Abstract
Peroxiredoxin 4 (Prx4) has a number of important biological functions, such as efficient antioxidant capacity and promotion of cell proliferation and differentiation. The purpose of this study was to investigate the expression and significance of Prx4 in human colorectal cancer (CRC). Quantitative polymerase chain reaction (qPCR) was performed to detect Prx4 in 8 freshly frozen specimens of CRC and their adjacent normal tissues. In addition, immunohistochemical analysis was performed to detect Prx4 in 59 specimens of CRC and 26 of adjacent normal tissues. The immunohistochemical and qPCR results demonstrated that the expressions of the Prx4 gene and protein were higher in CRC compared to those in the adjacent normal tissues. The expression intensity of the Prx4 protein was correlated with depth of invasion (P=0.001), lymph node metastasis (P=0.006) and Dukes’ classification (P=0.004) in CRC. The Kaplan-Meier survival curves revealed that high Prx4 expression was correlated with short survival time. However, the Cox proportional hazards regression analysis did not identify Prx4 as an independent prognostic marker for CRC (P>0.05). These results suggested that Prx4 may be associated with carcinogenesis and the development of CRC and it may be a prognostic marker for postoperative CRC patients.
Keywords: peroxiredoxin, colorectal cancer, expression, prognostic marker
Introduction
Colorectal cancer (CRC) is the third most common gastrointestinal malignancy worldwide (1,2). In China, with the improvement of living standards and changing of dietary habits, the incidence of CRC exhibits a rising trend each year, with the mortality rate rising to the fifth place (3). It has been demonstrated that early-stage CRC may be cured by minimally invasive radical surgical resection. However, a significant proportion of CRC patients are diagnosed at an advanced stage, when conventional treatment options are unavailable (4,5). Therefore, the early diagnosis of CRC is crucial, as it may significantly reduce the mortality rate of CRC. The pathogenesis of CRC is complicated. It was previously reported that the occurrence of CRC is a process involving genetic and environmental factors, including a variety of genetic mutations, such as oncogene activation and tumor suppressor gene inactivation (6). Therefore, the mechanisms underlying the occurrence and the molecular pathogenesis of CRC must be elucidated.
It was previously demonstrated that the expression of peroxiredoxins (Prxs) is significantly upregulated in the majority of tumors, suggesting that Prxs may play an important role in tumor stage, invasion, recurrence and prognosis; it is also hypothesized that the high expression of Prxs may be induced by high levels of reactive oxygen species within the tumor cells and may be associated with resistance to apoptosis (7). Therefore, Prxs may be used as tumor markers.
As a member of the Prx family, the newly discovered peroxiredoxin 4 (Prx4) has important biological functions (8,9), through acting as an antioxidant as well as promoting cell proliferation and differentiation and participating in cell signal transduction. However, the prognostic significance of Prx4 expression in CRC has yet to be investigated. The aim of this study was to elucidate the role of Prx4 in the development of CRC and the association between Prx4 and the clinical characteristics of CRC.
Patients and methods
Tissue samples
Fresh samples of CRC and adjacent normal tissues were obtained from 15 patients who underwent surgical resection at the Affiliated Hospital of Nantong University, Jiangsu, China. Upon surgical removal, the samples were immediately snap-frozen in liquid nitrogen. All the tissues were microdissected prior to RNA extraction. A total of 59 CRC tissue samples were obtained from 59 patients between April, 2004 and February, 2008. The median follow-up was 50 months. The patients comprised 36 men and 23 women, with a mean age of 65 years (range, 28–80 years). The tumors were classified as 33 right hemicolon carcinomas and 26 left hemicolon and rectal carcinomas, with 15 in Dukes’ stage A, 26 in Dukes’ stage B, 14 in Dukes’ stage C and 4 in Dukes’ stage D. In addition, 26 normal colorectal tissue samples were used as controls. The main clinicopathological variables of the patients are summarized in Table I. All the specimens were fixed in 10% formalin, embedded in paraffin and cut into 4-μm sections. All the patients had undergone surgical resection at the Surgery Department of the Affiliated Hospital of Nantong University. None of the patients had undergone chemotherapy prior to surgery. The diagnoses were confirmed by pathological examination.
