Abstract
Background
Schistosomiasis mansoni is a parasitic liver disease, which causes several metabolic disturbances. Here, we evaluate the influence of Apolipoprotein E (APOE) gene polymorphism, a known modulator of lipid metabolism, on plasma lipid levels in patients with hepatosplenic schistosomiasis.
Methodology/Principal Findings
Blood samples were used for APOE genotyping and to measure total cholesterol (TC), LDL-C, HDL-C and triglycerides. Schistosomiasis patients had reduced TC, LDL-C and triglycerides (25%, 38% and 32% lower, respectively; P<0.0001) compared to control individuals, whereas HDL-C was increased (10% higher; P = 0.0136). Frequency of the common alleles, ε2, ε3 and ε4, was similar (P = 0.3568) between controls (n = 108) and patients (n = 84), implying that APOE genotype did not affect susceptibility to the advanced stage of schistosomiasis. Nevertheless, while patient TC and LDL-C levels were significantly reduced for each allele (except TC in ε2 patients), changes in HDL-C and triglycerides were noted only for the less common ε2 and ε4 alleles. The most striking finding, however, was that accepted regulation of plasma lipid levels by APOE genotype was disrupted by schistosomiasis. Thus, while ε2 controls had higher TC and LDL-C than ε3 carriers, these parameters were lower in ε2 versus ε3 patients. Similarly, the inverse relationship of TG levels in controls (ε2>ε3>ε4) was absent in patients (ε2 or ε4>ε3), and the increase in HDL-C of ε2 or ε4 patients compared to ε3 patients was not seen in the control groups.
Conclusion/Significance
We confirm that human schistosomiasis causes dyslipidemia and report for the first time that certain changes in plasma lipid and lipoprotein levels depend on APOE gene polymorphism. Importantly, we also concluded that S. mansoni disrupts the expected regulation of plasma lipids by the different ApoE isoforms. This finding suggests ways to identify new metabolic pathways affected by schistosomiasis and also potential molecular targets to treat associated morbidities.
Introduction
Schistosomiasis, caused by Schistosoma mansoni worms, is one of the most prevalent parasitic diseases. More than 200 million people are infected and worldwide at least 280,000 people die because of schistosomiasis every year, most in developing countries [1], [2]. S. mansoni infections progress to hepatic fibrosis associated with portal blood hypertension [3] and 5–10% of patients present with the most severe form, hepatosplenic schistosomiasis [4], [5].
Previous studies have reported that human schistosomiasis alters plasma lipid composition [6]–[9] and metabolism [10]. From animal model studies, it is generally agreed that S. mansoni infection reduces levels of plasma cholesterol and triglycerides in both rodents [11], [12] and non-human primates [13], [14]. Nevertheless, the mechanisms behind these changes and the possible consequences for human health are not well understood.
One factor known to affect human plasma lipid concentrations is Apolipoprotein E (APOE, gene; ApoE, protein), which distributes between triglyceride-rich lipoproteins (very-low-density lipoproteins, VLDL and postprandial chylomicrons) and high-density lipoproteins (HDL), helping to regulate their metabolism and the plasma levels of cholesterol and triglyceride. The APOE gene is polymorphic with three major alleles, ε2, ε3 and ε4, arising from point mutations at a single gene locus to produce three common protein isoforms, ApoE2, E3, and E4. The parent form, ApoE3 has cysteine and arginine residues at positions 112 and 158, respectively, while ApoE2 (Arg158Cys) and ApoE4 (Cys112Arg) have single amino acid substitutions [15], [16]. These variant ApoE isoforms have different receptor binding activities, which affect lipoprotein clearance, while their differential affinity for triglyceride-rich lipoproteins influences lipolysis [17], [18].
In addition, ApoE has several biological functions not directly related to lipid transport, including roles in inflammation and the immune response [19], [20], which may be modulated in an isoform-dependent manner [21], [22]. Susceptibility and variable outcome of some infectious diseases is also linked to APOE gene polymorphism [23]–[26]. However, it remains unclear whether the plasma lipid changes induced by schistosomiasis depend on APOE genotype. Thus, the aim of our study was to determine whether the different APOE alleles influence plasma lipid levels and lipoprotein profiles in patients with hepatosplenic schistosomiasis mansoni.
Methods
Ethical Statement
The whole study was planned and executed following the Ethical Guidelines of the Helsinki Declaration. Participants were volunteers and all signed an informed consent statement after a full explanation about the scope of the study, including its objectives, procedures and potential risks. Ethical approval for all procedures was granted by the Human Research Ethics Committee, Center for Health Sciences, UFPE (Protocol No. 359/08).
Study Area and Subjects
Eighty-four patients diagnosed with hepatosplenic schistosomiasis and attending the Gastroenterology Outpatient Department at the “Hospital das Clínicas - UFPE” were recruited during 2009 and 2010. The control group comprised 108 individuals with an epidemiological history incompatible with schistosomiasis and were drawn from the same age group (18–65 years) and socioeconomic background, as judged by a standardized questionnaire that enabled family budget, education level and lifestyle to be matched with those of the patients. Three stool samples from all individuals in both groups were also analyzed for parasitological infections. Subjects were excluded from the study if there was any evidence of parasitic infections, hepatitis B or C virus infections, cardiovascular or chronic kidney diseases, thyroid dysfunction or cancer. Individuals who had taken lipid-lowering drugs at anytime within the previous year were also excluded.
