Abstract
Enteroendocrine cells (EEC) produce neuropeptides, which are crucially involved in the maintenance of the intestinal barrier. Hence, EEC dysfunction is suggested to be involved in the complex pathophysiology of inflammatory bowel disease (IBD), which is characterized by decreased intestinal barrier function. However, the underlying mechanisms for EEC dysfunction are not clear and suitable models for a better understanding are lacking. Here, we demonstrate that Carboxypeptidase E (CPE) is specifically expressed in EEC of the murine colon and ileum and that its deficiency is associated with reduced intestinal levels of Neuropeptide Y (NPY) and Peptide YY (PYY), which are both produced by EEC. Moreover, cpe−/− mice exhibit an aggravated course of DSS-induced chronic colitis compared to wildtype littermates. In addition, we observed elevated mucosal IL-6 and KC transcript levels already at baseline conditions in cpe−/− mice. Moreover, supernatants obtained from isolated intestinal crypts of cpe−/− mice lead to increased IL-6 and KC expression in MODE-K cells in the presence of LPS. This effect was reversible by co-administration of recombinant NPY, suggesting a CPE mediated immunosuppressive effect in the intestines by influencing the processing of specific neuropeptides. In this context, the chemotaxis of bone marrow derived macrophages towards respective supernatants was enhanced. In conclusion, our data point to an anti-inflammatory role of CPE in the intestine by influencing local cytokine levels and thus regulating the migration of myeloid immune cells into the mucosa. These findings highlight the importance of EEC for intestinal homeostasis and propose EEC as potential therapeutic targets in IBD.
Introduction
Inflammatory bowel diseases (IBD) are chronically recurring inflammatory disorders of the gastrointestinal tract (GIT) characterized by an impaired intestinal barrier function [1]. Although extensive research has been performed over the last decades, the underlying mechanisms are still unknown.
According to current knowledge, the maintenance of the intestinal barrier critically depends on the interplay of the gut microbiota, intestinal epithelial cells (IEC) and primary immune cells [2], [3]. The orchestration of these cells is tightly organized and depends on various messenger molecules such as cytokines and neuropeptides [3]–[5]. Consequently, a disturbance in this communication network is suggested to contribute to the pathogenesis of IBD [6].
In IBD patients, intestinal levels of neuropeptides expressed by enteroendocrine cells (EEC) are altered [7]–[10], leading to the hypothesis of EEC dysfunction as an underlying pathophysiological mechanism [11]. Supporting this theory is the association between Crohn's Disease (CD) and autoantibodies against the ubiquitination protein 4a (UbE4A), a protein with specifically high expression in EEC [12]. Additionally, gene polymorphisms in the EEC transcription factor Phox2B are present in CD, which might affect expression levels of EEC derived neuropeptides in CD [11], [13].
However, animal models employed to determine the role of neuropeptides in intestinal inflammation focused only on single neuropeptides and therefore disregarded the complex interplay of different neuropeptides for intestinal homeostasis. Considering that EEC function is exerted by a great number of different neuropeptides, modulators of neuropeptide synthesis turn up as potential tools to examine the influence of EEC on intestinal inflammation.
CPE is an exopeptidase crucial for the function of neuroendocrine cells by processing and sorting of many neuropeptides. Moreover, there is increasing evidence for a CPE expression in EEC [14] implicating a functional role for EEC function and therefore for intestinal homeostasis and barrier function.
In this study we were able to show the specific expression of CPE in EEC of the lower intestine by immunofluorescence staining. After we confirmed a relevant role of CPE on neuropeptide levels in the GIT, we used cpe −/− mice as a model of EEC dysfunction to examine the pathophysiologic mechanisms in experimental colitis. As a result we provide evidence for a relevant role of CPE for EEC function and intestinal homeostasis. As a new model for EEC dysfunction cpe −/− mice might contribute to new insights in the pathophysiology of IBD.
Materials and Methods
Ethics Statement
All mice were placed in the Laboratory Animal Unit of the University of Lübeck, kept in a climate-controlled environment with 12 hour light-dark-cycle and were provided with food and water ad libitum. All experiments were performed in strict accordance with the animal care guidelines of the University of Lübeck and with national and international laws and policies. The study protocol was approved by the Ministerium für Energiewende, Landwirtschaft, Umwelt und ländliche Räume des Landes Schleswig-Holstein (acceptance no.: V 312–72241.122-1 (70–6/11)). During the experiments all efforts were made to minimize suffering.
Animal models
All experiments were performed in accordance with the animal care guidelines of the University of Lübeck (acceptance no.: V 312–72241.122-1 (70–6/11)). Procedures involving animals and their care were conducted in accordance with national and international laws and policies. Cpe +/+ and cpe −/− mice were generated by crossing B6.HRS(BKS)-Cpefat/J+/− mice obtained from the Jackson Laboratory. In all experiments cpe −/− mice and their respective littermate controls (cpe +/+) were used. The animals were kept under standard conditions at the animal facility of the University to Lübeck and were provided with food and water ad libitum.
Immunofluorescence analysis of mouse intestine
Tissue slides of ileal and colonic biopsies were immunostained with anti-Chromogranin B (CgB), anti-CPE, anti-NPY, anti-CD3, anti-F4/80 and anti-Ly6G and anti-Peptide YY (PYY) according to standard protocols. Briefly, frozen tissue sections were fixed in methanol and acetone (1∶1) and then incubated with goat anti-CgB (Santa Cruz, 1∶100), rabbit anti-CPE (Sigma, 1∶200), goat anti-NPY (Santa Cruz, 1∶200), goat anti-CD3 (BioLegend, 1∶1000), goat anti-F4/80 (BioLegend, 1∶10000), or goat anti-PYY (Santa Cruz, 1∶200) primary antibodies for one hour. After several washing steps with PBS sections were incubated with appropriate secondary antibodies. Nuclear staining was done with Hoechst dye, and slides were evaluated using a confocal laser scanning microscope.
