Abstract
Alzheimer’s disease is the most common neurodegenerative disease and this disease induces progressive loss of memory function Scopolamine is a non-selective muscarinic cholinergic receptor antagonist and it induces impairment of learning ability. Exercise is known to ameliorate memory deficits induced by various brain diseases. In the present study, we investigated the effect of treadmill exercise on spatial learning ability in relation with cell proliferation in the hippocampus using the scopolamine-induced amnesia mice. For the induction of amnesia, 1 mg/kg scopolamine hydrobromide was administered intraperitoneally once a day for 14 days. Morris water maze test for spatial learning ability was conducted. Immonofluorescence for 5-bromo-2-deoxyuri-dine (BrdU) and western blot for brain-derived neurotrophic factor (BDNF) and its receptor tyrosine kinase B (TrkB) were performed. In the present results, scopolamine-induced amnesia mice showed deterioration of spatial learning ability. Inhibition of cell proliferation and suppression of BDNF and TrkB expressions were observed in the scopolamine-induced amnesia mice. Treadmill exercise improved spatial learning ability and increased cell proliferation through activating of BDNF-TrkB pathway in the amnesia mice. These findings offer a possibility that treadmill exercise may provide preventive or therapeutic value for the memory loss induced by variable neurodegenerative diseases including Alzheimer’s disease.
Keywords: Scopolamine, Amnesia, Treadmill exercise, Cell proliferation, Spatial learning ability, Brain-derived neurotrophic factor
INTRODUCTION
Alzheimer’s disease (AD) is most common neurodegenerative disease and this disease induces progressive loss of learning ability and memory function (Moosavi et al., 2012). The degree of memory loss is correlated with the severity of AD (Howes et al., 2003). Scopolamine is a non-selective muscarinic cholinergic receptor antagonist and it deteriorates learning ability and short-term memory. Scopolamine is used to create the amnesia animal model (Araujo et al., 2005; Heo et al., 2014). Scopolamine also inhibits central cholinergic neuronal activity (Konar et al., 2011). Central cholinergic system is closely associated with and neurogenesis and/or cell proliferation in the hippocampus (Kotani et al., 2006; Yoo et al., 2011).
Hippocampus plays a crucial brain area for the process of learning and memory (Kim et al., 2010). It is vulnerable to neuronal injury produced by scopolamine, resulting in the deficits of synaptic plasticity and spatial memory (Mattson et al., 2002). The generation of neurons in the hippocampus plays an important role in the learning ability and memory function. Neurogenesis in the hippocampal dentate gyrus is known to be enhanced by enriched environment, neurotrophic growth factors, and exercise, while adrenal steroids, opioid peptides, and stress inhibit it (Baek et al., 2012; Gould and Tanapat, 1999; Kim et al., 2003; van Praag et al., 1999).
Adult hippocampal neurogenesisis and neuroplasticity are modulated by many neurotrophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin-3 (Malberget al., 2000), Of these, BDNF has been intensively studied and shown to be involved in the learning and memory (Poo et al., 2001). BDNF is a neurotrophin involved in neuronal survival and plasticity that binds to high-affinity receptors, tyrosine kinase B (TrkB) (Givalois et al., 2004). BDNF-TrkB interaction promotes the survival and differentiation of neurons and synaptic plasticity of the central nervous systems (Kim et al., 2010; Lu et al., 2008).
The enhancing effects of exercise on learning ability and memory functions have been reported in many studies (Baek et al., 2012; Ji et al., 2014; Kim et al., 2010; van Praag et al., 1999). In the present study, we investigated the effect of treadmill exercise on spatial learning ability in relation with cell proliferation in the hippocampal dentate gyrus using the scopolamine-induced amnesia mice. For this study, Morris water maze test, immonofluorescence for 5-bromo-2’-deoxyuridine (BrdU) and western blot for brain-derived BDNF and TrkB were conducted.
MATERIALS AND METHODS
Experimental animals
Male ICR mice weighing 35±0.5 g were used in this experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The mice were housed under controlled temperature (20±2°C) and lighting (07:00 to 19:00 h) conditions with food and water available ad libitum. The mice were randomly divided into 4 groups (n=10 in each group): control group, exercise group, scopolamine-induced amnesia group, and scopolamine-induced amnesia and exercise group. All mice received 50 mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally once a day 30 min before the treadmill exercises for 5 consecutive days, starting experiment.