Table I.
Protein expression of peroxiredoxin 4 in colorectal cancer (CRC) and adjacent normal tissues.
| Tissue | Cases | Positive rate (%) | − | + | ++ | +++ | Za | Pa |
|---|---|---|---|---|---|---|---|---|
| CRC | 26 | 88.5 | 3 | 1 | 11 | 11 | 4.237 | 0.0000 |
| Adjacent tissues | 26 | 65.4 | 9 | 10 | 7 | 0 |
The Z and P-values were calculated using the rank sum test.
Approval for the study was obtained from the Ethics Committee of the Affiliated Hospital of Nantong University according to the relevant provisions of the Declaration of Helsinki and the people involved biomedical research ethics review method (trial).
Quantitative polymerase chain reaction (qPCR)
RNA was extracted from the fresh frozen cancer tissues and adjacent normal tissues using the Biozol RNA extraction kit (BioFlux, Tokyo, Japan). Total RNA (1 μg) was used to prepare cDNA using random hexamers (Fermentas, Glen Burnie, MD, USA) and was used as template in qPCR. Real-time amplifications, using SYBR-Green detection chemistry, were run in triplicate on 96-well reaction plates with the QuantStudio™ 7 FLex machine (Invitrogen Life Technologies, Carlsbad, CA, USA). The reactions were prepared in a total volume of 25 μl containing 2.0 μl cDNA, 1.0 μl of each 10 μM primer (Invitrogen Life Technologies), 12.5 μl of Maxima SYBR-Green Master mix (Fermentas, Glen Bernie, MD, USA) and 8.5 μl RNase/DNase-free sterile water. Blank controls were run in triplicate for each Master mix. The cycle conditions were set as follows: initial template denaturation at 95°C for 30 sec, followed by 40 cycles of denaturation at 95°C for 5 sec and combined primer annealing/elongation at 60°C for 30 sec. β-actin was used as the endogenous control for quantitation. The forward and reverse primers used for each molecule were as follows: Prx4: forward, 5′-TCTTTCAGATTTGACCCATCAG-3′ and reverse, 5′-AGGGCAGACTTCTCCGTGT-3′; β-actin: forward, 5′-AAGTACTCCGTGTGGATCGG-3′ and reverse, 5′-ATGCTATCACCTCCCCTGTG-3′. This cycle was followed by a melting curve analysis, ranging from 56 to 95°C, with temperature increasing by steps of 0.5°C every 10 sec. The QuantStudio™ 7 FLex software (Life Technologies) was used to determine baseline and threshold values automatically for all plates. Raw Ct values were transformed to quantities using an Excel spreadsheet generated by the authors, based on the comparative Ct method (10).
Immunohistochemistry
The immunohistochemical staining of Prx4 was performed using Polymer Detection system for immunohistolochemical staining (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The paraffin-embedded tissues were cut into 4-μm sections and placed on glass slides. The sections were deparaffinized in xylene, hydrated in descending concentrations of ethanol and rinsed with deionized water. For each washing, 0.05 mol/l Tris-buffered saline (pH 7.4) was used. To retrieve the antigen, the sections were boiled for 10 min in citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked for 30 min with 0.3% hydrogen peroxide. The sections were then incubated overnight at 4°C with mouse monoclonal antibodies against Prx4 (dilution 1:200; Abfrontier, Shanghai, China). All the sections were sequentially incubated with PV9003 I (polymer helper) for 20 min. After rinsing in phosphate-buffered saline, the sections were incubated with PV9003 II (polyperoxidase-anti-mouse IgG) for 30 min. Peroxidase activity was revealed by 3,3-diaminobenzidine staining for 10 min; counterstaining was performed with hematoxylin and the sections were dehydrated and coverslipped. Finally, the sections were dehydrated through graded ethanols, cleared in xylene and mounted on glass slides. Sections without primary antibodies were used as negative control.