Induction of Colitis and determination of clinical scores
In two independent experiments, chronic colitis induction was performed in cpe −/− mice and their littermates (cpe +/+) at an age of 9–10 weeks by feeding with 2% dextran sulfate sodium (DSS; molecular mass 40 kDa; TdB Consultancy; Uppsala; Batch DB001-27) dissolved in the drinking water for 5 days, followed by 5 days of normal drinking water. This cycle was repeated three times. Control mice received water without DSS. Mice were sacrificed on day 30 of the experiment as described previously [15]. Clinical parameters were assessed every other day. The disease activity index (DAI) was assessed as a combined score of weight loss, stool consistency and rectal bleeding as described elsewhere [16]. High resolution mouse endoscopy was employed (HOPKINS Optik 64019BA; Karl Storz AidaVet) to determine the murine endoscopic index of colitis severity (MEICS) as described previously [15], [17].
Histological analysis of mouse colon tissue
Ileal and colonic biopsies were fixed in 4% formalin and embedded in paraffin. Sections of 5 µm thickness were stained with haematoxylin-eosin using standard procedures. The grade of inflammation after chronic DSS-colitis induction was determined as described elsewhere [16]. Briefly, the grade and extent of tissue damage was assessed and the infiltration with inflammatory cells was estimated. The analysis was conducted by two independent observers in a blinded fashion.
Real time PCR
RNA was extracted using the innuPREP RNA mini kit (Analytik Jena AG, Germany) and transcribed to cDNA (RevertAid H Minus reverse transcriptase, Fermentas). Real-time quantitative PCR was carried out with 10 µl of Maxima SYBR Green qPCR Master Mix, plus 0.5 µM of each primer using a 96-well plate format. The amplification program consisted of: i) pre-incubation at 95°C for 5 min; ii) 40 cycles of denaturation at 95°C for 45 s and annealing at appropriate temperature (60°C) for 1 min using ABI PRISM 7000 (Applied Biosystems). To confirm amplification of specific transcripts, melting curve profiles were produced. Expression levels were normalized to β-Actin. The following primer pairs were used: β-Actin (for: GATGCTCCCCGGGCTGTATT, rev: GGGGTACTTCAGGGTCAGGA), IL-6 (for: CTCCCAACAGACCTGTCTATAC, rev: GTGCATCATCGTTGTTCATAC), KC (for: GCTGGGATTCACCTCAAGAA, rev: TGGGGACACCTTTTAGCATC), TNF-α (for: CCCTCACACTCAGATCATCTTCTC, rev: TGGCTCAGCCACTCCAG), CgB (for: CCCGCTGGCTGAACTTTTC, rev: GAGTTCTGACGGCGGAAGAG), PYY (for: TTCACAGACGACAGCGACA, rev: CACCACTGGTCCAAACCTTC), NPY (for: CCCTCGCTCTATCTCTGCTCGT, rev: GCGTTTTCTGTGCTTTCCTTCA), VIP (for: TGGATGACAGGATGCCGTTT, rev: CGGCATCAGAGTGTCGTTTG), CgA (for: AGCCAGACTACAGACCCACT, rev: TGACTTCCAGGACGCACTTC).
ELISA
Colonic punch biopsies were obtained from both genotypes and stimulated with lipopolysaccharide (LPS) (50 ng/ml) or forskolin (30 µM), an agent with previously described secretagogue effects [18], for two hours at 37°C. Culture supernatants were then harvested and assayed for NPY and PYY using commercially available kits according to the manufacturer's instructions (Mouse NPY ELISA KIT: Bio-Medical Assay Co.,Ltd. Cat#: 21176, Mouse PYY EIA KIT: Ray Biotech, Inc. Cat#. EIA-PYY-1).
Cell culture
Colonic crypts of both genotypes were isolated, treated with forskolin (30 µM) for one hour and supernatants were then added to an immortalized epithelial cell line derived from the murine duodenum (MODE-K) [19]. After 2 h of cultivation w/wo LPS (50 ng/ml) at 37°C and 5% CO2 cells were harvested for RNA preparation. In a subgroup of experiments recombinant NPY and PYY (Tocris Bioscience, Bristol, UK) were co-administered with LPS at a concentration of 1 µM.
Migration assay
Femurs and tibias from wildtype mice were harvested and flushed with DMEM-medium containing 1% L-glutamin, 1% antibiotics and 10% fetal calf serum. Bone marrow cells were collected and differentiated to bone marrow-derived macrophages (BMDM) in DMEM-Medium containing 33% L929-cell culture supernatants as a source of M-CSF for 8 days at 37°C and 5% CO2. The chemotactic effects of crypt supernatants, which were harvested from cpe +/+ and cpe −/− mice and pre-incubated with forskolin (30 µM) for two hours at 37°C, on bone marrow-derived macrophages were investigated as described elsewhere [20]. BMDM were resuspended in the same DMEM medium at a density of 3x106 cells/ml. The crypt supernatants were used as chemoattractants, placed in the bottom wells of a micro Boyden chemotaxis chamber (Neuroprobe) and overlaid with a 5 µm polycarbonate membrane. 50 µl of the BMDM cell suspension were then placed in the top wells and incubated for 45 min at 37°C and 10% CO2. Subsequently, the membranes were removed and the cells on the bottom side of the membrane were stained with Diff-Quick. The numbers of migrated cells per high-power field were counted by light microscopy. Results are expressed as percentage of the mean value of wildtype mice. The Assay was replicated twice independently.
