cutoff values were selected to maximize sensitivity, specificity and diagnostic accuracy. Sensitivity, specificity, positive predictive value and negative predictive value (NPV) were calculated using cutoffs obtained from the ROC curves. Variables that were found to be significant on the univariate analysis of factors affecting microsatellite distribution were included in a subsequent multivariate analysis using Cox’s proportional hazards model. P<0.05 was considered to indicate a statistically significant difference. The statistical analysis was performed using JMP statistical software package, version 9 (SAS Institute Japan Ltd., Tokyo, Japan). All the values are presented as means ± standard deviation.
Results
Clinical and pathological characteristics
A total of 89 patients (70 men and 19 women; median age, 68.4 years) were enrolled in this study. The patients’ clinical characteristics are presented in Table I. The median follow-up period was 12.1 months (range, 1–126.7 months). The median disease-free survival was 12.0 months and 45 patients (50.5%) developed postoperative recurrence. Intrahepatic recurrence occurred in 36 patients and extrahepatic recurrence occurred in 9. The locations of extrahepatic recurrence were the lungs (n=4), lymph nodes (n=3), bone (n=2) and adrenal gland (n=1). The median tumor SUVmax was 3.9 (range, 1.0–20.5). Of the 29 patients (32.6%) with microsatellite lesions in the resected specimens, 19 developed recurrence after resection.
Table I.
Clinical characteristics of patients in this study (n=89).
| Variables | Values |
|---|---|
| Patient characteristics | |
| Gender (male/female) | 70/19 |
| Age, years [median (range)] | 70 (38–87) |
| BMI, kg/m2 [median (range)] | 23.0 (17.5–42.1) |
| Etiology | |
| Hepatitis B/hepatitis C/other | 14/48/27 |
| Biochemical value, median (range) | |
| AST (IU/l) | 43 (5–134) |
| ALT (IU/l) | 34 (9–161) |
| PLT (104/μl) | 14.1 (3.6–31.3) |
| TBil (mg/dl) | 0.6 (0.2–2.6) |
| Alb (g/dl) | 4.0 (2.4–4.9) |
| PT (%) | 80.2 (58.4–117.3) |
| AFP (ng/dl) | 26.1 (1.4–75,000) |
| PIVKA-II (mAU/ml) | 160 (4.5–75,000) |
| HCC characteristics, median (range) | |
| Tumor size (cm) | 4.0 (1.2–21) |
| Number of tumors in one patient (range) | 1 (1–3) |
| Tumor stage (I/II/III/IVa) | 4/54/24/7 |
| SUVmax of tumor | 3.9 (1.0–20.5) |
| Number of satellite lesions | 29 |
| Follow-up | |
| Mean follow-up period (months) | 12.1 |
| Number of patients with recurrence | 45 |
| Location of extrahepatic metastasis (lung/bone/lymph nodule/adrenal gland) | 4/2/3/1 |
BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet count; TBil, total bilirubin; Alb, albumin; PT, prothrombin time; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; HCC, hepatocellular carcinoma; SUVmax, maximal standardized uptake value.
Parameters associated with microsatellite distance
In a linear regression model, the significant parameters included α-fetoprotein (AFP) (P<0.0003, r=0.37, 95% CI: 0.18–0.54), protein induced by vitamin K absence or antagonist-II (PIVKA-II) (P<0.0001, r=0.40, 95% CI: 0.20–0.56), tumor diameter (P<0.0001, r=0.44, 95% CI: −0.26–0.60) and tumor SUVmax (P<0.0001, r=0.58, 95% CI: 0.41–0.70) (Table II).
Table II.
Linear regression model for parameters associated with microsatellite distance.