All participants lived in Zona da Mata, an endemic area in the state of Pernambuco, northeast Brazil, and their grandparents and parents were also born in this same region. The study population comprised unrelated individuals. Hepatosplenic schistosomiasis was diagnosed by physical examination and upper abdominal ultrasound, conducted by a qualified and experienced professional according to the WHO protocol for ultrasound of schistosomiasis-related morbidity [27]. The patients with hepatosplenic schistosomiasis mansoni (SM) had typical hepatosplenomegaly and portal hypertension, and at least 6 months prior to the study had been treated with praziquantel (50 mg/Kg).
Sample Collection and Processing
Venous blood samples were drawn into evacuated tubes containing EDTA (0.562 M) after a 12 h fasting period. Plasma was separated within 2 h by centrifugation at 1500×g (10 min at 4°C), stored at −20°C and used for lipid analyses within 24 h. Whole blood samples were stored at 2–8°C and APOE genotype determined within 7 days.
Biochemical Measurement
Plasma total cholesterol (TC) and triglyceride (TG) concentrations were assayed by routine enzymatic methods. HDL cholesterol (HDL-C) was measured after precipitation of ApoB-containing lipoproteins from plasma with phosphotungstic acid in the presence of magnesium ions [28]. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula in subjects whose TG levels were ≤400 mg/dL [29]. Individuals whose TG levels were >400 mg/dL (six controls with the ε4-allele) were excluded from LDL-C analysis.
Determination of APOE Genotype
Genomic DNA was extracted from leukocytes in whole blood, following a standard salting-out technique [30]. Single nucleotide APOE polymorphisms (rs7412 and rs429358) were detected by polymerase chain reaction (PCR) [31]. Amplified sequences were digested with the enzyme HhaI (5 units/mL) for 3 h and the restriction fragments were separated by 4% agarose gel electrophoresis and stained with ethidium bromide (0.5 mg/L).
Genotyping was performed with blinding to subject identity. Sequence-proven controls were run with each PCR. A random 1/24 of samples were genotyped again on another day; no discrepancies were observed.
Statistical Analysis
The chi-square (χ2) goodness-of-fit test was used to assess deviation from Hardy-Weinberg equilibrium for each polymorphism and to compare categorical parameters among groups. All continuous variables were checked for normality and present a Gaussian distribution. Unpaired t-test was used to compare differences among continuous variables of SM patients and control individuals, while APOE allele groups were analyzed by one-way ANOVA followed by Fisher's Protected Least Significant Difference (PLSD). Lipid levels were adjusted for potentially confounding variables of age and gender. Pearson's Correlation test was used to estimate association between continuous parameters. Quantitative variables were expressed as mean ± standard error of media, while qualitative variables were expressed as absolute frequencies (percentage). P-values less than 0.05 were considered to be statistically significant. All statistical analyses were performed using StatView SAS Inc. (1998; NC, USA).
To evaluate APOE genotype effects on schistosomiasis mansoni, subjects were categorized into three groups: ε2 carriers (ε2/ε2+ε2/ε3 genotypes), ε3 carriers (ε3/ε3 genotype) and ε4 carriers (ε4/ε4+ε4/ε3 genotypes). In each model, the homozygous ε3/ε3 genotypes formed the reference group. Six individuals (ε2/ε4; 3.13%) were excluded from the analyses because of the putative opposing effects of these two alleles.
Results
For this cross-sectional study, the two groups were matched by age and gender, as shown in Table 1. The frequency of APOE alleles among all participants were: ε2 – 11.46%, ε3 – 71.35%, and ε4 – 17.19%, similar to other studies in Brazilian populations [32]–[34]; the detailed genotype frequency is given in Table 2. All SNPs were in accordance with Hardy-Weinberg equilibrium for both SM patients (χ2 = 3.4164, φ = 3, p = 0.3318) and controls (χ2 = 3.2518, φ = 3, p = 0.3544). Both control and patient groups showed similar mean age (control: P = 0.3803; SM: P = 0.4123) and gender frequency (control: χ2 = 2.960, φ = 2, P = 0.2776; SM: χ2 = 2.439, φ = 2, P = 0.2953) among the three different alleles. The allele frequencies were not statistically different between control and SM groups (P = 0.3568), indicating that APOE polymorphism was not able to affect the chance or course of schistosomiasis in this population.
Table 1. Participants, genotype and lipid parameters of participants.
| Parameters* | Control | SM | P-value |
| Age (years) | 47.0±3.2 | 55.0±2.3 | 0.0541 |
| Gender | |||
| Male | 30 | 21 | - |
| Female | 78 | 63 | - |
| N total | 108 | 84 | 0.7309 |
| ε2 | 14 (13.0) | 8 (9.5) | - |
| ε3 | 73 (67.6) | 64 (76.2) | - |
| ε4 | 21 (19.4) | 12 (14.3) | - |
| TC | 194.4±4.5 | 146.4±3.0 | <0.0001 |
| LDL-C | 129.0±4.5 | 79.8±2.7 | <0.0001 |
| HDL-C | 43.4±1.4 | 47.9±2.7 | 0.0136 |
| TG | 140.6±11.9 | 95.8±2.8 | <0.0007 |
SM, schistosomiasis mansoni patients; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; Continuous variables are presented as mean ± standard error and were compared by unpaired t-test, whereas categorical variables are presented as absolute (percentage) frequencies and were compared by the Chi-square test.
*Plasma lipids are expressed in mg/dL.