Statistical analysis
Results are expressed as means ± SEM. Values of p were calculated with Prism 5 (GraphPad) using a 2-tailed Student's t test for parametric values and MANN-Whitney-U test for non-parametrical values. Values of p<0.05 were considered statistically significant. Experiments and measurements were replicated at least twice.
Results
CPE co-localizes with CgB, NPY and PYY in the lower murine intestine
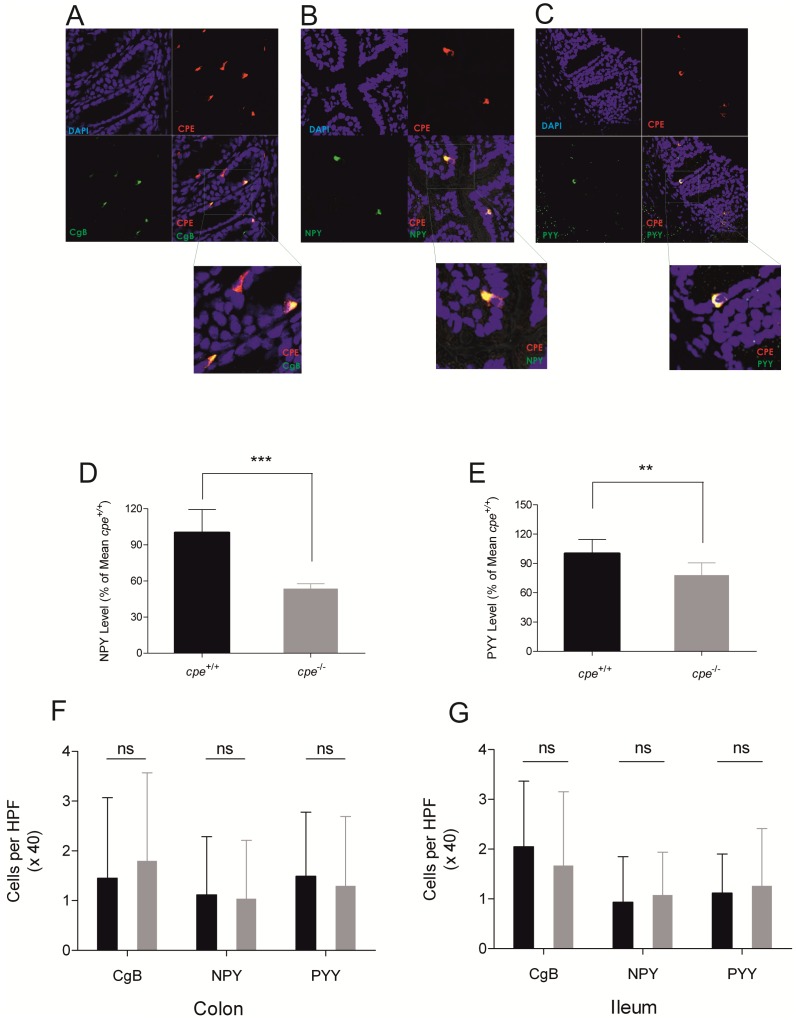
Although CPE has been detected in enteroendocrine cells of the upper GIT [14], nothing is known about CPE expression in the lower intestines. Therefore, we performed a CPE-specific staining of intestinal biopsies showing that CPE is located in defined cells of the ileal and colonic epithelium. Furthermore, staining for NPY, which is known to be processed by CPE [21]–[23], as well as CgB and PYY revealed a co-localization of these EEC markers with CPE (Figure 1A, B, C; ileum not shown). In conclusion, our results demonstrate a specific expression of CPE in enteroendocrine cells.
Figure 1. CPE is expressed in EEC and affects colonic NPY and PYY levels.
(A–C) Immunofluorescence staining of colonic biopsies from wildtype mice for CPE and the EEC marker CgB (A), NPY (B) and PYY (C). (D–E) NPY (D) and PYY (E) neuropeptide level assayed by ELISA from LPS treated colon punch biopsies. n = 8 per genotype. (F–G) Determination of enteroendocrine cell numbers in the colonic (F) and ileal (G) mucosa by counting CgB, NPY and PYY positive epithelial cells per high power field (magnification 40x), 5 random HPF per animal, n = 6 per genotype. **p<0.01, ***p<0.001, ns = not significant, by t-test.
CPE deficiency causes reduced colonic levels of NPY and PYY
In order to investigate the effects of CPE deficiency on EEC function, we next determined the levels of EEC-secreted neuropeptides NPY and PYY in supernatants of colonic biopsies from cpe −/− mice and wildtype controls. We did not detect relevant differences of neuropeptides in colonic supernatants at baseline (data not shown). Therefore, we examined the levels of neuropeptides after addition of LPS, a known inducer of neuropeptide secretion which is constantly present in the gut under physiological conditions [24], [25]. NPY and PYY level were found to be significantly decreased in cpe −/− mice compared to wildtype controls (Figure 1D, E) after LPS treatment (NPY: 53.64%±3.99 (cpe −/−) vs. 100%±19.33 (cpe +/+), p<0.005; PYY: 77,98%±12.53 (cpe −/−) vs. 100%±20.04 (cpe +/+), p<0,01). However, transcript levels of NPY and PYY as well as other neuropeptides such as Chromogranin A (CgA), Chromogranin B and vasoactive intestinal peptide (VIP) were slightly increased in cpe −/− mice, although not reaching statistical significance (Figure S1A). This could indicate a mechanism to compensate the reduced levels of neuropeptides due to CPE-deficiency.