Immunohistochemical evaluation
In Prx4-positive staining, the cytoplasm of the cells was stained brown-yellow. All the immunostained sections were evaluated by pathologists who were blinded to the clinical and pathological variables of the patients. For Prx4 assessment, staining intensity was scored as follows: 0, negative; 1, weak; 2, medium; and 3, strong. The extent of the staining was scored as follows: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%, according to the percentage of the positively-stained areas in relation to the entire carcinomatous area. The sum of the staining intensity and extent scores was used as the final staining score for Prx4: −, 0–2; +, 3; ++, 4; and +++, ≥5. Tumors with a final staining score of ≥3 were considered to be positive (11). In all the samples, staining was repeated twice to avoid possible technical errors, but similar results were obtained in these samples. The abovementioned evaluation procedures were performed by two independent investigators and a consensus was achieved.
Statistical analysis
The Stata v8.0 software (StataCorp LP, College Station, TX, USA) and the SPSS v15.0 software (SPSS Inc., Chicago, IL, USA) were used for statistical analysis. Materials with skewed distribution were tested with the rank sum test and expressed as median (measurement range). The survival time was estimated using the Kaplan-Meier method and the log-rank test was used for testing differences between groups. The multivariate Cox proportional hazards model was applied to detect independent predictors of survival. P<0.05 was considered to indicate a statistically significant difference.
Results
Prx4 gene and protein expression differences between CRC and adjacent normal tissues
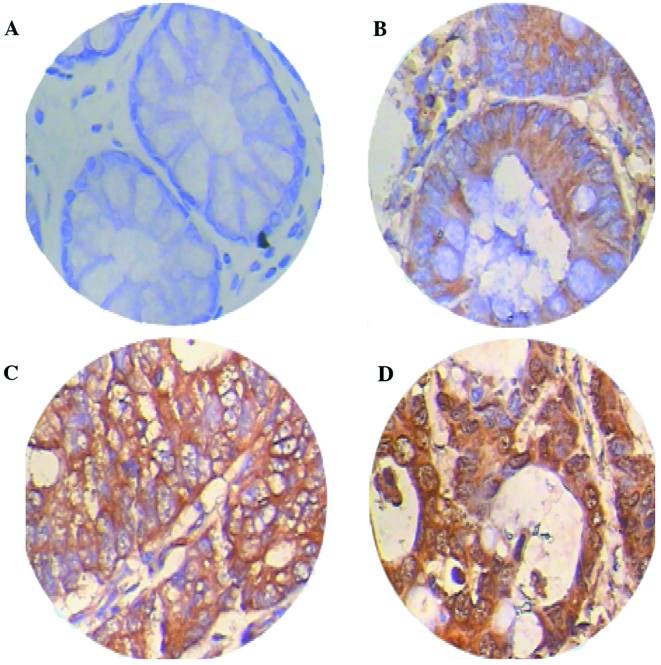
The qPCR demonstrated that the relative expression measurements of the Prx4 gene exhibited skewed distribution and the results for CRC tissues [0.1663 (0.0365–5.7038)] were significantly higher compared to those for adjacent normal colorectal tissues [0.0396 (0.0013–1.3397)] (Z=3.056, P=0.0022) (Fig. 1). The immunohistochemichal examination revealed that the Prx4 protein was mainly expressed in the endoplasmic reticulum and in the extracellular matrix, occasionally in the nucleus, with different expression intensities. Among the 26 samples of CRC and adjacent normal tissues, the positive rate of Prx4 expression was 88.5% (23/26) and 65.4% (17/26), respectively. The expression of Prx4 in the CRC tissues (23/26) was significantly higher compared to that in the adjacent normal tissues (17/26) (Z=4.237, P=0.0000) (Table I), with strongly positive expression areas (++, +++) mainly concentrated in the CRC tissues and weakly positive areas (+) mostly in the adjacent normal tissues (Fig. 2, Table I).