| Parameters | r value | 95% CI | t value | P-value |
|---|---|---|---|---|
| Age (years) | −0.04 | −0.30–0.18 | −0.35 | 0.727 |
| BMI (kg/m2) | −0.12 | −0.32–0.09 | −1.10 | 0.274 |
| AST (IU/l) | −0.14 | −0.07–0.34 | 1.32 | 0.192 |
| ALT (IU/l) | −0.005 | −0.21–0.20 | −0.01 | 0.988 |
| PLT (x104/μl) | −0.17 | −0.04–0.37 | 1.47 | 0.145 |
| TBil (mg/dl) | −0.06 | −0.27–0.14 | −0.56 | 0.579 |
| Alb (g/dl) | −0.06 | −0.26–0.16 | −0.45 | 0.651 |
| PT (%) | −0.04 | −0.30–0.18 | 0.08 | 0.935 |
| AFP (ng/ml) | 0.37 | 0.18–0.54 | 3.37 | 0.0003 |
| PIVKA-II (mAU/ml) | 0.40 | 0.20–0.56 | 4.16 | <0.0001 |
| HbA1c (%) | −0.15 | −0.33–0.08 | −1.47 | 0.145 |
| Diameter of tumor (cm) | 0.44 | −0.26–0.60 | 4.63 | <0.0001 |
| SUVmax | 0.58 | 0.41–0.70 | 6.53 | <0.0001 |
CI, confidence interval; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet count; TBil, total bilirubin; Alb, albumin; PT, prothrombin time; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; HbA1c, hemoglobin A1c; SUVmax, maximal standardized uptake value.
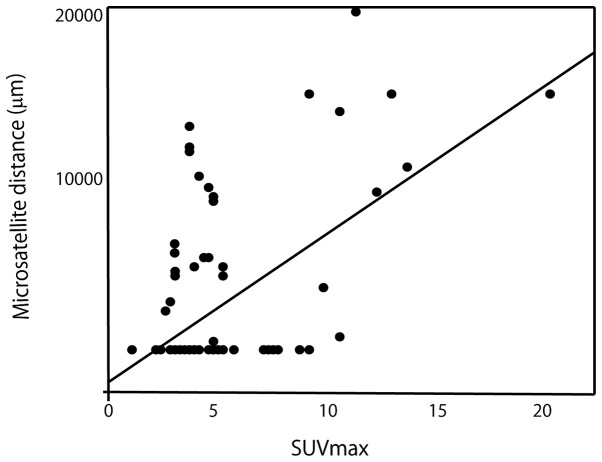
SUVmax exhibited the most significant correlation with microsatellite distance. The linear regression model is shown in Fig. 2.
Figure 2.
Linear correlation between microsatellite distance and maximum standardized uptake value (SUVmax). The correlation was found to be statistically significant (r=0.58, P<0.0001).
Independent risk factors for microsatellite distance >1 cm
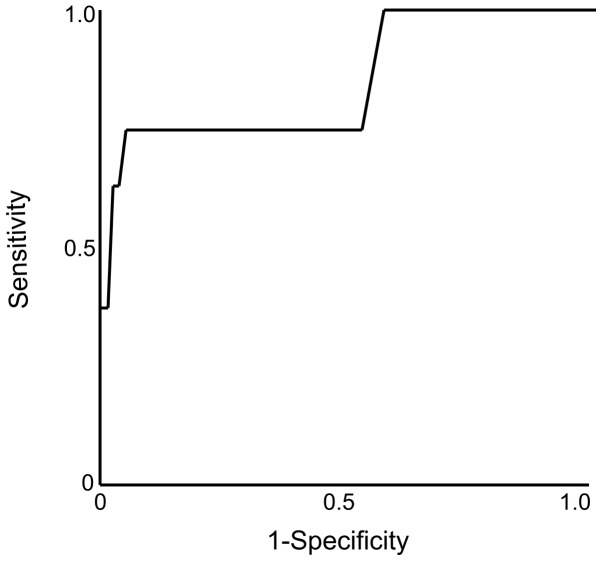
On the univariate analysis of the independent risk factors for microsatellite distance >1 cm, the significant factors were preoperative tumor diameter (P=0.017; hazard ratio=1.23; 95% CI: 1.04–1.39) and SUVmax (P=0.0003; hazard ratio=1.65; 95% CI: 1.30–2.29) (Table III). On the multivariate analysis, the only significant factor was SUVmax (P=0.002; hazard ratio=1.60; 95% CI: 1.23–2.26). ROC curves were constructed and are presented in Fig. 3.
Table III.