In order to check if the decreased neuropeptide levels in cpe −/− mice are due to a reduced quantity of enteroendocrine cells, we further counted CgB, NPY and PYY positive cells as cell specific markers for EEC. No significant differences in enteroendocrine cell counts could be observed in ileal and colonic biopsies (Figure 1F, G), indicating that indeed CPE deficiency is causative for the neuropeptide reduction.
CPE deficiency aggravates experimental chronic colitis
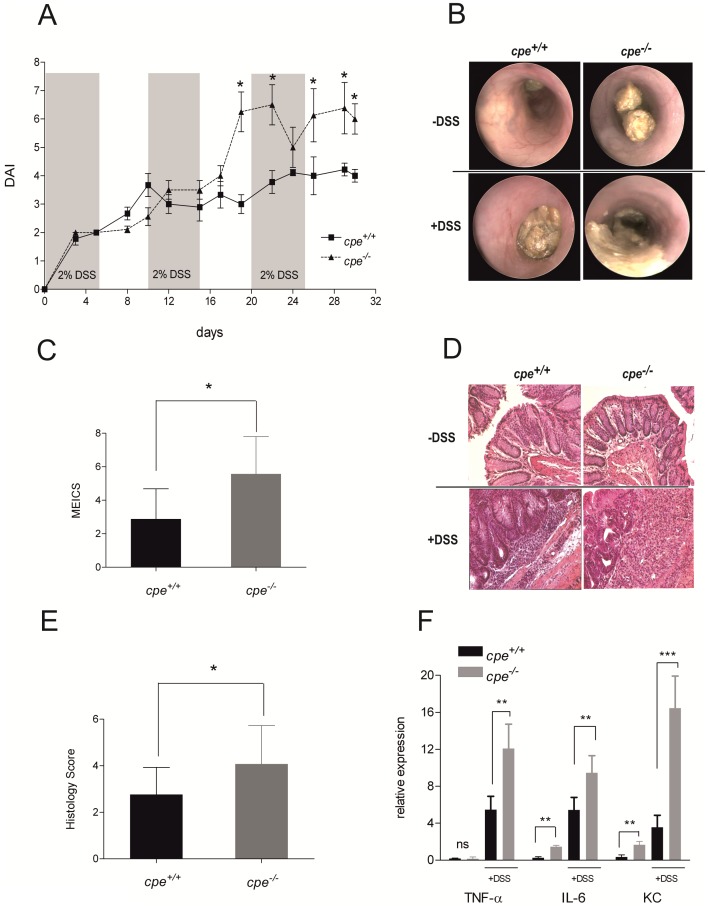
Considering a crucial role of EEC for intestinal barrier function, a feature that is highly disturbed in IBD [2], we next examined the effects of CPE deficiency on intestinal inflammation. Experimental chronic colitis was induced by cyclic administration of 2% DSS, and clinical parameters were evaluated. Regarding body weight loss, rectal bleeding and stool consistency, cpe −/− mice displayed a significantly more severe phenotype of colitis compared to wildtype controls (Figure 2A). Macroscopic assessment of colitis severity via mouse endoscopy confirmed the physical findings with a corresponding higher MEICS-Score in cpe −/− mice (5.57±2.23 (cpe −/−) vs. 2.88±1.81 (cpe +/+), p<0.05) (Figure 2B, C). Additionally, colonic biopsies were examined histologically and scored for mucosa morphology and inflammatory cell infiltration. Compared to wildtype controls, cpe −/− mice displayed a higher histology score comprised from the degree of mucosal edema, abscesses and ulcer as well as the numbers of infiltrating immune cells (4.07±1.66 (cpe −/−) vs. 2.77±1.17 (cpe +/+), p<0.05) (Figure 2D, E). Interestingly, at baseline conditions no visible histological differences of the mucosal/submucosal architecture were detected. Considering the proliferative potential of primary immune cells on EEC and their products as recently demonstrated by Worthington et al., we counted CD3 (89.64%±9.353 (cpe −/−) vs. 100%±11.84 (cpe +/+), n.s.) and F4/80 (107.6%±14.66 (cpe −/−) vs. 100%±9.65 (cpe +/+), n.s.) positive cells in intestinal biopsies in order to ensure that our strains do not exhibit relevant different myeloid cell compositions in the intestines (Figure S1B–E) [26]. In summary, CPE deficiency, although not leading to spontaneous intestinal inflammation, is associated with increased susceptibility to pro-inflammatory intestinal stimuli.
Figure 2. CPE deficiency aggravates experimental chronic colitis.
(A) Calculation of the disease activity index (DAI) by determining clinical parameters of inflammation (body weight development, stool consistency, rectal bleeding) through 30 days of experimental colitis. n = 9 (cpe +/+), n = 8 (cpe −/−). (B-C) Determination of macroscopic colitis severity via mouse endoscopy. Representative endoluminal pictures of the distal colon on day 30 of experimental colitis (B) and calculation of the murine endoscopic index of colitis severity (MEICS) by analyzing mucosal morphology, stool consistency and shape of the vascular pattern via mouse endoscopy (C). n = 9 (cpe +/+), n = 8 (cpe −/−). (D–E) Determination of microscopic colitis severity via histology. Representative histological pictures of the distal colon on day 30 of experimental colitis (D) and calculation of the histology score by analyzing mucosal architecture and infiltration of immune cells (E). n = 8 per genotype. (F) Determination of expression level of TNF-α, IL-6 and KC in colonic punch biopsies by real time RT-PCR after 30 days of experimental colitis and at baseline. n = 8 per genotype. *p<0.05, **p<0.01, ***p<0.001 by t-test.