Table 2. APOE genotype frequencies among patients with hepatosplenic schistosomiasis mansoni and controls.
| Genotype | Control | SM |
| ε2/ε2 | 2 | 0 |
| ε2/ε3 | 12 | 8 |
| ε2/ε4 | 2 | 4 |
| ε3/ε3 | 73 | 64 |
| ε3/ε4 | 18 | 11 |
| ε4/ε4 | 3 | 1 |
| Total | 108 | 84 |
When compared to healthy controls, the SM patients showed significant reductions (P<0.0001) in the plasma levels of TC (25%), LDL-C (38%) and TG (32%). By contrast, the concentration of HDL-C was significantly increased in the patients (10% higher; P = 0.0136) (Table 1).
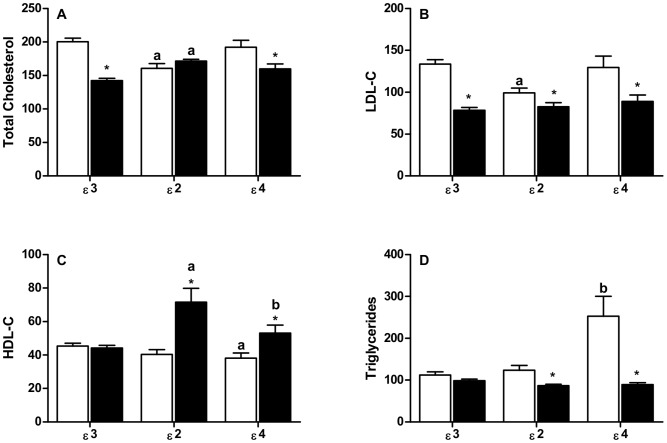
To assess the influence of APOE gene polymorphism on plasma lipid parameters, we repeated the analyses after subdividing each group on the basis of APOE alleles. Lower TC and LDL-C levels were found in the ε3 subgroup (ε3/ε3 genotype) of SM patients, as observed without allele differentiation (Figure 1A and 1B). However, the increases in HDL-C and reductions in TG noted for all SM patients were not seen, even though the ε3/ε3 genotype is carried by 70% of participants (Figure 1C and D). This analysis of the ε3/ε3 genotype allows the influence of schistosomiasis on human lipid metabolism to be evaluated without possible interfering factors from the inclusion of ε2 and ε4 alleles. All the values of P from comparisons showed in Figure 1 are shown in Table 3.
Figure 1. Effect of APOE gene polymorphism on plasma levels of Total Cholesterol (A), LDL-C (B), HDL-C (C), and Triglycerides (D) in Control subjects (open bars) and SM patients (filled bars).
Plasma lipids are expressed in mg/dL. * = P<0.05 for group of SM patients vs. Control of the same allele. Comparisons between the different alleles of the same SM patient group, or of the same Control group, are indicated as follows: a = P<0.05 vs. ε3; b = P<0.05 vs. ε2 and ε3. Exact P values are given in Table 3.
Table 3. Values of P from comparisons showed in Figure 1.
| TC | LDL-C | HDL-C | TG | ||
| Control vs SM | ε3 | <0.0001 | <0.0001 | 0.6337 | 0.1422 |
| ε2 | 0.8494 | 0.0098 | 0.0005 | 0.0166 | |
| ε4 | 0.0403 | 0.0219 | 0.0113 | 0.0152 | |
| Control | ε2 vs ε3 | 0.0052 | 0.0157 | 0.2533 | 0.7348 |
| ε2 vs ε4 | 0.0547 | 0.0842 | 0.6825 | 0.0018 | |
| ε3 vs ε4 | 0.4590 | 0.7536 | 0.0431 | <0.0001 | |
| SM | ε2 vs ε3 | 0.0458 | 0.9933 | <0.0001 | 0.3366 |
| ε2 vs ε4 | 0.8342 | 0.3445 | 0.0316 | 0.9945 | |
| ε3 vs ε4 | 0.0586 | 0.1883 | 0.0413 | 0.2727 |
ANOVA followed by Fisher's PLSD test.
Despite this significantly lower plasma TC in ε3 patients, the TC levels were similar (P = 0.5360) in ε2 patient and control groups. This reflected a marked HDL-C increase (77%) and a positive correlation between TC and HDL-C (R = 0.724; P = 0.0250) for patient ε2-carriers. By contrast, LDL-C was reduced in ε2 patients (as it was in ε3 patients; Figure 1A) and unrelated to TC levels (R = 0.225; P = 0.5750).
Plasma cholesterol changes associated with schistosomiasis were also noted for ε4-carriers. As with the ε2 allele, the ε4 SM patients had increased HDL-C (39% higher) compared to their control counterparts (Figure 1C); and like ε3-carriers they had reduced TC and LDL-C (Figure 1A and 1B).
Decreased plasma TG concentrations were seen in SM patients with the ε2 or ε4 variant alleles, but not for the ε3/ε3 genotype. However, the most striking difference in TG was noted in healthy ε4-carriers; their TG concentration was two-fold higher than the five other subgroups (Figure 1D).
Discussion
Our report is the first to identify a host genetic factor, APOE polymorphism, which influences the extent and nature of plasma lipid changes associated with schistosomiasis mansoni. In future studies, this finding will help in understanding how the parasite affects particular steps in host lipid metabolism and how host genetic background modifies disease progression and morbidity.