Preoperative predictive factors for microsatellite distance >1 cm on univariate and multivariate regression models.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Parameters | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | AUC |
| Age (years) | 0.97 (0.91–1.13) | 0.273 | |||
| BMI (kg/m2) | 0.86 (0.65–1.07) | 0.251 | |||
| AST (IU/l) | 1.00 (0.98–1.03) | 0.501 | |||
| ALT (IU/l) | 0.99 (0.96–1.02) | 0.863 | |||
| PLT (x104/μl) | 1.07 (0.96–1.19) | 0.180 | |||
| TBil (mg/dl) | 0.26 (0.01–2.11) | 0.327 | |||
| Alb (g/dl) | 1.34 (0.35–6.40) | 0.819 | |||
| PT (%) | 1.00 (0.94–1.07) | 0.868 | |||
| AFP (ng/ml) | 1.00 (0.99–1.00) | 0.182 | |||
| PIVKA-II (mAU/ml) | 1.00 (0.99–1.00) | 0.122 | |||
| HbA1c (%) | 0.41 (0.09–1.34) | 0.182 | |||
| Diameter of tumor (cm) | 1.23 (1.04–1.39) | 0.017 | 1.06 (0.82–1.32) | 0.591 | |
| SUVmax | 1.65 (1.30–2.29) | 0.003 | 1.60 (1.23–2.26) | 0.002 | 0.854 |
CI, confidence interval; AUC, area under the receiver operating characteristic curve; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet count; TBil, total bilirubin; Alb, albumin; PT, prothrombin time; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; HbA1c, hemoglobin A1c; SUVmax, maximal standardized uptake value.
Figure 3.
Receiver operating characteristic curve of maximum standardized uptake value for predicting the distance of the microsatellite lesion from the main tumor.
The predictive accuracy for microsatellite distance >1 cm was highest for SUVmax (0.854). The optimal cutoff value was 8.8. The sensitivity and specificity were 75.0 and 95.0%, respectively. The NPV was also found to be high (97.3%).
Microsatellite distance and SUVmax by postoperative recurrence pattern
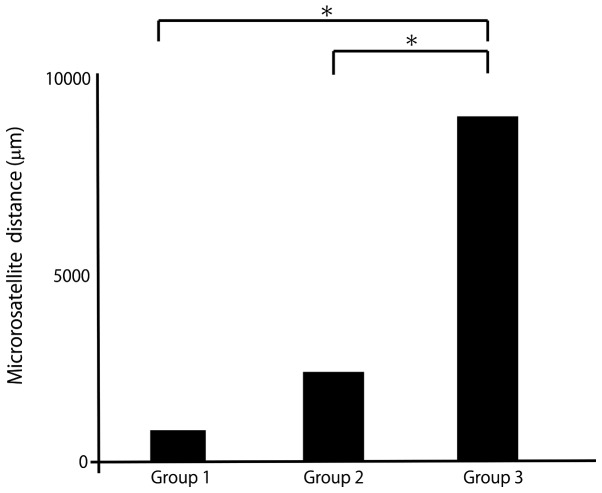
The patients were divided into 3 groups according to the initial recurrence pattern: No recurrence (group 1), intrahepatic recurrence (group 2) and extrahepatic recurrence (group 3).
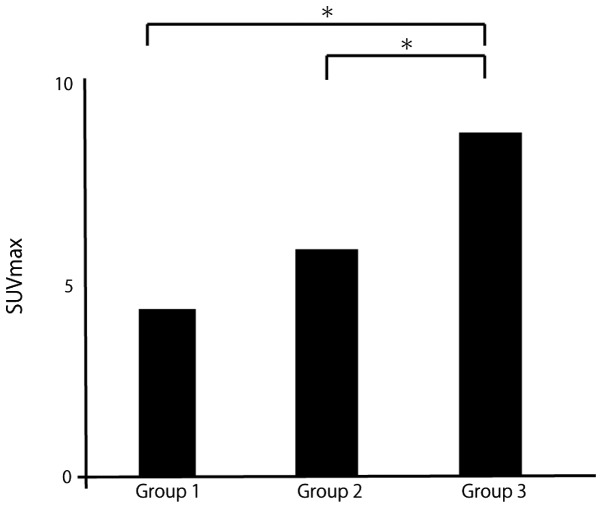
The mean distance was 841 μm in group 1, 2,098 μm in group 2 and 9,691 μm in group 3 (Fig. 4). The mean SUVmax was 4.0 in group 1, 5.1 in group 2 and 8.6 in group 3 (Fig. 5). The mean microsatellite distance and mean SUVmax were significantly higher with extrahepatic recurrence compared with other recurrence patterns.
Figure 4.
Mean microsatellite distance by recurrence pattern. Group 1, no recurrence; Group 2, intrahepatic recurrence; and Group 3, extrahepatic recurrence. *P<0.01.