Cytokine profile of CPE-deficient mice
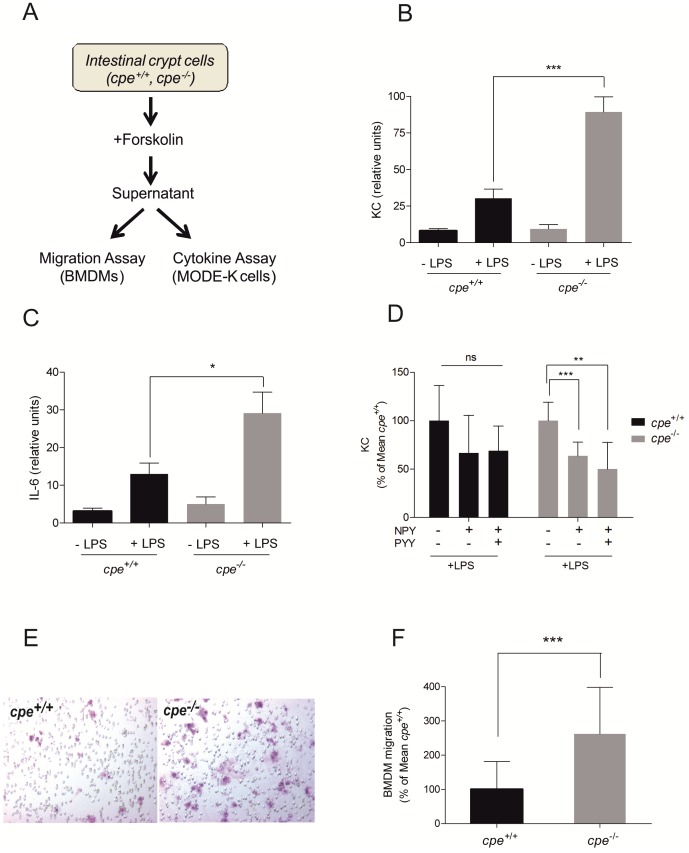
Cytokines are important factors in the communication of the local immune system in the gut and are tightly regulated by various factors, among them several neuropeptides [27]–[29]. Considering the decreased intestinal NPY and PYY levels in cpe −/− mice, we next analyzed colonic cytokine transcript levels of both genotypes at baseline conditions and after experimental colitis induction via quantitative RT-PCR. Under inflammatory conditions, cpe −/− mice showed significantly increased expression levels of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6) and chemokine (C-X-C motif) ligand 1 (KC) compared to wildtype controls (Figure 2F), whereas levels of interferon gamma (IFN-γ), IL-5, IL-13 and IL-17 were not different (data not shown). Surprisingly, IL-6 and KC transcripts in cpe −/− mice were already significantly elevated at baseline (Figure 2F). Moreover, as IL-6 and KC are known to be expressed by IEC [30]–[32], we asked whether the CPE mediated effects on cytokine production in the lower intestine could be locally limited to the intestinal epithelium. Therefore, we studied the effects of colonic crypt supernatants obtained from cpe −/− and cpe +/+ mice after incubation with forskolin on MODE-K cells, a murine duodenal IEC line. Similar to the supernatants of LPS stimulated colonic biopsies we found decreased levels of NPY and PYY as assessed by ELISA (data not shown). After incubation of MODE-K cells with the respective supernatants in the presence or absence of LPS (see Figure 3A for experimental setup) transcript levels of KC and IL-6 were determined via RT-PCR. No significant differences between the groups were measured for both KC and IL-6 without LPS. However, after addition of LPS the expression levels of KC and IL-6 were elevated. Remarkably, the LPS-dependent inductive potential of cpe −/− supernatants was significantly higher than the respective potential of supernatants obtained from cpe +/+ mice (Figure 3B, C). In order to study a potential link between the reduced levels of intestinal neuropeptides, we repeated the experiment in the presence of recombinant NPY and PYY. Notably, co-administration with NPY leads to an approximately 40% reduction of KC expression in MODE-K cells independently of the supernatant's origin (LPS: 100%±19.24 (cpe −/−) vs. 100%±36.32 (cpe +/+); LPS+NPY: 63.58%±14.34 (cpe −/−) vs. 66.6%±38.8 (cpe +/+); LPS+NPY+PYY: 50.08%±27.5 (cpe −/−) vs. 68.78%±25.76 (cpe +/+)) (Figure 3D). Similar, NPY co-treatment also reduced the expression levels of IL-6 (LPS: 100%±64.0 (cpe −/−) vs. 100%±115.54 (cpe +/+); LPS+NPY: 69.6%±58 (cpe −/−) vs. 62.0%±38.81 (cpe +/+); LPS+NPY+PYY: 70.48%±58.34 (cpe −/−) vs. 79.0%±105.34 (cpe +/+)), although not reaching statistical significance (Figure S1F). Finally, treatment with PYY together with NPY did not augment the effect of NPY alone. In conclusion, our data suggest that the lack of neuropeptides resulting from CPE deficiency alters IEC cytokine production.
Figure 3. Proinflammatory properties of colonic crypt supernatants of CPE-deficient mice.