Several studies have shown the APOE genotype to influence infection susceptibility and damage in certain diseases caused by viruses, including human immunodeficiency virus [18] and hepatitis C [26], [35] and B [36], protozoa [23] and fungi [24]. As allele frequencies were similar for patients and controls, we infer that the different ApoE isoforms do not affect progression of schistosomiasis to the chronic hepatosplenic condition. Conceivably, this conclusion may not hold for the earlier, less severe hepatointestinal stage or for hepatosplenic patients subdivided on the extent of liver fibrosis [3]. Though of interest, as APOE alleles are suggested to affect fibrosis progression in hepatitis C infection [26], [37], a much larger patient population would be needed to ensure adequate power for subtle genotype effects [35]. Gene studies of individuals infected with schistosomiasis have found significant associations of cytokines related to the immune response [38]–[40]. However, to date and similar to our result, no study has reported a link between the APOE gene polymorphism and schistosomiasis prevalence or severity.
The changes we report in plasma lipoprotein profiles, reductions in TC and LDL-C and an increase in HDL-C, is considered cardioprotective and hence can be regarded as a beneficial side-effect of schistosomiasis. We, and others, have previously reported low plasma total cholesterol in human studies [6], [7] and in infected animals [11]–[13], [41]. Doenhoff et al. [11] have shown that when fat-fed ApoE-deficient mice are infected with S. mansoni the decrease in plasma cholesterol is associated with a 50% reduction in atherosclerotic plaque progression, consistent with the low frequency of atherosclerosis noted in schistosomiasis patients [6], [42].
Schistosomes need, but do not synthesize, cholesterol and one explanation for reduced host plasma cholesterol is that adult worms internalize LDL, via tegumental proteins analagous to mammalian LDL receptors [43]. Another suggestion is that the worms shed antigenic glycosyl-phosphatidylinositol (GPI)-anchored proteins into the circulation, which are sequestered by host lipoprotein particles [44]. Subsequent, antibody attack leads to lipoprotein removal by neutrophil endocytosis, although any plasma cholesterol-lowering effect in vivo has yet to be assessed. Against both these mechanisms is the failure of same-sex worms to lower cholesterol during mouse infections [41], implying that adult worms alone are not responsible and that the parasite's eggs are hypocholesterolemic. This concept is supported by La Flamme et al. [12] who noted reduced plasma cholesterol in mice chronically exposed to schistosome eggs, while Stanley et al. [41] found that soluble factors released from S. mansoni eggs were responsible.
Although TC and LDL-C were decreased in SM patients, we noted increased levels of HDL-C, consistent with an early report that alpha-lipoproteins were significantly higher in patients with Bilharzial hepatic fibrosis [6]. By contrast, infection of ApoE-deficient mice with S. mansoni cercariae resulted in reduced HDL-C [11], although levels do not change during chronic exposure to schistosome eggs [12]. However, direct comparisons of mouse and human plasma lipoprotein metabolism are complex, as there is a marked difference in the LDL-C to HDL-C ratio [13]. Absence of cholesteryl ester transfer protein (CETP) in mice increases HDL levels compared to humans [45], while much of their LDL is cleared rapidly using ApoE as ligand rather than the slow ApoB100 pathway used by human LDL [46].
Effects of infection and inflammation on host lipoprotein metabolism are multi-faceted. Although the acute-phase response inhibits ApoAI synthesis and lowers HDL-C [47], [48], the most profound changes are in structure and composition, which transform the HDL from anti-inflammatory to proinflammatory particles [49]–[51]. Such pathological changes in HDL have yet to be studied in hepatosplenic schistosomiasis, although chronic inflammation is known to impair reverse cholesterol transport and the antioxidant capacity of HDL [52]–[54]. Indirect evidence suggests that HDL in schistosomiasis patients is a poor antioxidant, as we previously found elevated levels of erythrocyte lipid peroxidation [55].
In humans, APOE polymorphism is well-documented to affect plasma TC and LDL-C; for example, meta-analyses by Bennet et al. [56] found differences between ε2/ε3 and ε3/ε4 carriers, the most common genotypes after ε3/ε3, of 8% and 14%, respectively. As indicated earlier, differential binding affinities of the individual ApoE isoforms to receptors and for surfaces of triglyceride-rich lipoprotein particles underlie such variation [16], [18]. We also noted effects of APOE genotype on TC and LDL-C in our controls as mean values were significantly lower in ε2-carriers compared to the ε3/ε3 group (P = 0.0052 and P = 0.0157, respectively), though unchanged for the ε4-allele. Interestingly, schistosomiasis abolished, and indeed reversed, this relationship; ε2 patients had higher mean TC and LDL-C than ε3/ε3 patients, although only the TC difference reached significance (P = 0.0458).
The relation of APOE genotypes with HDL-C was reported by Bennet et al. [56] to be inverse and weak with a 5% difference between the ε2/ε3 and ε3/ε4 carriers. Despite small numbers, we also noted a slight but significant fall of HDL-C in control ε4 carriers compared to their ε3/ε3 counterparts. The small (10%) HDL-C increase in our schistosomiasis patients (Table 1) was due to higher levels in ε2- and ε4-carriers, as the HDL-C of ε3/ε3 genotypes was near-identical for controls and patients. Can these findings be explained? One difficulty is the complexity of HDL formation, maturation and clearance, namely reverse cholesterol transport, which though involving the major HDL protein, ApoAI, is influenced and assisted at each step by ApoE [57]. Thus, initial sequestration of excess cellular cholesterol [58], activation of the cholesterol esterifying enzyme, plasma lecithin-cholesterol acyltransferase (LCAT) [59] and cholesterol ester delivery to the liver [17], [60] are all processes that involve ApoE in an isoform-dependent manner. To this complexity, we can overlay HDL metabolic changes due to S. mansoni infection and associated inflammatory responses and fibrogenesis. For example, we have reported LCAT deficiency in human [7] and animal [13] schistosomiasis, while decreased CETP activity is a feature of the acute-phase response [47] and raises HDL levels, particularly ApoE-rich HDL [18], [61].
