Abstract
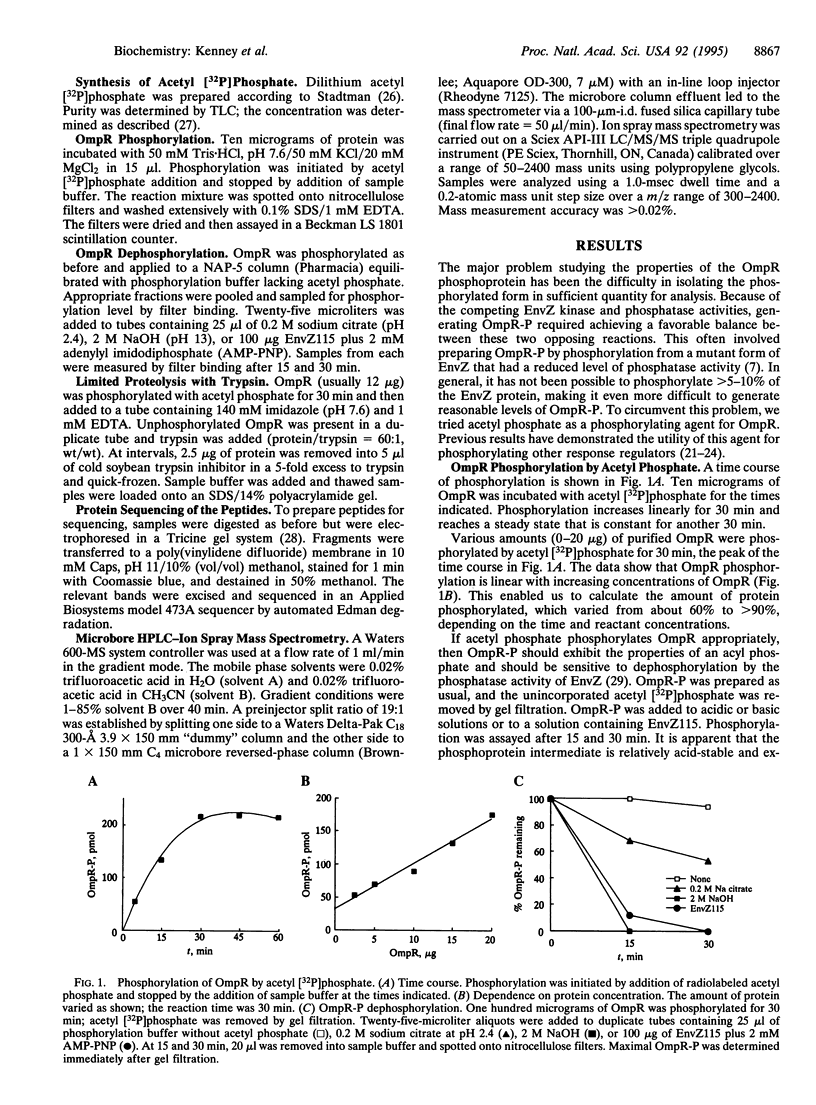
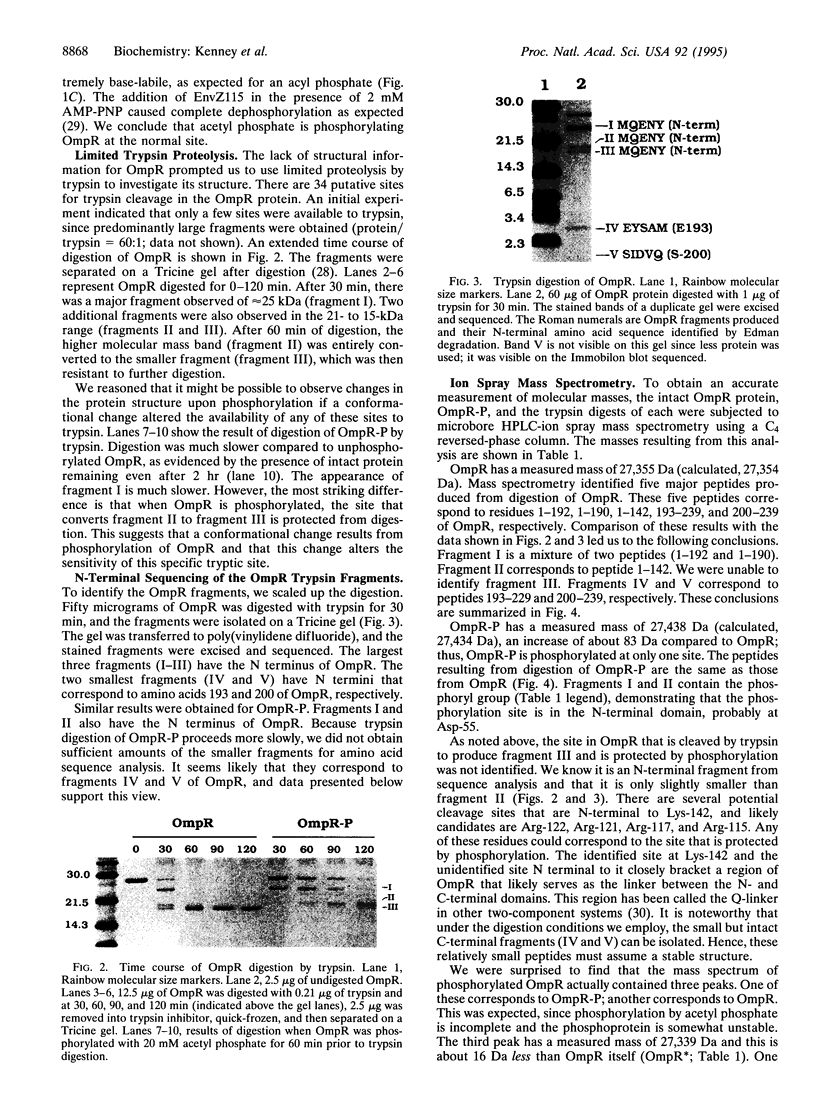
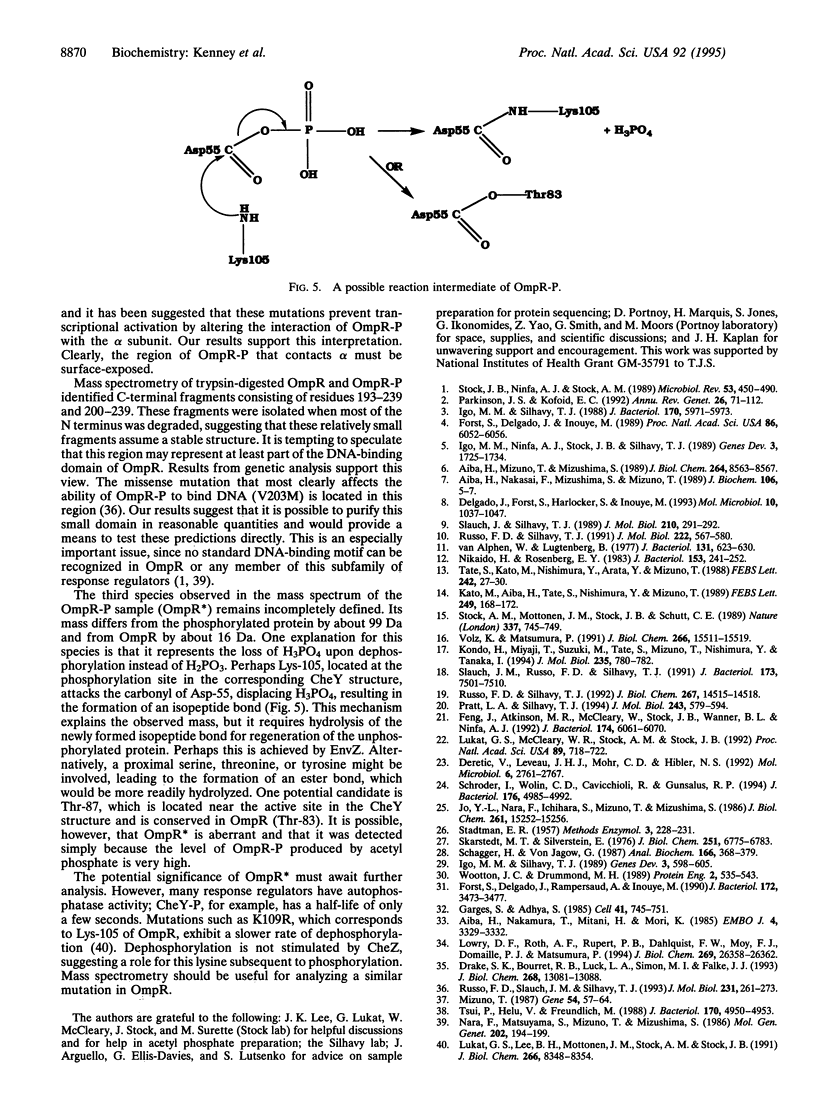
Osmoregulated porin gene expression in Escherichia coli is controlled by the two-component regulatory system EnvZ and OmpR. EnvZ, the osmosensor, is an inner membrane protein and a histidine kinase. EnvZ phosphorylates OmpR, a cytoplasmic DNA-binding protein, on an aspartyl residue. Phospho-OmpR binds to the promoters of the porin genes to regulate the expression of ompF and ompC. We describe the use of limited proteolysis by trypsin and ion spray mass spectrometry to characterize phospho-OmpR and the conformational changes that occur upon phosphorylation. Our results are consistent with a two-domain structure for OmpR, an N-terminal phosphorylation domain joined to a C-terminal DNA-binding domain by a flexible linker region. In the presence of acetyl phosphate, OmpR is phosphorylated at only one site. Phosphorylation induces a conformational change that is transmitted to the C-terminal domain via the central linker. Previous genetic analysis identified a region in the C-terminal domain that is required for transcriptional activation. Our results indicate that this region is within a surface-exposed loop. We propose that this loop contacts the alpha subunit of RNA polymerase to activate transcription. Mass spectrometry also reveals an unusual dephosphorylated form of OmpR, the potential significance of which is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mizuno T., Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989 May 25;264(15):8563–8567. [PubMed] [Google Scholar]
- Aiba H., Nakamura T., Mitani H., Mori H. Mutations that alter the allosteric nature of cAMP receptor protein of Escherichia coli. EMBO J. 1985 Dec 1;4(12):3329–3332. doi: 10.1002/j.1460-2075.1985.tb04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J Biochem. 1989 Jul;106(1):5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado J., Forst S., Harlocker S., Inouye M. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol Microbiol. 1993 Dec;10(5):1037–1047. doi: 10.1111/j.1365-2958.1993.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Deretic V., Leveau J. H., Mohr C. D., Hibler N. S. In vitro phosphorylation of AlgR, a regulator of mucoidy in Pseudomonas aeruginosa, by a histidine protein kinase and effects of small phospho-donor molecules. Mol Microbiol. 1992 Oct;6(19):2761–2767. doi: 10.1111/j.1365-2958.1992.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Drake S. K., Bourret R. B., Luck L. A., Simon M. I., Falke J. J. Activation of the phosphosignaling protein CheY. I. Analysis of the phosphorylated conformation by 19F NMR and protein engineering. J Biol Chem. 1993 Jun 25;268(18):13081–13088. [PMC free article] [PubMed] [Google Scholar]
- Feng J., Atkinson M. R., McCleary W., Stock J. B., Wanner B. L., Ninfa A. J. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Rampersaud A., Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Silhavy T. J. EnvZ, a transmembrane environmental sensor of Escherichia coli K-12, is phosphorylated in vitro. J Bacteriol. 1988 Dec;170(12):5971–5973. doi: 10.1128/jb.170.12.5971-5973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y. L., Nara F., Ichihara S., Mizuno T., Mizushima S. Purification and characterization of the OmpR protein, a positive regulator involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1986 Nov 15;261(32):15252–15256. [PubMed] [Google Scholar]
- Kato M., Aiba H., Tate S., Nishimura Y., Mizuno T. Location of phosphorylation site and DNA-binding site of a positive regulator, OmpR, involved in activation of the osmoregulatory genes of Escherichia coli. FEBS Lett. 1989 Jun 5;249(2):168–172. doi: 10.1016/0014-5793(89)80617-9. [DOI] [PubMed] [Google Scholar]
- Kondo H., Miyaji T., Suzuki M., Tate S., Mizuno T., Nishimura Y., Tanaka I. Crystallization and X-ray studies of the DNA-binding domain of OmpR protein, a positive regulator involved in activation of osmoregulatory genes in Escherichia coli. J Mol Biol. 1994 Jan 14;235(2):780–782. doi: 10.1006/jmbi.1994.1032. [DOI] [PubMed] [Google Scholar]