(A) Experimental set-up for the acquirement and utilization of forskolin-stimulated supernatants of isolated colonic crypts. (B–C) Determination of KC (B) and IL-6 (C) transcript levels produced in MODE-K cells after incubation with LPS (50 ng/ml) and forskolin-stimulated supernatants of cpe +/+ and cpe −/− colonic crypts via RT-PCR. (D). KC transcript levels produced in MODE-K cells after incubation with forskolin-stimulated supernatants of of cpe +/+ and cpe −/− mice and LPS together with recombinant NPY +/− PYY (1 µM/ml). KC expression levels are expressed in percent of the Mean of cpe+/+. (E–F) BMDM migration via Boyden chamber assay. Representative pictures of migrated BMDM (E) and quantification (F) of BMDM migration towards supernatants of forskolin-stimulated colonic crypts of cpe +/+ and cpe −/− mice. *p<0.05, **p<0.01, ***p<0.001 by t-test.
Differential chemotactic potential of colonic crypt supernatants
In order to study a possible mechanistic link between the modulation of EEC function by CPE through processing and sorting of gastrointestinal neuropeptides and primary immune cells, which drive intestinal inflammation, we finally tested the effect of supernatants obtained from forskolin-treated crypt cells from cpe −/− and cpe +/+ mice on the migratory capacity of BMDM. In relation to supernatants obtained from cpe +/+ mice, those from cpe −/− mice exhibited a significantly higher chemotactic effect on wildtype BMDM (261.99%±136,27, p<0,001) as shown by the numbers of migrated BMDM (Figure 3E, F). Interestingly, migration of BMDM isolated from cpe +/+ and cpe −/− mice was similar (data not shown), indicating that the chemoattractive supernatants from cpe −/− mice are responsible for the observed effect and not an altered migratory capacity of the respective BMDM.
Discussion
EEC are crucially involved in the maintenance of intestinal homeostasis, and EEC dysfunction is discussed to play a relevant pathophysiologic role in IBD [11]. With CPE being a possible modulator of EEC function, cpe −/− mice represent a potential model for the investigation of EEC dysfunction and its relevance in intestinal inflammation.
In the present study, we demonstrate a cellular co-localization of CPE with the EEC markers NPY, PYY and CgB in the lower GIT. This result indicates the specific expression of CPE in EEC of the intestinal mucosa. Moreover, our data provide evidence that the intestinal levels of neuropeptides are significantly influenced by CPE. Both analysed neuropeptides, NPY and PYY, are reduced in cpe −/− mice compared to wildtype littermates. According to current knowledge, only precursors of NPY and not PYY are directly processed by CPE in neuroendocrine tissue [33], implicating a more general effect of CPE for EEC function. Here, the known sorting function of CPE for neuropeptides could be a factor of higher relevance than the exopeptidase function on single neuropeptides [34], [35]. Considering that in IBD patients the levels of many different neuropeptides have been found to be altered, we suggest that the cpe −/− model might be a valuable tool to study the complex interplay of EEC derived neuroendocrine factors in the intestines.
In order to investigate the phenotype of cpe −/− mice in the context of intestinal inflammation, we induced chronic DSS colitis as an established murine model of human IBD. The major finding here was a significantly more severe phenotype of colitis in cpe −/− mice as shown by MEICS, DAI and histology, indicating that CPE deficiency modulates the susceptibility of respective mice to experimental colitis.
Moreover, by analyzing intestinal biopsies obtained from DSS treated mice for cytokine expression, we accordingly found significantly increased levels of the proinflammatory cytokines TNF-α, IL-6 and KC as compared to the control group. Surprisingly, we also detected increased KC and IL-6 levels in cpe −/− mice under baseline conditions, suggesting a relevant role of CPE for basal cytokine production. In this regard, several neuropeptides expressed in the intestine have already been shown to affect cytokine levels in vivo [28], [36], [37].
The number of primary immune cells in the mucosa, as the main producers of many cytokines [38], was low at baseline conditions and did not significantly differ between both mouse strains (data not shown). This raises the question of alternative sources for the increased IL-6 and KC levels in cpe −/− mice at baseline. In this regard, both IL-6 and KC have been reported to also be expressed by IEC [30]–[32], [39], suggesting that the source of the observed cytokine expression is located in the epithelium and not in primary immune cells, such as lymphocytes and macrophages. In order to test this hypothesis, we used MODE-K cells, a murine intestinal epithelial cell line, and incubated them with supernatants of forskolin-stimulated intestinal crypts isolated either from cpe −/− mice or wildtype littermates. Additionally, cells were treated with LPS, which is constantly present under physiological conditions in the intestine. As the result, MODE-K cells showed significantly increased expression level of KC and IL-6 when stimulated with supernatants of cpe−/− mice. Interestingly, this effect was reversible by co-administration of recombinant NPY to the supernatants. However, additional administration of PYY did not show a statistical significant effect on KC expression levels.
These data indicate that the elevated cytokine levels observed under baseline conditions in cpe −/− mice might originate rather specifically from IEC, highlighting an important role of CPE and its downstream targets for the immunological environment in the intestinal mucosa, orchestrated by IEC themselves. Moreover, our results highlight the role of NPY as an anti-inflammatory molecule, a property which has been recently demonstrated on myeloid derived primary immune cells [25]. Considering that the cellular processing of NPY is known to be dependent on CPE, our data establish a new link between CPE and NPY as executers of intestinal immune regulation.
In order to study the consequences of altered mucosal cytokine expression in cpe −/− mice, we finally employed a chemotaxis experiment with respective crypt supernatants of cpe−/− and cpe +/+ mice. As the result, bone marrow-derived macrophages showed increased migratory behavior towards the supernatants obtained from cpe−/− mice, irrespective of BMDM origin from cpe −/− or cpe +/+ mice. This might be a further explanation of the observed proinflammatory phenotype of cpe −/− mice upon DSS administration. In this context, primary immune cells invade the intestinal mucosa and have been described to be crucially involved in chronic DSS colitis [40]–[42].