Administration of scopolamine
To induce amnesia, 1 mg/kg scopolamine hydrobromide (Sigma Chemical Co.) dissolved in physiological saline was injected intraperitoneally once a day for 14 consecutive days, as the previously described method (Heo et al., 2014). The mice in the control group received saline instead of scopolamine. Morris water-maze test was carried out at 30 min after last injection of scopolamine.
Treadmill exercise protocol
The mice in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 14 days. Exercise load for the running groups consisted of running at a speed of 5 meters/min with the 0° inclination. The mice in the non-exercise groups were left on the treadmill without running for the same duration as the exercise groups.
Morris water-maze test
In order to evaluate the spatial learning ability in mice, occupancy time in the Morris water maze test was determined, as the previously described method (Kim et al., 2010; Sim et al., 2014). The water maze was a circular pool (diameter=115 cm, height=22 cm) constructed of fiberglass. The water was maintained at a temperature of 22±2°C. During testing in the water maze, a platform (diameter=9 cm, height=20 cm) was located 2 cm below the water surface in one of four locations in the pool. Clearly visible cues outside the basin were provided for orientation. The test consisted of three acquisition phases and two probe trials. In the acquisition phase, the mice had daily four trials for 3 consecutive days to find the platform submerged 2 cm under the water surface. For each trial, the mice were placed in the water, facing the wall of the tank, in one of the four start locations. The mice were allowed to search for the platform for 60 sec. If the mice found the platform, remaining on the platform for 10 sec was permitted. If the mice did not find the platform within 60 sec, the mice were guided and allowed to remain on the platform for 10 sec. The interval between trials was 20 sec. After each training session, the mice were dried with a towel and returned to their cages. To assess spatial learning ability, the animals were subjected to the 60 sec probe trial following the last training session, and then the platform was removed from the pool. The occupancy time in the quadrant platform was recorded automatically by a video tracking system (SMART; Pan-Lab, Barcelona, Spain).
Tissue preparation
The mice were sacrificed immediately after determining occupancy time. The mice were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were dissected and post-fixed in the same fixative method overnight, and transferred to a 30% sucrose solution for cryoprotection. Then, 40 μm thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany). On average 10 slice sections in the hippocampal region were collected from each mice.
BrdU immunofluorescence
Immunofluorescence for the BrdU-positive and NeuN-positive cells in the hippocampal dentate gyrus was performed, according to the previously described method (Shin et al., 2013). The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2 X standard saline citrate (SSC) at 65°C for 2 h, denatured in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). After washing in PBS for 15 min at 27°C, the sections were followed by wash for 10 min three times at room temperature. The sections were incubated overnight with rat anti-BrdU antibody (1:200; Abcam, Cambridge, Cambridgeshire, UK) and mouse anti-NeuN antibody (1:400; Chemicon, Temecula, CA, USA). The sections were next incubated for 2 h with cy3-conjugated anti-rat secondary antibody (1:200; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for the BrdU antibody and fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary antibody (1:200; Jackson Immuno Research Laboratories) for the NeuN antibody. The sections were then mounted on gelatin-coated glass slides, and the cover slips were mounted using fluorescent mounting medium (Dako Cytomation, Carpinteria, CA, USA). The slides of the fluorescent images were captured using a confocal laser scanning microscopy (LSM-700; Carl Zeiss, München-Hallbergmoos, Germany).
Western blot analysis
Western blot analysis was conducted for the determination of BDNF and TrkB expressions, as the previously described method (Kim et al., 2010). Hippocampal tissues were collected and then immediately frozen at −70°C. Hippocampal tissues were homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodiumfluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Hercules, CA, USA). Protein (30 μg) was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse beta-actin antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit BDNF antibody, and rabbit TrkB antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody (1:3,000; Vector Laboratories, Burlingame, CA, USA) for BDNF and TrkB were used as the secondary antibodies. Experiments were performed under normal laboratory conditions and at room temperature, except for the transfer to membranes. The transfer was performed at 4°C with a cold pack and pre-chilled buffer. Band detection was performed using enhanced chemiluminescence (ECL) detection kits (Santa Cruz Biotechnology).
Data analysis
The numbers of BrdU-positive cells in the hippocampal dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the numbers of cells per mm2 in the hippocampal dentate gyrus. To compare the relative expressions of BDNF and TrkB, the detected bands were calculated densitometrically using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The data are expressed as the number of cells per mm2 of the hippocampus. Statistical analysis was performed using one-way ANOVA followed by Duncan’s post-hoc test. The results are presented as the mean±standard error of the mean (SEM). Significance was set as P<0.05.