Figure 1.
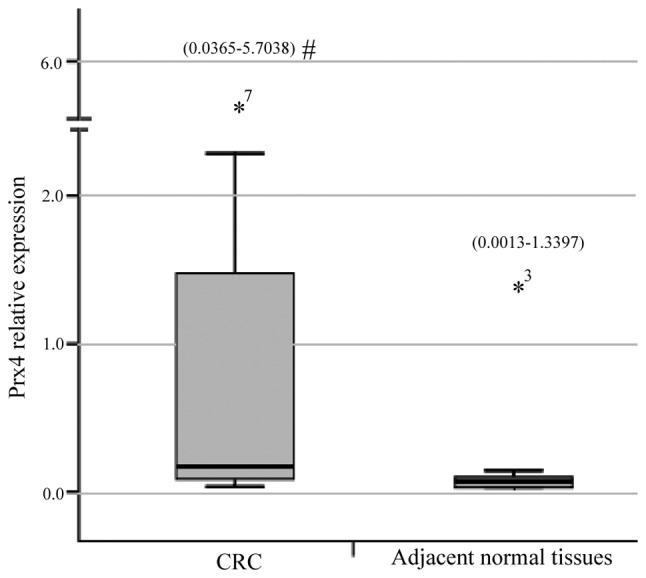
Relative expression of the peroxiredoxin 4 (Prx4) gene in colorectal cancer (CRC) and adjacent normal tissues (n=15). Relative expression measurements of the Prx4 gene using quantitative polymerase chain reaction were performed using the comparative Ct method. The measurement ranges are expressed in brackets at the top of each bar. *7, the highest value (5.7038) in CRC. *3, the highest value (1.3397) in adjacent normal tissues. #, P<0.01.
Figure 2.
Immunohistochemical staining for peroxiredoxin 4 (Prx4) in (A) adjacent normal tissues and (B–D) colorectal cancer (CRC) tissues. Paraffin-embedded tissue sections were stained with antibodies against Prx4 and counterstained with hematoxylin. (A) Prx4-negative staining in adjacent normal tissues. Prx4 immunoreactivity was detected in (B) well-differentiated, (C) moderately differentiated and (D) poorly differentiated CRC (magnification, ×400).
Correlation of Prx4 expression with clinicopathological variables in CRC
The clinicopathological data of the patients are summarized in Table II. The associations of Prx4 expression with clinicopathological variables were evaluated. The Prx4 protein expression in CRC tissues was significantly correlated with infiltration depth (P=0.0012), lymph node metastasis (P=0.0061) and Dukes’ stage (P=0.0041). No significant association was identified between overexpression of Prx4 and gender, age, tumor location, tumor diameter, gross type, tumor differentiation degree and distant metastasis.
Table II.
Association between peroxiredoxin 4 expression and clinicopathological characteristics.
