Since the observation that cholesterol-lowering statins could induce T cell immune modulation and reverse paralysis in mice with experimental autoimmune encephalomyelitis (EAE) 1, there has been substantial interest in determining whether this class of medications may be beneficial in MS therapy. One question, which has not yet been resolved, is whether a statin used alone is beneficial in MS therapy. More energy has been devoted to determining whether a statin can provide added benefit when combined with IFNβ, the most commonly prescribed class of medication in MS therapy. Results from trials have been mixed 2–5. While one study demonstrated that the combination of a statin with IFNβ was antagonistic 2, other small studies have suggested that there could be a benefit 4, 5.
In this issue, Sorensen and colleagues tested whether oral simvastatin could augment the benefit of intramuscular (IM) IFNβ-1a in MS therapy 6. A total of 307 treatment-naive patients with relapsing-remitting MS (RRMS) were enrolled in the SIMCOMBIN trial, representing the largest study-to-date which has evaluated the combination of a statin with IFNβ in MS. After a three-month run-in period with weekly IM IFNβ-1a, patients were randomized to receive simvastatin 80 mg (n=151) or placebo (n=156) in addition to IFNβ, then followed for clinical exacerbations and development of demyelinating lesions on brain MRI for one to three years. Although the combination of simvastatin and IM IFNβ-1a was tolerated, at 12 months no significant difference was observed in annualized relapse rate, the measure used for the primary endpoint, or in development of new or enlarging T2 brain MRI lesions. Not only was there a lack of benefit, again, the results raised concern that there may be some level of antagonism when combining a statin and IFNβ in MS treatment.
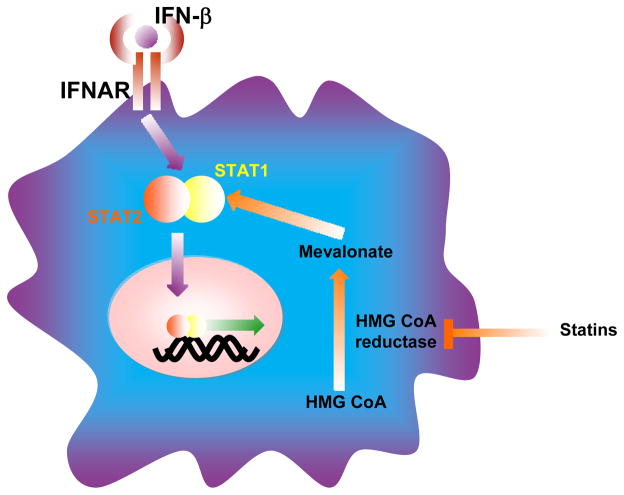
IFNβ binds to the type I IFN receptor (IFNAR) expressed on nearly all cells, which leads to activation of signal transducers and activators of transcription (STAT) 1 and STAT2, the initial steps required in the biochemical cascade leading to immune activation and regulation by these medications (see Figure). By inhibiting the HMG CoA reductase, statins inhibit synthesis of molecular intermediates that can block the activation of STAT1. Thus, opposing effects on STAT1 represent one point for potential antagonism. Here, it may have been helpful to have first conducted pre-clinical evaluation of the combination of an IFNβ and a statin. In this regard, when tested in EAE, statins have enhanced the activity of other agents 7, 8. For example, atorvastatin enhanced the clinical activity of glatiramer acetate (GA) in EAE, providing support to test this combination in MS. Interestingly, GA, like statins, inhibits STAT1 activation in myeloid cells 9.
Figure.
A molecular intersection where cholesterol-lowering statins may oppose IFNβ. Binding of IFNβ to the cell surface type I IFN receptor (IFNAR) leads to activation (phosphorylation) of signal transducers and activators of transcription (STAT) 1 and STAT2, which then form heterodimers that enter the nucleus where they activate transcription of genes involved in immune modulation, anti-viral and anti-proliferative responses. Statins passively enter cells, bind and inhibit their target HMG-CoA reductase from generating mevalonate and its isoprenoid derivatives that serve as building blocks in cholesterol synthesis. The attachment of isoprenoids to small GTP binding proteins (ras, rho and rac) is also necessary for these molecules to activate other proteins involved in cellular function and immunity. Statins can also inhibit expression or activation of STAT1, providing a focus for potential antagonism between IFNβ and a statin.