We can speculate, therefore, that HDL-C increases in schistosomiasis are a multi-step process, promoted by low CETP activity and enhanced further by the ε2-allele: ApoE2 has a higher affinity than ApoE3 or ApoE4 for HDL [62], allowing the particles to expand in size [18], [63], while defective ApoE2 receptor binding delays their clearance from plasma via hepatic LDL-receptors [17], [60]. A different scenario is required to explain the HDL-C increase in the ε4-carrying patients, since ApoE4 associates poorly with HDL and has high affinity for LDL receptors, properties predicted to reduce HDL-C. One tentative possibility is that the poor antioxidant capacity of cysteine-negative ApoE4 [64] allows excessive formation of oxidized HDL, particularly as S. mansoni infections markedly increase oxidative stresses [65]. As oxidized HDL impedes normal reverse cholesterol transport [51]–[53], this delay in maturation may increase HDL-C in patients with ε4-alleles.
The plasma triglycerides change has inconsistent results in humans, as seen in mice earlier reports [9], [11], [12]. We observed 30% reduction in plasma triglycerides in SM patients. The mechanism(s) causing reduced plasma TG is uncertain. It may simply reflect lower levels of non-HDL lipoproteins since the acquired LCAT deficiency of human schistosomiasis increases the TG:CE ratio of core lipids [9], or be independent of infection-related responses, as pulmonary fibrosis with non-infectious origins results in low plasma TG [64]. A direct effect is also possible, as S. mansoni infected mice had reduced hepatic expression of acetyl coenzyme A acyltransferase, an enzyme involved in fatty acid metabolism [67]. Nevertheless, data from animal studies are inconsistent. Infection of non-human primates resulted in TG rises >10% after 30 or 60 days, whereas in mice TG levels were reported to rise two-fold [11] or be unchanged 7–10 weeks post-infection [66], or to significantly decline from the 4th week [68].
Meta-analyses to assess association of APOE genotypes with plasma triglycerides report non-linear relationships, the ε2- and ε4-carriers having higher levels than those with the ε3/ε3 genotype [56], [69], [70]. For ApoE2 the simplest explanation is reduced binding and delayed hepatic clearance of VLDL remnants, whereas a dual mechanism is invoked for ApoE4; impaired lipolysis because ApoE4 has higher affinity for VLDL [17] and, paradoxically as it has high receptor binding, by the failure of ApoE4 to accelerate hepatic removal of VLDL remnants due to inefficient recycling of the ApoE4 protein into the Space of Disse [71]. Consistent with the data from meta-analyses [56], [69], [70], we found that the mean TG values for control individuals with ε4- and ε2-alleles were higher than the ε3/ε3 group (though P<0.05 only for ε4-carriers; Figure 1D). Unexpectedly, the mean TG (252±48 mg/dL) of this ε4 control group was much higher than seen in other studies, including different Brazilian populations [30]–[32], [72], an unexpected finding for which we have no immediate explanation. Of greater interest, however, was that the mean TG level in the ε3/ε3 patients was higher, albeit not significant, than levels in patients carrying ε2- or ε4-alleles (Figure 1D). These data suggest that the mechanism(s) which promotes increased plasma TG in healthy ε2- and ε4-carriers is either inoperative or ineffective in schistosomiasis patients.
One limitation of this study is that it was conducted only at a single hospital, the Hospital das Clinicas, UFPE, which is the reference hospital for schistosomiasis in Pernambuco State, Brazil. Here, the Gastroenterology Outpatient Department receives the most severe cases of schistosomiasis, usually patients with a history of one or more episodes of gastrointestinal bleeding and hence most of the patients have the hepatosplenic form of the disease. Moreover, we had no information on plasma lipid levels before infection to compare with levels after the patients had developed the hepatosplenic form of schistosomiais. Therefore, the findings from the present study may not be extrapolated to all patients from other endemic areas who present with the hepatosplenic form of the disease.
In summary, we confirm that human schistosomiasis causes dyslipidemia and, for the first time, report that certain changes in plasma lipid levels and lipoprotein profiles are dependent on patient APOE gene polymorphism. Importantly, we also conclude that the normal regulation of plasma lipid levels by APOE genotype is disrupted by schistosomiasis mansoni. This finding merits further investigation; it may uncover new metabolic pathways and pathological processes associated with human schistosomiasis. In turn, these may identify molecular targets to aid treatment of schistosomiasis morbidity, and perhaps also inform other lipid-associated diseases, including atherosclerosis and diabetes.