RESULTS
Effect of treadmill exercise on the time spent in the probe quadrant of Morris water maze test
Spatial learning ability was measured using the Morris-water maze test (Fig. 1). The time spent in the probe quadrant was 28.69±0.63% in the control group, 32.66±2.49% in control and exercise group, 22.27±0.72% in the scopolamine-induced amnesia group, and 28.00±1.39% in the scopolamine-induced amnesia and exercise group. The present results revealed that spatial learning ability in the mice was deteriorated by the induction of amnesia (P<0.05). In contrast, treadmill exercise improved spatial learning ability in the scopolamine-induced amnesia mice (P<0.05). In the normal mice, treadmill exercise enhanced spatial learning ability.
Fig. 1.
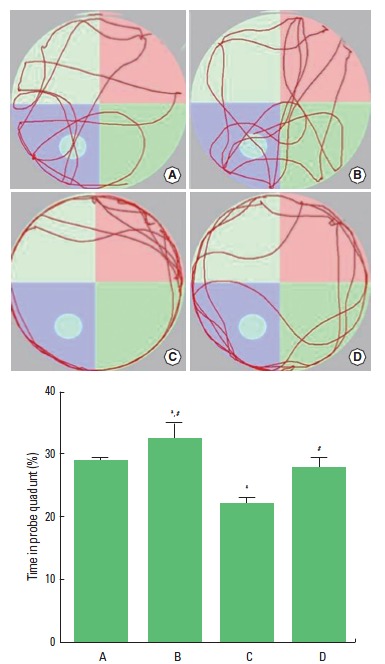
Effect of treadmill exercise on spatial learning ability. (A) Control group, (B) Exercise group, (C) Scopolamine-induced amnesia group, and (D) Scopolamine-induced amnesia and exercise group. The data are presented as the mean±standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the scopolamine-induced amnesia group.
Effect of treadmill exercise on the number of BrdU-positive in the dentate gyrus of hippocampus
Cell proliferation in the hippocampal dentate gyrus is presented in Fig. 2. The number of BrdU-positive cells in the hippocampal dentate gyrus was 167.70±3.44/mm2 in the control group, 244.22±8.00/mm2 in control and exercise group, 93.33±5.11/mm2 in the scopolamine-induced amnesia group, and 134.24±11.46/mm2 in the scopolamine-induced amnesia and exercise group. The present results showed that cell proliferation in the hippocampal dentate gyrus was significantly suppressed in the scopolamine-induced amnesia mice (P<0.05). In contrast, tread-mill exercise enhanced cell proliferation in the scopolamine-induced amnesia mice (P<0.05). In the normal rats, treadmill exercise increased cell proliferation in the hippocampal dentate gyrus (P<0.05).
Fig. 2.
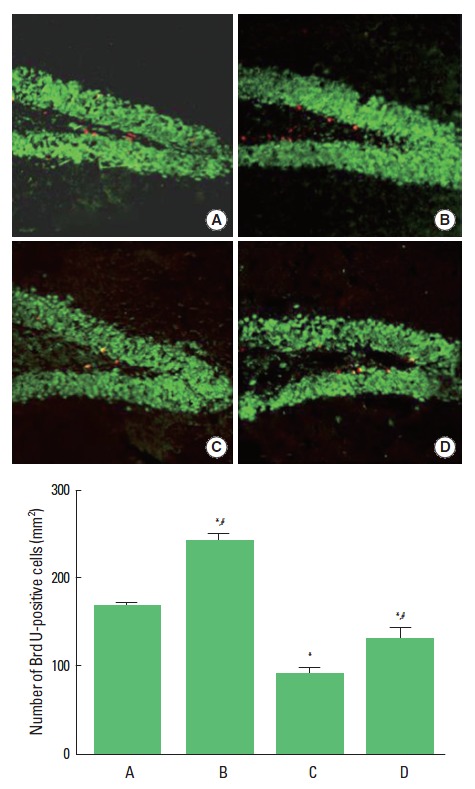
Effect of treadmill exercise on cell proliferation in the hippocampal dentate gyrus. (A) Control group, (B) Exercise group, (C) Scopolamine-induced amnesia group, and (D) Scopolamine-induced amnesia and exercise group. The scale bar represents 100 μm. The data are presented as the mean±standard error of the mean (SEM). *P < 0.05 compared to the control group. #P < 0.05 compared to the scopolamine-induced amnesia group.
