Abstract
The concept of cognitive reserve emerged from observed disparities between brain pathology and clinical symptoms. It may explain better neuropsychological performance in healthy individuals. The objectives of this study were to measure reserve in healthy subjects using a new Cognitive Reserve Scale (CRS), analyze the internal consistency of the CRS, and analyze validity evidence. A total of 117 healthy individuals were divided into two groups: 87 adults (aged 18–64 years) and 30 elderly adults (≥65 years). All subjects completed the CRS and a comprehensive neuropsychological battery. The internal consistency of the scale was satisfactory (α = 0.77). No significant differences were observed between genders (t = 0.51, p = 0.611), and age was corrected by averaging the CRS score. The study of validity evidence showed that education affected the CRS (t = −2.98, p = 0.004, partial h2 = 0.07) and there was no significant relationship between the CRS and IQ (r = 0.09, p = 0.33). Occupational attainment and the CRS were not related (F2,116 = 0.11, p = 0.898). In line with previous studies on reserve, heterogeneity was observed in the analyses of relationships between the CRS and cognitive performance. There were significant relationships between CRS score and the Verbal Learning Spanish–Complutense Test last trial (r = 0.24, p = 0.009), sum (r = 0.32, p = 0.000), short-term (r = 0.29, p = 0.002) and long-term memory (r = 0.22, p = 0.018), Matrix Reasoning subtest (r = 0.20, p = 0.027) and Block Design subtest (r = 0.20, p = 0.029). No other neuropsychological variables correlated with the CRS (p>0.05). The CRS is a reliable instrument that reflects the frequency of participation in brain-stimulating activities across the lifetime. The associations between the CRS and education and neuropsychological performance support validity evidence.
Introduction
The concept of reserve emerged from the lack of correlation between brain pathology and clinical symptoms. The reserve theory postulates that individuals with a greater reserve will cope with brain damage more successfully than those with low levels of reserve [1]. Reserve provides protection that contributes toward delaying age-related changes and clinical symptoms related to underlying neuropathological processes, such as Alzheimer's disease [2]–[6]. However, this protection does not prevent some symptoms appearing in the long term [7].
In recent decades, the number of studies on reserve has increased notably [8]–[15]. The interest that reserve initially aroused in the field of Alzheimer's disease [1], [16] has spread to a wide variety of studies related to healthy aging, multiple sclerosis, mild cognitive impairment, heart failure and schizophrenia [17]–[21].
The reserve theory distinguishes between two models: cerebral reserve and cognitive reserve (CR). The passive or cerebral reserve model postulates that brain pathology can accumulate to a critical threshold at which symptoms appear [22]. The active or cognitive reserve model, from the perspective of cerebral plasticity, postulates that the brain has the capacity to cope with damage through compensatory mechanisms, or through flexible and adaptive networks [1]. The active model has repercussions for healthy people. Individuals with a higher CR may have more efficient networks, which would allow them to achieve better performance on cognitive tasks than individuals with a lower CR [1], [23]. Some authors have suggested studying the passive and active models in combination, because they complement each other [13], [24]–[27].
In the passive model, cerebral reserve is mainly quantified by brain size or intracranial volume and by head circumference [26]–[28]. In contrast, in the active model, years of education and premorbid intelligence quotient (IQ) are used as CR proxies. IQ is usually measured by the Vocabulary subtest of the Wechsler Adult Intelligence Scale [13], [23], [29]–[31] or by reading tests such as the National Adult Reading Test [30], [32]–[35]. Educational attainment either takes into account the total number of years of education or is classified into high and low educational levels [11], [17], [28], [36], [37]. Occupational attainment is another commonly used proxy of CR [8], [14], [38].
CR may be attained through an active cognitive lifestyle, which involves engaging in cognitively stimulating activities. Participation in intellectual, social, physical or leisure activities contributes toward delaying or attenuating symptoms related to brain damage and reduces the risk of dementia [2], [39]–[43]. The methodological instruments used in CR studies, such as the Cognitive Activities Scale [40], Activities Scale [9] or Lifetime of Experiences Questionnaire [42], have influenced the development of the Cognitive Reserve Questionnaire [44], Cognitive Reserve Index Questionnaire [45] and Cognitive Reserve Scale (CRS) [46]. In 2013, a new measure of premorbid cognitive abilities in subjects with low educational attainment, the Premorbid Cognitive Abilities Scale, was suggested as another proxy of CR [47].