Funding Statement
Financial support for this study was received from Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco - FACEPE (Grant: FACEPE-PPSUS-PE APQ-1369-4.00/08), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, et al. (2003) Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop 86: 125–139. [DOI] [PubMed] [Google Scholar]
- 2. Gryseels B (2012) Schistosomiasis. Infect Dis Clin North Am 26: 383–397. [DOI] [PubMed] [Google Scholar]
- 3. Leite LA, Pimenta Filho AA, Martins da Fonseca CS, Santana dos Santos B, Ferreira R de C, et al. (2013) Hemostatic dysfunction is increased in patients with hepatosplenic schistosomiasis mansoni and advanced periportal fibrosis. PLoS Negl Trop Dis 7: e2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambertucci JR, Cota GF, Pinto-Silva RA, Serufo JC, Gerspacher-Lara R, et al. (2001) Hepatosplenic schistosomiasis in field-based studies: a combined clinical and sonographic definition. Mem Inst Oswaldo Cruz 96: 147–150. [DOI] [PubMed] [Google Scholar]
- 5. Correia MCB, Domingues ALC, Lacerda HR, Santos EM, Machado CGF, et al. (2009) Platelet function and the von Willebrand factor antigen in the hepatosplenic form of schistosomiasis mansoni. Roy Soc Trop Med Hyg 103: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 6. Ghanem MH, Fahmy MH, Aboul-Kheir F, Mikhail MN, Guirgis FK (1970) Lipid metabolism in bilharzial cirrhosis. Atherosclerosis 12: 55–61. [DOI] [PubMed] [Google Scholar]
- 7.Owen JS, Gillett MP (1978) Lecithin:cholesterol acyltransferase deficiency associated with hepatic schistosomiasis mansoni. Scand J Clin Lab Invest Suppl 150: 194–198 [PubMed]
- 8. Gillett MP, Sibrian AM, Dimenstein R, Carvalho VC, Gonçalves MM, et al. (1989) Alterations in the fatty acyl composition of plasma cholesteryl esters in Brazilian patients with hepatosplenic schistosomiasis mansoni. Braz J Med Biol Res 22: 949–957. [PubMed] [Google Scholar]
- 9. Dimenstein R, Carvalho VC, Oliveira DN, Gillett MP (1992) Alterations in the levels and lipid composition of plasma lipoproteins (VLDL, LDL and HDL) in Brazilian patients with hepatosplenic schistosomiasis mansoni. Braz J Med Biol Res 25: 1091–1102. [PubMed] [Google Scholar]
- 10. Silva CA, Oliveira KF, Carvalho VCO, Domingues ALC, Brandt CT, et al. (2001) Surgical treatment effect on the liver lecithin: cholesterol acyltransferase (LCAT) in schistosomiasis mansoni. Acta Cir Bras 17: 28–30. [Google Scholar]
- 11. Doenhoff MJ, Stanley RG, Griffiths K, Jackson CJ (2002) An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology 125: 415–421. [DOI] [PubMed] [Google Scholar]
- 12. La Flamme AC, Harvie M, Kenwright D, Cameron K, Rawlence N, et al. (2007) Chronic exposure to schistosome eggs reduces serum cholesterol but has no effect on atherosclerotic lesion development. Parasite Immunol 29: 259–266. [DOI] [PubMed] [Google Scholar]
- 13. Lima VLM, Sena VLM, Stewart B, Owen JS, Dolphin PJ (1998) An evaluation of the marmoset Callithrix jacchus (sagüi) as an experimental model for the dyslipoproteinemia of human Schistosomiasis mansoni. Biochim Biophys Acta 1393: 235–243. [DOI] [PubMed] [Google Scholar]
- 14. Ramos TMB, Vasconcelos AS, Carvalho VC, Lima VLM (2004) Alterations in cholesterol, triglyceride and total phospholipid levels in plasma of Callithrix jacchus (sagüi) reinfected by Schistosoma mansoni . Rev Soc Bras Med Trop 37: 37–40. [DOI] [PubMed] [Google Scholar]
- 15. Mahley RW, Weisgraber KH, Huang Y (2009) Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res 50: S183–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papaioannou I, Simons JP, Owen JS (2012) Targeted in situ gene correction of dysfunctional APOE alleles to produce atheroprotective plasma ApoE3 protein. Cardiol Res Pract 2012: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Dhanasekaran P, Alexander ET, Rader DJ, Phillips MC, et al. (2013) Molecular mechanisms responsible for the differential effects of apoE3 and apoE4 on plasma lipoprotein-cholesterol levels. Arterioscler Thromb Vasc Biol 33: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahley RW, Huang Y, Rall AC Jr (1999) Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res 40: 1933–1949. [PubMed] [Google Scholar]
- 19. Harris JD, Evans V, Owen JS (2006) ApoE gene therapy to treat hyperlipidemia and atherosclerosis. Curr Opin Mol Ther 8: 275–287. [PubMed] [Google Scholar]
- 20. Getz GS, Reardon CA (2009) Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res 50: S156–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang KJ, Zhang HL, Zhang XM, Zheng XY, Quezada HC, Zhang D, et al. (2011) Apolipoprotein E isoform-specific effects on cytokine and nitric oxide production from mouse Schwann cells after inflammatory stimulation. Neurosci Lett 499: 175–180. [DOI] [PubMed] [Google Scholar]
- 22. Zhang H, Wu LM, Wu J (2011) Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm 2011: 949072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wozniak MA, Faragher EB, Todd JA, Koram KA, Riley EM, et al. (2003) Does apolipoprotein E polymorphism influence susceptibility to malaria? J Med Genet 40: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tursen U, Kaya TI, Eskandari G, Bocekli E, Muslu N, et al. (2004) Apolipoprotein E gene polymorphism and serum lipids in patients with superficial fungal disease. Yonsei Med J 45: 375–379. [DOI] [PubMed] [Google Scholar]
- 25. Burt TD, Agan BK, Marconi VC, He W, Kulkarni H, et al. (2008) Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci U S A 105: 8718–8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wozniak MA, Itzhaki RF, Faragher EB, James MW, Ryder SD, et al. (2002) Apolipoprotein E-ε4 protects against severe liver disease caused by hepatitis C virus. Hepatology 36: 456–463. [DOI] [PubMed] [Google Scholar]
- 27.Niamey Working Group. (2000) Ultrasound in schistosomiasis: a practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis-related morbidity. Geneva: World Health Organization. TDR/STR/SCH/00.1. Available: http://apps.who.int/iris/bitstream/10665/66535/1/TDR_STR_SCH_00.1.pdf.