In summary, we demonstrated increased susceptibility of cpe −/− mice against chronic DSS colitis highlighting an important role of CPE for intestinal homeostasis. As a potential mechanism we identified CPE to modulate epithelial cytokine production and thus to be involved in the orchestration of the local immunological environment regulating the migration of primary immune cells. However, more studies are needed to dissect the executers and molecular mechanisms of the CPE mediated effects. Furthermore, future investigation is necessary in order to examine whether the patients with CD who have autoantibodies against EEC or genetic polymorphisms in the Phox2B gene, also show a defective local immune system as demonstrated here in cpe −/− mice. Potentially, these patients might especially benefit from local substitution with GI-neuropeptides or pharmacological modification of EEC function.
Supporting Information
(A) Determination of expression levels of different neuropetides in colonic punch biopsies by real time RT-PCR at baseline. n = 6 per genotype. (B-E) Immunofluorescence staining of colonic biopsies from cpe+/+ and cpe−/− mice for CD3, F4/80 and Ly6G (B) and quantification of immune cells in colonic biopsies by counting CD3 (C), F4/80 (D) and Ly6G (E) positive cells per high power field (magnification 40x). 5 random HPF per animal, n = 6 per genotype. (F) IL-6 transcript levels produced in MODE-K cells after incubation with forskolin-stimulated supernatants of of cpe +/+ and cpe −/− mice and LPS together with recombinant NPY +/− PYY (1 µM/ml). IL-6 expression levels are expressed in percent of the Mean of cpe+/+. *p<0.05; ns = not significant, by t-test.
(JPG)
Acknowledgments
We thank Ann-Kathrin Brethack, Lidija Gutjahr, Petra Langenstrassen and Harry Manfeldt for the excellent technical support. MODE-K cell line was kindly provided by D. Kaiserlian, INSERM U851, Lyon.
Funding Statement
This work was supported by the Section of Medicine, University of Lübeck (E06-2011; P01-2012 to C.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28: 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G (2009) Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis 15(1): 100–113. [DOI] [PubMed] [Google Scholar]
- 3. Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9(11): 799–809. [DOI] [PubMed] [Google Scholar]
- 4. Artis D (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 8(6): 411–420. [DOI] [PubMed] [Google Scholar]
- 5. Stead RH, Bienenstock J, Stanisz AM (1987) Neuropeptide regulation of mucosal immunity. Immunol Rev 100: 333–359. [DOI] [PubMed] [Google Scholar]
- 6. Chandrasekharan B, Nezami BG, Srinivasan S (2013) Emerging neuropeptide targets in inflammation: NPY and VIP. Am J Physiol Gastrointest Liver Physiol 304(11): 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koch TR, Roddy DR, Carney JA, Go VL (1988) Peptide YY concentrations in normal ileum and colon and in idiopathic inflammatory bowel disease. Dig Dis Sci 33(10): 1322–1328. [DOI] [PubMed] [Google Scholar]
- 8. Mazumdar S, Das KM (1992) Immunocytochemical localization of vasoactive intestinal peptide and substance P in the colon from normal subjects and patients with inflammatory bowel disease. Am J Gastroenterol 87(2): 176–181. [PubMed] [Google Scholar]
- 9. Tari A, Teshima H, Sumii K, Haruma K, Ohgoshi H, et al. (1988) Peptide YY abnormalities in patients with ulcerative colitis. Jpn J Med 27(1): 49–55. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe T, Kubota Y, Sawada T, Muto T (1992) Distribution and quantification of somatostatin in inflammatory disease. Dis Colon Rectum 35(5): 488–494. [DOI] [PubMed] [Google Scholar]
- 11.Moran GW, Pennock J, McLaughlin JT (2012) Enteroendocrine cells in terminal ileal Crohn's disease. J Crohns Colitis 6(9): Available: http://www.sciencedirect.com/science/article/pii/S1873994612000190. Accessed 2013 Nov 18. [DOI] [PubMed]
- 12. Sakiyama T, Fujita H, Tsubouchi H (2008) Autoantibodies against ubiquitination factor E4A (UBE4A) are associated with severity of Crohn's disease. Inflamm Bowel Dis 14(3): 310–317. [DOI] [PubMed] [Google Scholar]
- 13. Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, et al. (2007) Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39(5): 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hougaard DM, Larsson LI (2004) Carboxypeptidase E in rat antropyloric mucosa: distribution in progenitor and mature endocrine cell types. Histochem Cell Biol 121(1): 55–61. [DOI] [PubMed] [Google Scholar]
- 15. Sina C, Arlt A, Gavrilova O, Midtling E, Kruse ML, et al. (2010) Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: role for gly96/iex-1 in the regulation of NF-kappaB. Inflamm Bowel Dis 16(2): 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siegmund B, Lehr HA, Fantuzzi G, Dinarello CA (2001) IL-1-converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci USA 98(23): 13249–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becker C, Fantini MC, Neurath MF (2006) High resolution colonoscopy in live mice. Nat Protoc 1(6): 2900–2904. [DOI] [PubMed] [Google Scholar]
- 18. Brubaker PL, Drucker DJ, Asa SL, Greenberg GR (1991) Regulation of peptide-YY synthesis and secretion in fetal rat intestinal cultures. Endocrinology 129(6): 3351–3358. [DOI] [PubMed] [Google Scholar]
- 19. Vidal K, Grosjean I, Evillard JP, Gespach C, Kaiserlian D (1993) Immortalization of mouse intestinal epithelial cells by the SV40-large T gene. Phenotypic and immune characterization of the MODE-K cell line. J Immunol Methods 166(1): 63–73. [DOI] [PubMed] [Google Scholar]