Psychometric analyses of questionnaires and scales on CR, along with assessment of their reliability and validity evidence [40], [42], [45], [46], have provided evidence for their application. Significant statistical correlations have been observed between these CR proxies and a decline in cognitive domains, which reflects the relevance of such research studies, although they do not seem to follow any clear or consistent pattern [40], [42]. Indeed, the theory of CR implies that this construct enables coping with challenges in general, and not only on a specific cognitive domain [23]. In addition, reserve is a hypothetical construct that cannot be measured directly; therefore, its measurement presents a challenge to the scientific community [28], [48].
This study was based on the active or CR model and its influence on healthy individuals [1], [23]. The CRS is a new instrument that can be used to obtain a measure of reserve focused on accounts of participation in stimulating activities throughout life. Based on previous studies, it was hypothesized that: (i) participants with more years of education would have higher CRS scores; (ii) occupational attainment would influence the CRS score; (iii) IQ and the CRS would not be associated; and (iv) relationships between neuropsychological scores and the CRS score would be heterogeneous, with significant relations expected between CRS score and memory tasks.
The overall aim was to study the psychometric properties of the CRS and support CR studies based on cognitively stimulating activities across the lifetime.
Materials and Methods
Ethics statement
This study was approved by the Ethics Committee of the University of Almeria, and conducted in compliance with the Declaration of Helsinki and Spanish legislation on personal data protection. All subjects were volunteers and they provided written consent.
Subjects
Participants were recruited from social clubs, social centers, entertainment centers and the University of Almeria. The sample (n = 154) was split into two age groups according to the study design: adults (aged 36–64 years) and elderly adults (≥65 years). The traditional Spanish retirement age (65 years) determined the classification of the two age groups in this study. Individuals were excluded from the analysis if they had a history of psychiatric or neurological illness, drug consumption or head injury, or if they were non-native Spanish. Elderly adults (≥65 years) were also excluded if they gained a score of 27 or lower on the Spanish adaptation of the Mini-Mental State Examination [49], Mini-Examen Cognoscitivo [50]. Following these criteria, and after removing subjects who decided not to complete the study, 37 participants (24%) were excluded. This affected the gender distribution in both age groups. The remaining sample of 117 subjects comprised 87 adults (mean ± SD age = 48.76±0.758 years), 54 (62.1%) of whom were women, and 30 elderly adults (age 72.9±1.102 years), 22 (73.3%) of whom were women. Table 1 shows the sociodemographic characteristics of the participants.
Table 1. Sociodemographic characteristics of the study participants (N = 117).
| n | % | |
| Gender | ||
| Male | 41 | 35.0 |
| Female | 76 | 65.0 |
| Age group | ||
| Adults (36–64 years) | 87 | 74.4 |
| Elderly adults (≥65 years) | 30 | 25.6 |
| Educational attainment | ||
| High (>8 years) | 29 | 24.8 |
| Low (≤8 years) | 88 | 75.2 |
| Occupational attainment | ||
| High | 19 | 16.2 |
| Medium | 36 | 30.8 |
| Low | 62 | 53.0 |
Educational and occupational attainment
Educational attainment was stratified into high level (>8 years of education) and low level (≤8 years of education) [2]. Regarding occupational attainment, each subject's primary occupation was recorded using the Spanish National Classification of Occupations (Clasificación Nacional de Ocupaciones) [51] and stratified into high, medium and low levels following similar classifications used in previous studies on CR [3], [24]. High level occupations included managers, scientific and intellectual technicians and professionals; medium level included clerks, accountants and related professionals, and professionals in the armed forces; and low level included sales agents and customer service employees, skilled workers in the agricultural, forestry and fishery industry, workers in crafts and related trades, plant and machine operators, elementary occupations and home-makers (Table 1).