Effect of treadmill exercise on BDNF and TrkB expressions in the hippocampus
We determined the relative expression of BDNF in the hippocampus (Fig. 3). When the level of BDNF (14 kDa) in the control group was set at 1.00, the level of BDNF was 1.65±0.52 in the control and exercise group, 0.35±0.22 in the scopolamine-induced amnesia group, and 0.88±0.27 in the scopolamine-induced amnesia and exercise group. The present results demonstrated that the expression of BDNF in the hippocampus was decreased in the scopolamine-induced amnesia mice (P<0.05). In contrast, treadmill exercise remarkably increased the BDNF expression in the scopolamine-induced amnesia mice (P<0.05). In the normal mice, treadmill exercise increased the BDNF expression in the hippocampus (P<0.05).
Fig. 3.
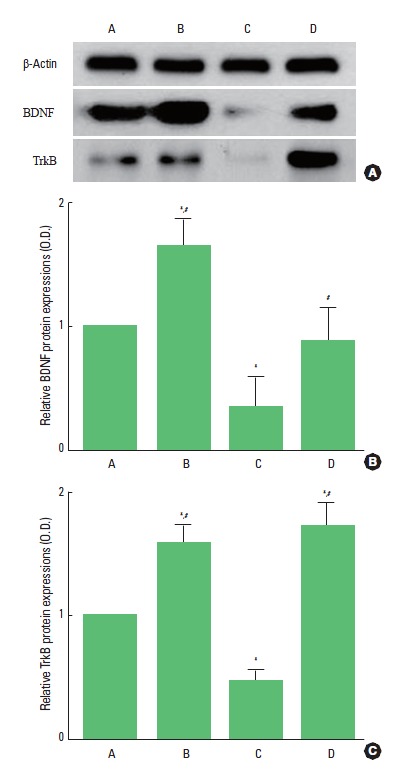
Effect of treadmill exercise on brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) expressions in the hippocampus. (A) Control group, (B) Exercise group, (C) Scopolamine-induced amnesia group, and (D) Scopolamine-induced amnesia and exercise group. The data are presented as the mean± standard error of the mean (SEM). *P< 0.05 compared to the control group. #P< 0.05 compared to the scopolamine-induced amnesia group.
We determined the relative expression of TrkB in the hippocampus (Fig. 3). When the level of TrkB (95–145 kDa) in the control group was set at 1.00, the level of TrkB was 1.59±0.14 in the control and exercise group, 0.47±0.29 in the scopolamine-induced amnesia group, and 1.73±0.56 in the scopolamine-induced amnesia and exercise group. The present results showed that the expression of TrkB in the hippocampus was decreased in the scopolamine-induced amnesia mice (P<0.05). In contrast, treadmill exercise remarkably increased the TrkB expression in the scopolamine-induced amnesia mice (P<0.05). In the normal mice, treadmill exercise increased the TrkB expression in the hippocampus (P<0.05).
DISCUSSION
AD is the most common cause of progressive loss of memory in the elderly (Rubio et al., 2007). Scopolamine dysregulates cholinergic activity, resulting in the deficits of the learning ability and memory tasks (Elvander et al., 2004). Scopolamine-induced memory impairment is closely associated with the cholinergic dysfunction (Sharma et al., 2010). Short-term memory was deteriorated in the scopolamine-induced amnesia mice (Heo et al., 2014). In the present results, spatial learning ability was deteriorated in the scopolamine-induced amnesia mice, while treadmill exercise ameliorated spatial learning deficits in the amnesia mice.
Lesion in the cholinergic nuclei leads to the suppression of neurogenesis in the hippocampus (Cooper-Kuhn et al., 2004). Scopolamine also inhibits neurogenesis in the hippocampus (Kotani et al., 2006). Enhanced hippocampal neurogenesis by physical exercise is closely associated with improving memory function (van Praag et al., 2005; Kim et al., 2010). In the present results, cell proliferation in the hippocampal dentate gyrus was decreased in the scopolamine-induced amnesia mice, while treadmill exercise increased neurogenesis in the amnesia mice.
Increased neurogenesis and improved memory function by exercise are induced by BDNF up-regulation in the brain (Cotman and Berchtold, 2002). Exercise increases the level of BDNF in the hippocampus and stimulates hippocampal neurogenesis (Rhodes et al., 2003). BDNF has been suggested as the potential mediator for the exercise-derived hippocampal plasticity and neurogenesis (Cotman and Berchtold, 2002; Kim et al., 2010). Decrement in BDNF and TrkB contributed memory deficits in the AD (Li et al., 2010).