- 20. Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, et al. (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 18(9): 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brakch N, Rist B, Beck-Sickinger AG, Goenaga J, Wittek R, et al. (1997) Role of prohormone convertases in pro-neuropeptide Y processing: coexpression and in vitro kinetic investigations. Biochemistry 36(51): 16309–16320. [DOI] [PubMed] [Google Scholar]
- 22. Funkelstein L, Toneff T, Hwang SR, Reinheckel T, Peters C, et al. (2008) Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem 106(1): 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paquet L, Massie B, Mains RE (1996) Proneuropeptide Y processing in large dense-core vesicles: manipulation of prohormone convertase expression in sympathetic neurons using adenoviruses. J Neurosci 16(3): 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giusti-Paiva A, Elias LL, Antunes-Rodrigues J (2005) Inhibitory effect of gaseous neuromodulators in vasopressin and oxytocin release induced by endotoxin in rats. Neurosci Lett 381(3): 320–324. [DOI] [PubMed] [Google Scholar]
- 25.Singer K, Morris DL, Oatmen KE, Wang T, DelProposto J, et al. (2013) Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PLoS One 8(3): Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%1372Fjournal.pone.0057929;jsessionid=0059088ED0057929AE0057924EE0057025E0057314E0059541AC0057926E0057073D. Accessed 2013 Nov 05. [DOI] [PMC free article] [PubMed]
- 26.Worthington JJ, Samuelson LC, Grencis RK, McLaughlin JT (2013) Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PLoS Pathog 9: Available: http://www.plospathogens.org/article/info%3Adoi%2F10.1371%1372Fjournal.ppat.1003122. Accessed 2013 Nov 10. [DOI] [PMC free article] [PubMed]
- 27. Dickerson C, Undem B, Bullock B, Winchurch RA (1998) Neuropeptide regulation of proinflammatory cytokine responses. J Leukoc Biol 63(5): 602–605. [DOI] [PubMed] [Google Scholar]
- 28.Levite M, Chowers Y (2001) Nerve-driven immunity: neuropeptides regulate cytokine secretion of T cells and intestinal epithelial cells in a direct, powerful and contextual manner. Ann Oncol (Suppl. 2): 19–25. [DOI] [PubMed]
- 29. Lotz M, Vaughan JH, Carson DA (1988) Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 241(4870): 1218–1221. [DOI] [PubMed] [Google Scholar]
- 30. Krueger J, Ray A, Tamm I, Sehgal PB (1991) Expression and function of interleukin-6 in epithelial cells. J Cell Biochem 45(4): 327–334. [DOI] [PubMed] [Google Scholar]
- 31. Parikh AA, Salzman AL, Kane CD, Fischer JE, Hasselgren PO (1997) IL-6 production in human intestinal epithelial cells following stimulation with IL-1 beta is associated with activation of the transcription factor NF-kappa B. J Surg Res 69(1): 139–144. [DOI] [PubMed] [Google Scholar]
- 32. Song F, Ito K, Denning TL, Kuninger D, Papaconstantinou J, et al. (1999) Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol 162(4): 2275–2280. [PubMed] [Google Scholar]
- 33. Bures EJ, Courchesne PL, Douglass J, Chen K, Davis MT, et al. (2001) Identification of incompletely processed potential carboxypeptidase E substrates from CpEfat/CpEfat mice. Proteomics 1(1): 79–92. [DOI] [PubMed] [Google Scholar]
- 34. Cool DR, Loh YP (1998) Carboxypeptidase E is a sorting receptor for prohormones: Binding and kinetic studies. Mol Cell Endocrinol 139: 7–13. [DOI] [PubMed] [Google Scholar]
- 35. Cool DR, Normant E, Shen F, Chen HC, Pannell L, et al. (1997) Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88(1): 73–83. [DOI] [PubMed] [Google Scholar]
- 36. Selleri S, Palazzo M, Deola S, Wang E, Balsari A, et al. (2008) Induction of pro-inflammatory programs in enteroendocrine cells by the Toll-like receptor agonists flagellin and bacterial LPS. Int Immunol 20(8): 961–970. [DOI] [PubMed] [Google Scholar]
- 37. Wheway J, Mackay CR, Newton RA, Sainsbury A, Boey D, et al. (2005) A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med 202(11): 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Curfs JH, Meis JF, Hoogkamp-Korstanje JA (1997) A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev 10(4): 742–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eckmann L, Kagnoff MF, Fierer J (1993) Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun 61(11): 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andres PG, Beck PL, Mizoguchi E, Mizoguchi A, Bhan AK, et al. (2000) Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol 164(12): 6303–6312. [DOI] [PubMed] [Google Scholar]
- 41. Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, et al. (2006) Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World J Gastroenterol 12(2): 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sina C, Gavrilova O, Förster M, Till A, Derer S, et al. (2009) G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol 183(11): 7514–7522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Determination of expression levels of different neuropetides in colonic punch biopsies by real time RT-PCR at baseline. n = 6 per genotype. (B-E) Immunofluorescence staining of colonic biopsies from cpe+/+ and cpe−/− mice for CD3, F4/80 and Ly6G (B) and quantification of immune cells in colonic biopsies by counting CD3 (C), F4/80 (D) and Ly6G (E) positive cells per high power field (magnification 40x). 5 random HPF per animal, n = 6 per genotype. (F) IL-6 transcript levels produced in MODE-K cells after incubation with forskolin-stimulated supernatants of of cpe +/+ and cpe −/− mice and LPS together with recombinant NPY +/− PYY (1 µM/ml). IL-6 expression levels are expressed in percent of the Mean of cpe+/+. *p<0.05; ns = not significant, by t-test.
(JPG)