Figure 5.
Mean maximum standardized uptake value (SUVmax) by recurrence pattern. Group 1, no recurrence; Group 2, intrahepatic recurrence; and Group 3, extrahepatic recurrence. *P<0.01.
Independent risk factors for prediction of the postoperative recurrence pattern
On the univariate analysis for the independent risk factors for postoperative extrahepatic recurrence, the significant factors were preoperative AFP level (P=0.019, hazard ratio 1.00, 95% CI: 1.00–1.00), PIVKA-II level (P=0.012, hazard ratio 1.00, 95% CI:1.00–1.00), SUVmax (P=0.001, hazard ratio 1.26, 95% CI: 1.03–1.53), and Diameter of tumor (P=0.033, hazard ratio 1.19, 95% CI: 1.01–1.42) (Table IV).
Table IV.
Preoperative predictive factors for postoperative extrahepatic recurrence on univariate and multivariate regression models.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Parameters | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | AUC |
| Age (years) | 1.01 (0.95–1.09) | 0.641 | |||
| BMI (kg/m2) | 1.04 (0.88–1.20) | 0.584 | |||
| AST (IU/l) | 1.01 (0.99–1.04) | 0.122 | |||
| ALT (IU/l) | 1.01 (0.99–1.03) | 0.158 | |||
| PLT (x104/μl) | 1.04 (0.94–1.14) | 0.414 | |||
| TBil (mg/dl) | 1.45 (0.28–5.22) | 0.593 | |||
| Alb (g/dl) | 1.46 (0.42–6.08) | 0.573 | |||
| PT (%) | 1.00 (0.95–1.04) | 0.854 | |||
| AFP (ng/ml) | 1.00 (1.00–1.00) | 0.019 | 1.00 (1.00–1.00) | 0.125 | |
| PIVKA-II (mAU/ml) | 1.00 (1.00–1.00) | 0.012 | 1.00 (0.99–1.00) | 0.387 | |
| HbA1c (%) | 0.55 (0.16–1.52) | 0.286 | |||
| Diameter of tumor (cm) | 1.19 (1.01–1.42) | 0.033 | 0.93 (0.64–1.20) | 0.620 | |
| SUVmax | 1.26 (1.03–1.53) | 0.001 | 1.24 (1.01–1.55) | 0.033 | 0.846 |
CI, confidence interval; AUC, area under the receiver operating characteristic curve; BMI, body mass index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLT, platelet count; TBil, total bilirubin; Alb, albumin; PT, prothrombin time; AFP, α-fetoprotein; PIVKA-II, protein induced by vitamin K absence or antagonist-II; HbA1c, hemoglobin A1c; SUVmax, maximal standardized uptake value.
Discussion
The rate of HCC recurrence following treatment remains high (15,16). In previous studies, the 5-year disease-free survival rate following liver resection was reported to be 72% (2). Therefore, it is crucial to assess the risk of postoperative recurrence. The presence of microsatellite lesions (intrahepatic metastasis and microvascular invasion) is an independent risk factor for postoperative recurrence (15,17–19). Sumie et al (20) reported that microvascular invasion was an independent risk factor for recurrence-free survival. The 5-year recurrence-free survival rates for patients with and without microvascular invasion were 20.8 and 52.6%, respectively. Microvascular invasion and intrahepatic metastases were identified as independent predictors of disease-specific survival.
Determining the extent of the treatment margin is critical, as an insufficient treatment margin causes disease recurrence after treatment. It is crucial to determine the treatment margin within the microsatellite lesion to prevent postoperative recurrence as much as possible. In previous studies, the microsatellite distance was associated with prognosis. Sasaki et al (7) reported that the overall survival rate of patients with a microsatellite distance >5 mm was lower compared to that of patients with a microsatellite distance <5 mm.
Even if the presence of a microsatellite lesion can be predicted preoperatively, it is difficult to predict the microsatellite distance. The extent of the treatment margin remains controversial. However, US, CT, or MRI cannot predict the presence of microsatellite lesions and the microsatellite distance preoperatively.