- Lowry D. F., Roth A. F., Rupert P. B., Dahlquist F. W., Moy F. J., Domaille P. J., Matsumura P. Signal transduction in chemotaxis. A propagating conformation change upon phosphorylation of CheY. J Biol Chem. 1994 Oct 21;269(42):26358–26362. [PubMed] [Google Scholar]
- Lukat G. S., Lee B. H., Mottonen J. M., Stock A. M., Stock J. B. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J Biol Chem. 1991 May 5;266(13):8348–8354. [PubMed] [Google Scholar]
- Lukat G. S., McCleary W. R., Stock A. M., Stock J. B. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T. Static bend of DNA helix at the activator recognition site of the ompF promoter in Escherichia coli. Gene. 1987;54(1):57–64. doi: 10.1016/0378-1119(87)90347-7. [DOI] [PubMed] [Google Scholar]
- Nara F., Matsuyama S., Mizuno T., Mizushima S. Molecular analysis of mutant ompR genes exhibiting different phenotypes as to osmoregulation of the ompF and ompC genes of Escherichia coli. Mol Gen Genet. 1986 Feb;202(2):194–199. doi: 10.1007/BF00331636. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Pratt L. A., Silhavy T. J. OmpR mutants specifically defective for transcriptional activation. J Mol Biol. 1994 Nov 4;243(4):579–594. doi: 10.1016/0022-2836(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Russo F. D., Silhavy T. J. Alpha: the Cinderella subunit of RNA polymerase. J Biol Chem. 1992 Jul 25;267(21):14515–14518. [PubMed] [Google Scholar]
- Russo F. D., Silhavy T. J. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991 Dec 5;222(3):567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- Russo F. D., Slauch J. M., Silhavy T. J. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J Mol Biol. 1993 May 20;231(2):261–273. doi: 10.1006/jmbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- Schröder I., Wolin C. D., Cavicchioli R., Gunsalus R. P. Phosphorylation and dephosphorylation of the NarQ, NarX, and NarL proteins of the nitrate-dependent two-component regulatory system of Escherichia coli. J Bacteriol. 