- 28. Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA (1982) Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res 23: 1206–1223. [PubMed] [Google Scholar]
- 29. Dos Santos BS, Melo Junior MR, Paiva MHS, Pimenta Filho AA, Araujo TF, et al. (2009) Comparative analysis of lipid profile in men from Pernambuco State, according to III and IV Brazilian Dyslipidemias Guidelines. Rev Bras Anal Clin 41(4): 295–297. [Google Scholar]
- 30. Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SW, Heo JH, Kim CH, Yoo DC, Won DH, et al. (2010) Rapid and direct detection of apolipoprotein E genotypes using whole blood from humans. J Toxicol Environ Health A 73: 1502–1510. [DOI] [PubMed] [Google Scholar]
- 32. Mendes-Lana A, Pena GG, Freitas SN, Lima AA, Nicolato RL, et al. (2007) Apolipoprotein E polymorphism in Brazilian dyslipidemic individuals: Ouro Preto study. Braz J Med Biol Res 40: 49–56. [DOI] [PubMed] [Google Scholar]
- 33. Fuzikawa AK, Peixoto SV, Taufer M, Moriguchi EH, Lima-Costa MF (2008) Association of ApoE polymorphisms with prevalent hypertension in 1406 older adults: the Bambuí Health Aging Study (BHAS). Braz J Med Biol Res 41: 89–94. [DOI] [PubMed] [Google Scholar]
- 34. Alvim RO, Freitas SR, Ferreira NE, Santos PC, Cunha RS, et al. (2010) APOE polymorphism is associated with lipid profile, but not with arterial stiffness in the general population. Lipids Health Dis 9: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Price DA, Bassendine MF, Norris SM, Golding C, Toms GL, et al. (2006) Apolipoprotein ε3 allele is associated with persistent hepatitis C virus infection. Gut 55: 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ahn SJ, Kim DK, Kim SS, Bae CB, Cho HJ, et al. (2012) Association between apolipoprotein E genotype, chronic liver disease, and hepatitis B virus. Clin Mol Hepatol 18: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabris C, Vandelli C, Toniutto P, Minisini R, Colletta C, et al. (2011) Apolipoprotein E genotypes modulate fibrosis progression in patients with chronic hepatitis C and persistently normal transaminases. J Gastroenterol Hepatol 26: 328–333. [DOI] [PubMed] [Google Scholar]
- 38. Marquet S, Abel L, Hillaire D, Dessein H, Kalil J, et al. (1996) Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31–q33. Nat Genet 14: 181–184. [DOI] [PubMed] [Google Scholar]
- 39. Dessein AJ, Hillaire D, Elwali NE, Marquet S, Mohamed-Ali Q, et al. (1999) Severe hepatic fibrosis in Schistosoma mansoni infection is controlled by a major locus that is closely linked to the interferon-gamma receptor gene. Am J Hum Genet 65: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dessein A, Arnaud V, He H, Li J, Dessein H, et al. (2013) Genetic analysis of human predisposition to hepatosplenic disease caused by schistosomes reveals the crucial role of connective tissue growth factor in rapid progression to severe hepatic fibrosis. Pathol Biol (Paris) 61: 3–10. [DOI] [PubMed] [Google Scholar]
- 41. Stanley RG, Jackson CL, Griffiths K, Doenhoff MJ (2009) Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis 207: 131–138. [DOI] [PubMed] [Google Scholar]
- 42.Brandt CT, Godoi ET, Valença A, Mascena GV, Godoi JT (2013) Atherogenesis: diseases that may affect the natural history “schistosomiasis and HIV infection”. In: Rezzani R, editor. Current Trends in Atherogenesis. InTech Europe, Croatia. pp. 81–96. [Google Scholar]
- 43. Tempone AJ, Bianconi ML, Rumjanek FD (1997) The interaction of human LDL with the tegument of adult Schistosoma mansoni. Mol Cell Biochem 177: 139–144. [DOI] [PubMed] [Google Scholar]
- 44. Sprong H, Suchanek M, van Dijk SM, van Remoortere A, Klumperman J, et al. (2006) Aberrant receptor-mediated endocytosis of Schistosoma mansoni glycoproteins on host lipoproteins. PLos Med 3: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, et al. (1991) Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem 266: 10796–10801. [PubMed] [Google Scholar]
- 46. Powell-Braxton L, Véniant M, Latvala RD, Hirano KI, Won WB, et al. (1998) A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nat Med 4: 934–938. [DOI] [PubMed] [Google Scholar]
- 47. Khovidhunkit W, Kim M-S, Memon RA, Shigenaga JK, Moser AH, et al. (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45: 1169–1196. [DOI] [PubMed] [Google Scholar]
- 48. Haas MJ, Mooradian AD (2010) Regulation of high-density lipoprotein by inflammatory cytokines: establishing links between immune dysfunction and cardiovascular disease. Diabetes Metab Res Rev 26: 90–99. [DOI] [PubMed] [Google Scholar]
- 49. G HB, Rao VS, Kakkar VV (2011) Friend turns foe: transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response. Cholesterol 2011: 274629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pruzanski W, Stefanski E, de Beer FC, de Beer MC, Ravandi A, et al. (2000) Comparative analysis of lipid composition of normal and acute-phase high density lipoproteins. J Lipid Res 41: 1035–1047. [PubMed] [Google Scholar]