| Characteristics | No. (n=59) | Positive rate (%) | − | + | ++ | +++ | Za | Pa |
|---|---|---|---|---|---|---|---|---|
| Gender | −0.108 | 0.9141 | ||||||
| Male | 36 | 31 (86.1) | 5 | 4 | 11 | 16 | ||
| Female | 23 | 22 (95.7) | 1 | 3 | 10 | 9 | ||
| Age, years | −1.660 | 0.0969 | ||||||
| <69 | 29 | 24 (82.8) | 5 | 4 | 10 | 10 | ||
| ≥69 | 30 | 29 (96.7) | 1 | 3 | 11 | 15 | ||
| Tumor location | −0.310 | 0.7567 | ||||||
| Right hemicolon | 33 | 31 (93.9) | 2 | 4 | 15 | 12 | ||
| Left hemicolon and rectum | 26 | 22 (84.6) | 4 | 3 | 6 | 13 | ||
| Tumor diameter, cm | −1.837 | 0.0663 | ||||||
| <5 | 32 | 26 (81.3) | 6 | 2 | 14 | 10 | ||
| ≥5 | 27 | 27 (100) | 0 | 5 | 7 | 15 | ||
| Gross type | 0.098 | 0.9223 | ||||||
| Massive | 27 | 25 (92.6) | 2 | 4 | 10 | 11 | ||
| Ulcerative | 32 | 28 (87.5) | 4 | 3 | 11 | 14 | ||
| Histological differentiation | 1.028 | 0.5982 | ||||||
| High | 10 | 9 (90.0) | 1 | 1 | 3 | 5 | ||
| Moderate | 42 | 37 (88.1) | 5 | 5 | 16 | 16 | ||
| Poor | 7 | 7 (100) | 0 | 1 | 2 | 4 | ||
| Lymph node metastasis | −2.744 | 0.0061 | ||||||
| Absent | 42 | 37 (88.1) | 5 | 7 | 17 | 13 | ||
| Present | 17 | 16 (94.1) | 1 | 0 | 4 | 12 | ||
| Distant metastasis | −1.252 | 0.2106 | ||||||
| Absent | 52 | 46 (88.5) | 6 | 7 | 18 | 21 | ||
| Present | 7 | 7 (100) | 0 | 0 | 3 | 4 | ||
| Infiltration depth | 15.844 | 0.0012 | ||||||
| T1 | 2 | 1 (50) | 1 | 1 | 0 | 0 | ||
| T2 | 12 | 10 (83.3) | 2 | 3 | 7 | 0 | ||
| T3 | 41 | 38 (92.7) | 3 | 3 | 14 | 21 | ||
| T4 | 4 | 4 (100) | 0 | 0 | 0 | 4 | ||
| Dukes’ stage | 13.248 | 0.0041 | ||||||
| A | 15 | 12 (80.0) | 3 | 4 | 7 | 1 | ||
| B | 26 | 24 (92.3) | 2 | 3 | 10 | 11 | ||
| C | 14 | 13 (92.9) | 1 | 0 | 3 | 10 | ||
| D | 4 | 4 (100) | 0 | 0 | 1 | 3 |
The Z and P-values were calculated using the rank sum test.
Univariate and multivariate analysis of prognostic variables
At the end of the clinical follow-up, survival information was available for all the patients. The univariate analysis for overall survival using the log-rank test identified histological differentiation (P=0.015), lymph node metastasis (P=0.000), distant metastasis (P=0.024), infiltration depth (P=0.001), Dukes’ stage (P=0.000) and positive Prx4 expression (P=0.010) as significant prognostic predictors. However, gender, age, tumor location, tumor diameter and gross type were of no prognostic value (Table III). In 59 patients with CRC, the survival rates for Prx4-positive patients were significantly lower compared to those with negative expression of Prx4 (log-rank test; P=0.010); the survival curve constructed according to the Kaplan-Meier method is shown in Fig. 3. However, the multivariate Cox regression analysis results revealed that Prx4 and other prognostic markers by univariate analysis, including lymph node metastasis, distant metastasis, histological differentiation, infiltration depth and Dukes’ stage, were not independent unfavorable prognostic factors for survival of CRC patients (P>0.05, Table III).
Table III.
Univariate and multivariate analyses of prognostic variables.