Heo et al. (2014) demonstrated that treadmill exercise ameliorated short-term memory impairment through suppressing acetylcholine esterase and enhancing angiogenesis in the scopolamine-induced amnesia mice. In the present results, BDNF and TrkB expressions in the hippocampus were suppressed in the scopolamine-induced amnesia mice, while treadmill exercise enhanced BDNF and TrkB expressions in the amnesia mice.
In the present study, induction of scopolamine-induced amnesia showed inhibition of cell proliferation and suppression of BDNF and TrkB expressions in the hippocampus, and resulted in the impairment of spatial learning ability. Treadmill exercise improved spatial learning ability by increasing cell proliferation through activation of BDNF-TrkB pathway. The present results offer a possibility that treadmill exercise may provide preventive or therapeutic value for the memory loss induced by variable neurodegenerative diseases including AD.
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2011-327-G00124).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY, Kim CJ. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal separated rat pups. Brain Dev. 2012;34:45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Elvander E, Schött PA, Sandin J, Bjelke B, Kehr J, Yoshitake T, Ogren SO. Intraseptal muscarinic ligandsand galanin: Influence on hippocampal acetylcholine and cognition. Neuroscience. 2004;126:541–557. doi: 10.1016/j.neuroscience.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Givalois L, Arancibia S, Alonso G, Tapia-Arancibia L. Expression of brain-derived neurotrophic factor in the rat median eminence cells with sensitivity to stress. Endocrinology. 2004;145:4737–4747. doi: 10.1210/en.2004-0616. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Heo YM, Shin MS, Lee JM, Kim CJ, Baek SB, Kim KH, Baek SS. Treadmill exercise ameliorates short-term memory disturbance in scopolamine-induced amnesia rats. Int Neurourol J. 2014;18:16–22. doi: 10.5213/inj.2014.18.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes MJ, Perry NS, Houghton PJ. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother Res. 2003;17:1–18. doi: 10.1002/ptr.1280. [DOI] [PubMed] [Google Scholar]
- Ji ES, Kim CJ, Park JH, Bahn GH. Duration-dependence of the effect of treadmill exercise on hyperactivity in attention deficit hyperactivity disorder rats. J Exerc Rehabil. 2014;10:75–80. doi: 10.12965/jer.140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24:114–117. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, Thakur MK. Protective role of Ashwagandha leaf extract and its component witha-none on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6:27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–514. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Li F, Gong QH, Wu Q, Lu YF, Shi JS. Icariin isolated from Epimediumbrevicornum Maxim attenuates learning and memory deficits induced by D-galactose in rats. Pharmacol Biochem Behav. 2010;96:301–305. doi: 10.1016/j.pbb.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL, Duan W. Modification of brain aging and neuro-degenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Khales GY, Abbasi L, Zarifkar A, Rastegar K. Agmatine protects against scopolamine-induced water maze performance impairment and hippocampal ERK and Akt inactivation. Neuropharmacology. 2012;62:2018–2023. doi: 10.1016/j.neuropharm.2011.12.031. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Rubio J, Dang H, Gong M, Liu X, Chen SL, Gonzales GF. Aqueous and hydroalcoholic extracts of black maca (Lepidiummeyenii) improve scopolamine-induced memory impairment in mice. Food Chem Toxicol. 2007;45:1882–1890. doi: 10.1016/j.fct.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Sharma D, Puri M, Tiwary AK, Singh N, Jaggi AS. Antiamnesic effect of stevioside in scopolamine-treated rats. Indian J Pharmacol. 2010;42:164–167. doi: 10.4103/0253-7613.66840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Ko IG, Kim SE, Kim BK, Kim TS, Lee SH, Hwang DS, Kim CJ, Park JK, Lim BV. Treadmill exercise ameliorates symptoms of methimazole-induced hypothyroidism through enhancing neurogenesis and suppressing apoptosis in the hippocampus of rat pups. Int J Dev Neurosci. 2013;31:214–223. doi: 10.1016/j.ijdevneu.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer’s disease rats. J Exerc Rehabil. 2014;10:81–88. doi: 10.12965/jer.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo DY, Kim W, Yoo KY, Lee CH, Choi JH, Kang IJ, Yoon YS, Kim DW, Won MH, Hwang IK. Effects of Nelumbonucifera rhizome extract on cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia animal model. Phytother Res. 2011;25:809–815. doi: 10.1002/ptr.3337. [DOI] [PubMed] [Google Scholar]