- 51. Natarajan P, Ray KK, Cannon CP (2010) High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol 55: 1283–1299. [DOI] [PubMed] [Google Scholar]
- 52. Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, et al. (1995) Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest 96: 2758–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saemann MD, Poglitsch M, Kopecky C, Haidinger M, Horl WH, et al. (2010) The versatility of HDL: a crucial anti-inflammatory regulator. Eur J Clin Invest 40: 1131–1143. [DOI] [PubMed] [Google Scholar]
- 54. Feingold KR, Grunfeld C (2010) The acute phase response inhibits reverse cholesterol transport. J Lipid Res 51: 682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Facundo HTF, Brandt CT, Owen JS, Lima VLM (2004) Elevated levels of erythrocyte conjugated dienes indicate increased lipid peroxidation in schistosomiasis mansoni patients. Braz J Med Biol Res 37: 957–962. [DOI] [PubMed] [Google Scholar]
- 56. Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, et al. (2007) Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA 298: 1300–1311. [DOI] [PubMed] [Google Scholar]
- 57. Krimbou L, Tremblay M, Davignon J, Cohn JS (1997) Characterization of human plasma apolipoprotein E-containing lipoproteins in the high density lipoprotein size range: focus on pre-β1-LpE, pre-β2-LpE, and α-LpE. J Lipid Res 38: 35–48. [PubMed] [Google Scholar]
- 58. Huang Y, von Eckardstein A, Wu S, Assmann G (1995) Effects of the apolipoprotein E polymorphism on uptake and transfer of cell-derived cholesterol in plasma. J Clin Invest 96: 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen CH, Albers JJ (1985) Activation of lecithin: cholesterol acyltransferase by apolipoproteins E-2, E-3, and A-IV isolated from human plasma. Biochim Biophys Acta 836: 279–285. [DOI] [PubMed] [Google Scholar]
- 60. Cassel DL, Phillips MC, Rostron P, Rothblat GH, Utermann G (1984) The conformation of apolipoprotein E isoforms in phospholipid complexes and their interaction with human Hep G2 cells. Atherosclerosis 52: 203–218. [DOI] [PubMed] [Google Scholar]
- 61. Hirata H, Yimin, Segawa S, Ozaki M, Kobayashi N, et al. (2012) Xanthohumol prevents atherosclerosis by reducing arterial cholesterol content via CETP and Apolipoprotein E in CETP-transgenic mice. PLoS ONE 7: e49415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Steinmetz A, Jakobs C, Motzny S, Kaffarnik H (1989) Differential distribution of apolipoprotein E isoforms in human plasma lipoproteins. Arteriosclerosis 9: 405–411. [DOI] [PubMed] [Google Scholar]
- 63. Mahley RW, Huang Y, Weisgraber KH (2006) Putting cholesterol in its place: apoE and reverse cholesterol transport. J Clin Invest 116: 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miyata M, Smith JD (1996) Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloids peptides. Nat Genet 14: 55–61. [DOI] [PubMed] [Google Scholar]
- 65. de Oliveira RB, Senger MR, Vasques LM, Gasparotto J, dos Santos JP, et al. (2013) Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation endproduct (RAGE) and tau levels in multiple organs in mice. Int J Parasitol 43: 371–379. [DOI] [PubMed] [Google Scholar]
- 66. Iannello S, Cavaleri A, Camuto M, Pisano MG, Milazzo P (2002) Low fasting serum triglyceride and high free fatty acid levels in pulmonary fibrosis: a previously unreported finding. MedGenMed 4: 5. [PubMed] [Google Scholar]
- 67. Harvie M, Jordan TW, La Flamme AC (2007) Differential liver protein expression during schistosomiasis. Infect Immun 75: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. el-Marzouki ZM, Amin AM (1997) Changes in serum lipids of mice experimentally infected with Schistosoma mansoni. J Egypt Soc Parasitol 27: 419–429. [PubMed] [Google Scholar]
- 69. Dallongeville J, Lussier-Cacan S, Davignon J (1992) Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J Lipid Res 33: 447–454. [PubMed] [Google Scholar]
- 70. Khan TA, Shah T, Prieto D, Zhang W, Price J, et al. (2013) Apolipoprotein E genotype, cardiovascular biomarkers and risk of stroke: systematic review and meta-analysis of 14,015 stroke cases and pooled analysis of primary biomarker data from up to 60,883 individuals. Int J Epidemiol. 42: 475–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Heeren J, Beisiegel U, Grewal T (2006) Apolipoprotein E recycling implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol 26: 442–448. [DOI] [PubMed] [Google Scholar]
- 72. Almeida S, Fiegenbaum M, Andrade FM, Osório-Wender MC, Hutz MH (2006) ESR1 and APOE gene polymorphisms, serum lipids, and hormonal replacement therapy. Maturitas 54: 119–126. [DOI] [PubMed] [Google Scholar]