Cognitive Reserve Scale
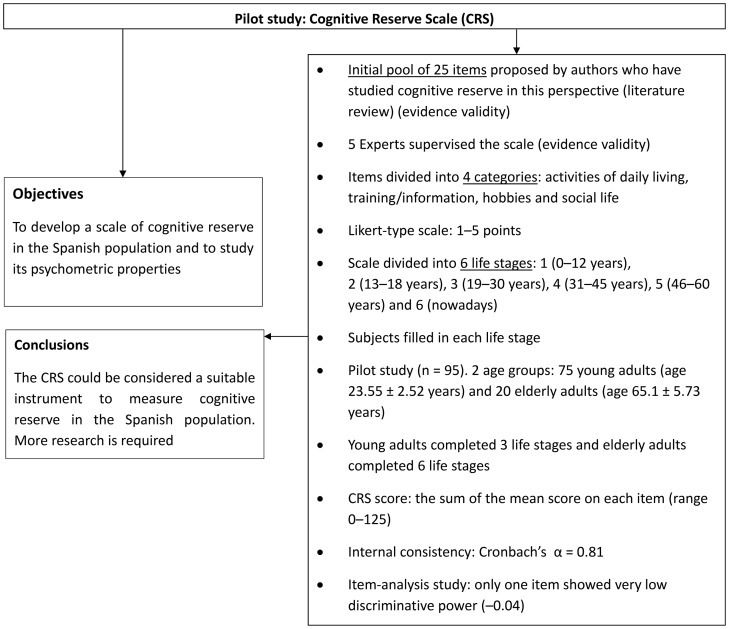
The CRS is a new test that measures participation in cognitively stimulating activities throughout a person's lifetime [46]. A pilot study on the CRS is summarized in Figure 1. There were 24 items in total and subjects completed each one several times, according to their age, because the CRS was divided into three different life stages (Figure 2).
Figure 1. Flow diagram of the pilot study of the Cognitive Reserve Scale (CRS) (León et al., 2011) [46].
Figure 2. The three life stages included in the Cognitive Reserve Scale (CRS).
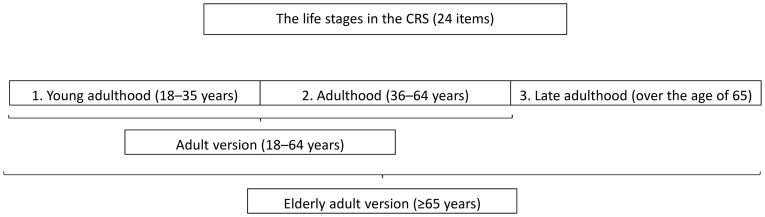
The specific age cut-offs for the life stages were established according to the results of previous studies on CR and stimulating cognitive activities throughout the lifetime [42], [46]. Items included a diverse variety of activities such as reading, playing a musical instrument, collecting things, practicing other language or dialects, traveling or taking part in sport. These activities have been proposed by authors studying CR from this perspective. The CRS was divided into four categories: activities of daily living, training–information, hobbies and social life. Table 2 shows examples of translated items in each category of the scale. The CRS is available upon request from the authors.
Table 2. Examples of translated items on the Cognitive Reserve Scale (CRS).
| Category | Example items |
| Activities of daily living | Controlling my own life (e.g. what to wear each day, hotel bookings, doctor's appointments) |
| Controlling financial matters at home (e.g. bills, mortgage) | |
| Training/information | Taking a course (e.g. language, Internet use) |
| Speaking a non-native language or dialect | |
| Hobbies | Reading (e.g. newspapers, magazines, books) |
| Playing games (e.g. crosswords, sudoku, cards, draughts, chess) | |
| Writing for pleasure (e.g. letters, personal diary) | |
| Listening to music or watching television | |
| Playing a musical instrument (e.g. guitar, flute) | |
| Collecting things (e.g. stamps, coins) | |
| Social life | Visiting relatives, friends, neighbors, etc. |
| Volunteering, going to church, etc. |
Subjects completed each item several times according to their age (Figure 2). A Likert-type scale of 0–4 points was used and the total CRS score was the sum of the mean score on each item (24 items). It is important to note that this mean score on each item corrected for the possible effect of the additional period, called late adulthood, for elderly adults. As a result, the CRS gave scores varying from 0 to 96, with higher CRS scores indicating more frequent participation.