The use of 18F-FDG PET/CT, which is based on the fact that a number of malignant tumors exhibit facilitated glucose uptake, was found to be effective for detecting and staging a variety of malignant diseases. However, in HCC, 18F-FDG PET/CT is not effective for detecting tumors, particularly well-differentiated HCC. However, FDG accumulation reflects tumor aggressiveness (8,9). Patients with tumors with a high accumulation of FDG have a poor prognosis after therapy (10,11). In other malignant tumors (e.g., malignant lymphoma or breast cancer), it is possible to diagnose not only the localization but also disease activity and biological characteristics. 18F-FDG PET/CT was found to be useful for predicting disease activity and biological characteristics in HCC, including pathological evaluation. 18F-FDG accumulation in HCC also reflects malignant potential. Hiraoka et al (21) demonstrated that high accumulation of FDG in HCC increased the risk of early postoperative recurrence following curative resection and the appearance of microsatellite lesions.
In the present study, the efficacy of 18F-FDG PET/CT for the prediction of microsatellite distance was investigated. To the best of our knowledge, this is the first study to demonstrate the correlation between microsatellite distance and median SUVmax; microsatellite distance correlated with SUVmax (r=0.58). On the multivariate analysis, the only significant factor was SUVmax (P=0.002; hazard ratio=1.60; 95% CI: 1.23–2.26). When the SUVmax was >9.3, the microsatellite distance was >1 cm.
There are various patterns of postoperative recurrence of HCC, including intrahepatic recurrence (within the same or a different segment of the primary tumor) or extrahepatic recurrence. The patterns of postoperative recurrence are associated with the prognosis of HCC. Intrahepatic metastasis may be satisfactorily treated and controlled by resection or RFA. Shiina et al (22) reported that the 5-year survival rate in patients treated by RFA was 60.2%. In addition, Ng et al (23) reported that the overall survival rates were significantly higher in patients with same segment recurrence of the primary tumor compared to different segment recurrence. Among the various recurrence patterns, extrahepatic recurrence has the worst prognosis following curative therapy. In previous reports, the 1-year survival rate was 40–70% in patients with extrahepatic recurrence (24,25). It is crucial to predict the postoperative recurrence pattern. However, the number of available studies investigating the imaging modalities useful for predicting the postoperative recurrence pattern is currently limited. In the present study, the SUVmax was found to be significantly higher in patients with postoperative extrahepatic recurrence of HCC compared to that in other patients.
It was previously demonstrated that HCCs with a higher SUVmax exhibit a higher malignant potential. There are two patterns of metastasis in HCC, via the portal veins or the systemic circulation. The mechanism of distant metastasis is based on various factors, according to previous studies (26,27). In microsatellite lesions with a microsatellite distance >1 cm, the pattern of metastasis is via the systemic circulation in almost all cases. Epithelial-to-mesenchymal transition (EMT) has been proposed as playing an important role in distant metastasis in cancer. During the process of EMT, epithelial cells lose polarity and intercellular junctions and acquire mesenchymal-like characteristics, such as motility, becoming able to detach from the original tissue. The activation of EMT induces more distant metastases as microsatellite distance is >1 cm. In a previous study, FDG uptake was found to reflect the activation of glucose metabolism. Amann et al (28) reported that HCC with a higher uptake of glucose via glucose transporter 1 (GLUT-1) into cancer cells exhibited higher proliferative activity and that 18F-FDG PET/CT reflects proliferative activity in HCC. The uptake of 18F-FDG and the expression of GLUT-1 were increased during the process of EMT, stimulated by transforming growth factor β (29). Therefore, it was suggested that high FDG accumulation may primarily reflect activation of EMT, tumor aggressiveness and distant metastasis in the present study.
There were several limitations to the present study. First, the design was retrospective. Second, the mean HCC diameter was relatively large; therefore, a validation study for microsatellite distance in small HCCs would be required as a further experiment. Third, our sample size was small; therefore, further investigations, including a larger patient sample, are required.
In conclusion, SUVmax may be a valid predictor of microsatellite distance and postoperative recurrence pattern. SUVmax may also be an indicator for determining the treatment protocol. There is currently no standard safety margin for liver resection or RFA. Generally, if the SUVmax is >8.8, we select anatomic liver resection with a margin >1 cm. In the future, if the prediction of extrahepatic metastasis is feasible using the SUVmax, SUVmax may become the standard for the introduction of systemic chemotherapy.
Acknowledgements
The authors would like to thank Dr Yoshiko Soga (Department of Pathology, Ehime University Graduate School of Medicine) and Mr Kenji Tanimoto (Department of Gastroenterology and Metabology, Ehime University Graduate School of Medicine) for their valuable technical assistance.