1994 Aug;176(16):4985–4992. doi: 10.1128/jb.176.16.4985-4992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Skarstedt M. T., Silverstein E. Escherichia coli acetate kinase mechanism studied by net initial rate, equilibrium, and independent isotopic exchange kinetics. J Biol Chem. 1976 Nov 10;251(21):6775–6783. [PubMed] [Google Scholar]
- Slauch J. M., Russo F. D., Silhavy T. J. Suppressor mutations in rpoA suggest that OmpR controls transcription by direct interaction with the alpha subunit of RNA polymerase. J Bacteriol. 1991 Dec;173(23):7501–7510. doi: 10.1128/jb.173.23.7501-7510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Stock A. M., Mottonen J. M., Stock J. B., Schutt C. E. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989 Feb 23;337(6209):745–749. doi: 10.1038/337745a0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S., Kato M., Nishimura Y., Arata Y., Mizuno T. Location of DNA-binding segment of a positive regulator, OmpR, involved in activation of the ompF and ompC genes of Escherichia coli. FEBS Lett. 1988 Dec 19;242(1):27–30. doi: 10.1016/0014-5793(88)80978-5. [DOI] [PubMed] [Google Scholar]
- Tsui P., Helu V., Freundlich M. Altered osmoregulation of ompF in integration host factor mutants of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4950–4953. doi: 10.1128/jb.170.10.4950-4953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K., Matsumura P. Crystal structure of Escherichia coli CheY refined at 1.7-A resolution. J Biol Chem. 1991 Aug 15;266(23):15511–15519. doi: 10.2210/pdb3chy/pdb. [DOI] [PubMed] [Google Scholar]
- Wootton J. C., Drummond M. H. The Q-linker: a class of interdomain sequences found in bacterial multidomain regulatory proteins. Protein Eng. 1989 May;2(7):535–543. doi: 10.1093/protein/2.7.535. [DOI] [PubMed] [Google Scholar]