| Univariatea | Multivariateb | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variables | χ2 | P | Risk ratio | 95% CI | Z | P |
| Gender | 0.096 | 0.757 | ||||
| Male/female | ||||||
| Age, years | 2.451 | 0.117 | ||||
| <65/≥65 | ||||||
| Tumor location | −0.310 | 0.7567 | ||||
| Right/left hemicolon and rectum | ||||||
| Tumor diameter, cm | 0.826 | 0.363 | ||||
| <5/≥5 | ||||||
| Gross type | 0.389 | 0.533 | ||||
| Massive/ulcerative | ||||||
| Histological differentiation | 8.418 | 0.015 | 1.375 | 0.410–4.605 | 0.266 | 0.606 |
| High/moderate/poor | ||||||
| Lymph node metastasis | 18.624 | 0.000 | 7.255 | 0.860–61.200 | 3.317 | 0.069 |
| Absent/present | ||||||
| Distant metastasis | 5.072 | 0.024 | 5.053 | 0.735–34.744 | 2.712 | 0.100 |
| Absent/present | ||||||
| Infiltration depth | 16.469 | 0.001 | 3.869 | 0.668–22.399 | 2.281 | 0.131 |
| T1/T2/T3/T4 | ||||||
| Dukes’ stage | 18.746 | 0.000 | 0.365 | 0.085–1.565 | 1.840 | 0.175 |
| A/B/C/D | ||||||
| Prx4 | 11.307 | 0.010 | 1.916 | 0.601–6.114 | 1.207 | 0.272 |
| −/+/++/+++ | ||||||
Statistical analyses performed using the log-rank test;
Statistical analyses performed using the Cox regression model.
CI, confidence interval; Prx4, peroxiredoxin 4.
Figure 3.
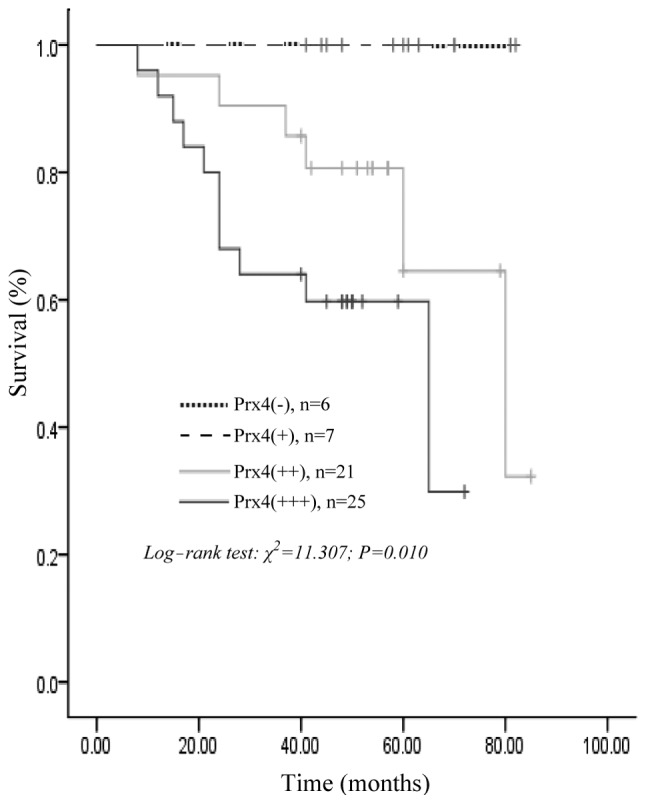
Kaplan-Meier survival curves for peroxiredoxin 4 (Prx4) negative vs. positive expression (+, ++, +++) in 59 patients with colorectal cancer showing a highly significant separation (P=0.010, log-rank test).
Discussion
Despite the advances in surgical techniques and chemotherapy in the treatment of CRC, the cure rate for advanced CRC remains low and the mortality rate remains high. Therefore, it is crucial to elucidate the molecular mechanisms underlying the development of this malignancy. The identification of the molecules involved in the initiation and progression of CRC may be of value for the diagnosis, targeted treatment and prediction of prognosis.
Prxs belong to the antioxidant protein superfamily and are abundant in prokaryotes and eukaryotes. All the Prx proteins contain a conserved cysteine (Cys) residue in the NH2-terminal portion of the molecule and the majority contain an additional conserved Cys in the COOH-terminal region. A small number of Prx proteins lack the COOH-terminal Cys. According to the different number of conserved Cys residues, six Prx isoforms in mammalian cells are divided into two subgroups, the 1-Cys Prx and the 2-Cys Prx subgroups (8). Prx6 belongs to 1-Cys Prx, whereas the other members belong to 2-Cys Prx. According to the different structures and mechanisms in oxidation-reduction reactions, the 2-Cys Prx may be further subdivided into two subgroups as follows: the designated 2-Cys subgroup, including Prx1 through Prx4 and Prx5, the atypical 2-Cys subgroup (12).