This study was supported in part by Grants-in-Aid for Scientific Research (Japan Society for the Promotion of Science, KAKENHI no. 24590980 to Y.H. and KAKENHI no. 25860541 to Y.K.).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330–339. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 4.Kanda M, Tateishi R, Yoshida H, et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int. 2008;28:1256–1263. doi: 10.1111/j.1478-3231.2008.01864.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagasue N, Uchida M, Makino Y, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. doi: 10.1016/0016-5085(93)90724-q. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki K, Soyama A, Hidaka M, et al. Ex vivo hepatic venography for hepatocellular carcinoma in livers explanted for liver transplantation. World J Surg Oncol. 2011;9:111. doi: 10.1186/1477-7819-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005;103:299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]
- 8.Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Jr, Pinson CW. Evaluation of benign vs. malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510–515. doi: 10.1001/archsurg.133.5.510. [DOI] [PubMed] [Google Scholar]
- 9.Iwata Y, Shiomi S, Sasaki N, et al. Clinical usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose in the diagnosis of liver tumors. Ann Nucl Med. 2000;14:121–126. doi: 10.1007/BF02988591. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682–687. doi: 10.2967/jnumed.108.060574. [DOI] [PubMed] [Google Scholar]
- 11.Kornberg A, Freesmeyer M, Barthel E, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawaoka T, Aikata H, Takaki S, et al. FDG positron emission tomography/computed tomography for the detection of extrahepatic metastases from hepatocellular carcinoma. Hepatol Res. 2009;39:134–142. doi: 10.1111/j.1872-034X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 13.Teras M, Tolvanen T, Johansson JJ, Williams JJ, Knuuti J. Performance of the new generation of whole-body PET/CT scanners: Discovery STE and Discovery VCT. Eur J Nucl Med Mol Imaging. 2007;34:1683–1692. doi: 10.1007/s00259-007-0493-3. [DOI] [PubMed] [Google Scholar]
- 14.Kanai T, Hirohashi S, Upton MP, et al. Pathology of small hepatocellular carcinoma. A proposal for new gross classification. Cancer. 1987;60:810–819. doi: 10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Adachi E, Maehara S, Tsujita E, et al. Clinicopathologic risk factors for recurrence after a curative hepatic resection for hepatocellular carcinoma. Surgery. 2002;131(Suppl 1):S148–S152. doi: 10.1067/msy.2002.119496. [DOI] [PubMed] [Google Scholar]
- 16.Lam CM, Lo CM, Yuen WK, et al. Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg. 1998;85:1198–1200. doi: 10.1046/j.1365-2168.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- 17.Shimada K, Sakamoto Y, Esaki M, et al. Analysis of prognostic factors affecting survival after initial recurrence and treatment efficacy for recurrence in patients undergoing potentially curative hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2007;14:2337–2347. doi: 10.1245/s10434-007-9415-7. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Nagano H, Ota H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753–758. doi: 10.1016/j.jamcollsurg.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 21.Hiraoka A, Ochi H, Hidaka S, et al. FDG positron emission tomography/computed tomography findings for prediction of early recurrence of hepatocellular carcinoma after surgical resection. Exp Ther Med. 2010;1:829–832. [Google Scholar]
- 22.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng KK, Poon RT, Lo CM, et al. Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg. 2008;12:183–191. doi: 10.1007/s11605-007-0276-y. [DOI] [PubMed] [Google Scholar]
- 24.Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang GT, Lee HS, Chen CH, et al. Correlation of E-cadherin expression and recurrence of hepatocellular carcinoma. Hepatogastroenterology. 1999;46:1923–1927. [PubMed] [Google Scholar]
- 27.Sakon M, Nagano H, Nakamori S, et al. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94–99. doi: 10.1001/archsurg.137.1.94. [DOI] [PubMed] [Google Scholar]
- 28.Amann T, Maegdefrau U, Hartmann A, et al. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544–1552. doi: 10.2353/ajpath.2009.080596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Wei Z, Liu Y, et al. Increased 18F-FDG uptake and expression of Glut1 in the EMT transformed breast cancer cells induced by TGF-beta. Neoplasma. 2010;57:234–240. doi: 10.4149/neo_2010_03_234. [DOI] [PubMed] [Google Scholar]