Clinical MS studies have now tested different statins and preparations of IFNβ preparations in combination. Just as individual IFNβ preparations differ in pharmacologic characteristics, statins vary in their capability to reduce cholesterol, and may differ in the potential immune modulatory capabilities. Thus, each combination may not be the same. Antagonism was observed when atorvastatin, a potent statin, was tested high dose subcutaneous IFNβ-1a 2. When simvastatin, which does not reduce cholesterol as efficiently as atorvastatin, was tested in combination with IM IFNβ, a low dose IFNβ in SIMCOMBIN, no benefit was observed. Of the studies to evaluate whether a statin should be combined with IFNβ, SIMCOMBIN is the only one to yield class I evidence. The authors concluded that the combination of IFNβ and statins should not be used as a treatment for RRMS.
We are now beginning to learn in whom IFNβ works and in whom it may actually worsen disease. We are starting to unravel who might be a responder and who will be a non-responder to various therapies for RRMS, with predictive biomarkers7 and characteristic patterns on imaging. As predictive medicine becomes a standard for choice of drugs, it will be worthwhile to examine potential synergies between drugs approved for MS, to see whether inexpensive drugs like statins approved for other conditions, can enhance efficacy in responder populations. Such studies testing combinations of drugs, can be first validated in pre-clinical experimental models7,10.
One ought not be too freewheeling combining medications in MS. Given the lack of benefit, and the potential for antagonism, one may wish to think twice before adding a statin to IFNβ in MS treatment.
Contributor Information
Scott S. Zamvil, Email: zamvil@ucsf.neuroimmunol.org, Department of Neurology and Program in Immunology, University of California, San Francisco, San Francisco, CA
Lawrence Steinman, Department of Neurology and Neurological Sciences and Interdepartmental Program in Immunology, Stanford University, Stanford, CA.
References
- 1.Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420(6911):78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum G, Cree B, Altafullah I, Zinser M, Reder AT. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology. 2008 doi: 10.1212/01.wnl.0000319698.40024.1c. [DOI] [PubMed] [Google Scholar]
- 3.Paul F, Waiczies S, Wuerfel J, Bellmann-Strobl J, Dorr J, Waiczies H, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PLoS ONE. 2008;3(4):e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Togha M, Karvigh SA, Nabavi M, Moghadam NB, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Multiple sclerosis. 2010;16(7):848–54. doi: 10.1177/1352458510369147. [DOI] [PubMed] [Google Scholar]
- 5.Lanzillo R, Orefice G, Quarantelli M, Rinaldi C, Prinster A, Ventrella G, et al. Atorvastatin combined to interferon to verify the efficacy (ACTIVE) in relapsing-remitting active multiple sclerosis patients: a longitudinal controlled trial of combination therapy. Multiple sclerosis. 2010;16(4):450–4. doi: 10.1177/1352458509358909. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen PS, zlycke E, Eralinna J-P, Edland A, Wu X, Frederiksen JL, et al. No beneficial effect of simvastatin as add-on threrapy to IM IFNβ-1a for relapsing-remitting MS: a placebo-controlled randomised study (SIMCOMBIN Study) Lancet Neurology. 2011 doi: 10.1016/S1474-4422(11)70144-2. [DOI] [PubMed] [Google Scholar]
- 7.Stuve O, Youssef S, Weber MS, Nessler S, von Budingen HC, Hemmer B, et al. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116(4):1037–44. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paintlia AS, Paintlia MK, Singh I, Singh AK. Immunomodulatory effect of combination therapy with lovastatin and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside alleviates neurodegeneration in experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169(3):1012–25. doi: 10.2353/ajpath.2006.051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, et al. Type II monocytes modulate T cell-mediated central nervous sys tem autoimmune disease. Nat Med. 2007;13(8):935–43. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 10.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nature medicine. 2010;16(4):406–12. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]