Prx4 (also referred to as AOE372 or TRANK) belongs to the typical 2-Cys Prx that mainly localize to the endoplasmic reticulum and extracellular matrix (13). Prx4 is overexpressed in the pancreas, liver and cardiac tissues and its lowest expression was found to be in brain tissue and blood granulocytes (14). Prx4 plays a key role in several cellular functions, such as protein and lipid protection against oxidative injury, cell proliferation, differentiation and cell signaling transduction. Accumulating evidence also demonstrates that Prx 4 is associated with the pathogenesis of tumors. Parkin (15) proposed that ~20% of human cancers are caused by infection or inflammatory conditions, such as CRC caused by inflammatory bowel diseases (16), pancreatic cancer induced by chronic pancreatitis (17) and liver cancer generated from alcoholic liver disease (18). Under these conditions, the activation of inflammatory cells may produce high levels of various types of reactive oxygen species. Several studies reported that the Prx4 protein is overexpressed in breast (19), ovarian (20), prostate (21) and other types of cancer (22) and Prx4 has been associated with invasion, recurrence, prognosis and other characteristics of cancer (23,24). It is generally believed that the high expression of Prx proteins may be induced by high levels of reactive oxygen species in tumor cells and is associated with its anti-apoptotic function.
In this study, we used qPCR to investigate Prx4 mRNA expression in CRC and adjacent normal tissues. The results demonstrated that Prx4 mRNA expression in cancer tissues was significantly higher compared to that in adjacent normal colorectal tissues, suggesting that the upregulated Prx4 expression may play a role in the occurrence and development of CRC. The immunohistochemical examination revealed that the Prx4 protein expression is higher in CRC compared to that in adjacent normal tissues, with strongly positive staining areas concentrated in CRC and weak expression areas mainly in adjacent normal tissues. This finding suggested that a high expression of the Prx4 protein may be associated with the pathogenesis and development of CRC. This study also demonstrated that the expression of Prx4 was significantly correlated with infiltration depth, lymph node metastasis and Dukes’ stage. Although the results of the Cox regression analysis revealed that Prx4, histological differentiation, lymph node metastasis, distant metastasis, infiltration depth and Dukes’ stage were not independent unfavorable prognostic factors in CRC, they were consistently associated with survival time in CRC cases, suggesting that Prx4 may play a role in cell proliferation, infiltration and lymph node metastasis of CRC and its effect on survival time may be synergistic with that of other clinicopathological variables.
In summary, this study demonstrated that Prx4 is overexpressed in CRC tissues and is correlated with the survival time of postoperative CRC patients, indicating that Prx4 may be associated with the pathogenesis and development of CRC and bears potential as a prognostic marker and therapeutic target for CRC.
Acknowledgements
This study was supported by grants from the Foundation for Supporting Talents in Six Fields of Jiangsu Province (no. 2012-WSN-065), the Health Project of Jiangsu Province (no. H201318) and the Social Development Foundation of Nantong City (nos. HS2011004 and BK2013069).
References
- 1.Esteban-Jurado C, Garre P, Vila M, et al. New genes emerging for colorectal cancer predisposition. World J Gastroenterol. 2014;20:1961–1971. doi: 10.3748/wjg.v20.i8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gingras D, Beliveau R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011;4:133–139. doi: 10.1007/s12307-010-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao Z, Sun J, Feng B, et al. The metastasis suppressor, N-myc downregulated gene 1 (NDRG1), is a prognostic biomarker for human colorectal cancer. PLoS One. 2013;8:e68206. doi: 10.1371/journal.pone.0068206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popa F, Bratucu M, Radu P. Present and future tense in operable rectal cancer. Chirurgia (Bucur) 2011;106:11–16. [PubMed] [Google Scholar]
- 6.Cherry LM. The genetic etiology of familial and nonfamilial colorectal cancer. Proc (Bayl Univ Med Cent) 2011;24:139–141. doi: 10.1080/08998280.2011.11928702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang KE, Park DS, Kim YS, et al. Prx1 modulates the chemosensitivity of lung cancer to docetaxel through suppression of FOXO1-induced apoptosis. Int J Oncol. 2013;43:72–78. doi: 10.3892/ijo.2013.1918. [DOI] [PubMed] [Google Scholar]
- 8.Fujii J, Ikeda Y. Advance in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002;7:123–130. doi: 10.1179/135100002125000352. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Kojima R, Okumura M, et al. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci Rep. 2013;3:2456. doi: 10.1038/srep02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spinsanti G, Zannolli R, Panti C, et al. Quantitative real-time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol Pain. 2008;4:51. doi: 10.1186/1744-8069-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie L, Ni WK, Chen XD, et al. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138:1035–1043. doi: 10.1007/s00432-012-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chae HZ, Robison K, Poole LB, et al. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci USA. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavender TJ, Bulleid NJ. Peroxiredoxin IV protects cells from oxidative stress by removing H2O2produced during disulphide formation. J Cell Sci. 2010;123:2672–2679. doi: 10.1242/jcs.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulte J. Peroxiredoxin 4: a multifunctional biomarker worthy of further exploration. BMC Med. 2011;9:137. doi: 10.1186/1741-7015-9-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 16.Lovasz BD, Lakatos L, Golovics PA, et al. Risk of colorectal cancer in Crohn’s disease patients with colonic involvement and stenosing disease in a population-based cohort from Hungary. J Gastrointestin Liver Dis. 2013;22:265–268. [PubMed] [Google Scholar]
- 17.Wu Y, Antony S, Hewitt SM, et al. Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol. 2013;42:1229–1238. doi: 10.3892/ijo.2013.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machida K, Chen CL, Liu JC, et al. Cancer stem cells generated by alcohol, diabetes, and hepatitis C virus. J Gastroenterol Hepatol. 2012;27(Suppl 2):19–22. doi: 10.1111/j.1440-1746.2011.07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karihtala P, Kauppila S, Soini Y, Arja-Jukkola-Vuorinen Oxidative stress and counteracting mechanisms in hormone receptor positive, triple-negative and basal-like breast carcinomas. BMC Cancer. 2011;11:262. doi: 10.1186/1471-2407-11-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karihtala P, Soini Y, Vaskivuo L, Bloigu R, Puistola U. DNA adduct 8-hydroxydeoxyguanosine, a novel putative marker of prognostic significance in ovarian carcinoma. Int J Gynecol Cancer. 2009;19:1047–1051. doi: 10.1111/IGC.0b013e3181ad0f0d. [DOI] [PubMed] [Google Scholar]
- 21.Basu A, Banerjee H, Rojas H, et al. Differential expression of peroxiredoxins in prostate cancer: consistent upregulation of PRDX3 and PRDX4. Prostate. 2011;71:755–765. doi: 10.1002/pros.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KP, Yu JS, Chien KY, et al. Identification of PRDX4 and P4HA2 as metastasis-associated proteins in oral cavity squamous cell carcinoma by comparative tissue proteomics of microdissected specimens using iTRAQ technology. J Proteome Res. 2011;10:4935–4947. doi: 10.1021/pr200311p. [DOI] [PubMed] [Google Scholar]
- 23.Soini Y, Haapasaari KM, Vaarala MH, Turpeenniemi-Hujanen T, Karja V, Karihtala P. 8-hydroxydeguanosine and nitrotyrosine are prognostic factors in urinary bladder carcinoma. Int J Clin Exp Pathol. 2011;4:267–275. [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii T, Warabi E, Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr. 2012;50:91–105. doi: 10.3164/jcbn.11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]