Neuropsychological assessment
Six cognitive domains from different subtests of the Wechsler Adult Intelligence Scale [29] and other neuropsychological tests were evaluated. Each cognitive domain included a minimum of two tests: (1) memory was assessed with the Verbal Learning Spanish–Complutense Test (TAVEC) [52] and Rey–Osterrieth Complex Figure (ROCF) [53]–[55]; (2) working memory was tested with the Digit Span subtest (backward) [29], [56], [57] and Corsi's Block-Tapping test (backward) [56], [57]; (3) attention was evaluated with the Digit Span subtest (forward) [29], [56], [57], Corsi's Block-Tapping test (forward) [56], [57] and the Stroop test [58]–[60]; (4) executive functions were assessed with the FAS test and Animals [61]–[63] and the Matrix Reasoning subtest [29]; (5) visuoconstruction and visuoperception were tested with the Block Design subtest [29] and the copy of ROCF [53]–[55]; and (6) processing speed (seconds) was evaluated with the copy of ROCF and the Trail Making Test (parts A and B) [53]–[57]. In addition, the Vocabulary subtest was applied to obtain an IQ score [29]. The direct scores of all neuropsychological tests were converted to standard scores adjusted for age and educational level (scale scores, z-scores or percentile scores) following normative studies in the Spanish population. The exception was the FAS test, which followed a normative study in an English population which was stratified for age and years of education [61].
Elderly adults (≥65 years) also completed the Spanish version of Mini-Mental State [49], Mini-Examen Cognoscitivo [50], which includes items about abstract reasoning and working memory. A trained psychologist performed the assessments.
Statistical analysis
A descriptive analysis of the distribution of total CRS scores was carried out for the whole healthy sample. The internal coherence of the scale was estimated using Cronbach's alpha test. The t-test was used to study the effects of gender and age on the CRS score. The possible effect of age was corrected by averaging the CRS score, and the t-test was also used to support the lack of effect of age on the score. To verify whether educational attainment (low/high) affected the CRS score, the t-test was used. One-way analysis of variance (ANOVA) was used to examine the influence of occupational attainment on the CRS. Relationships between the total CRS score and IQ, and the total CRS score and cognitive tests, were evaluated with Pearson's correlations. Analyses were carried out with the statistical package IBM-SPSS (version 20.0 for Windows). Results with p<0.05 were considered statistically significant.
Results
Cognitive Reserve Scale scores
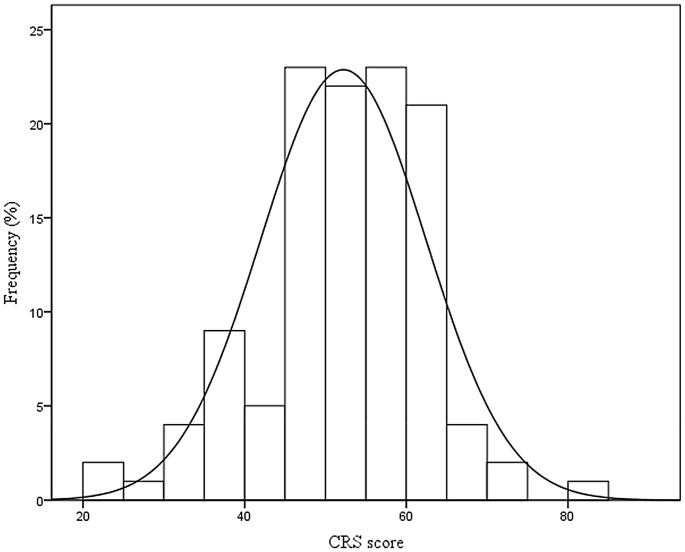
A composite measure of frequency of activities on the CRS (24 items) across the three life stages (young adulthood, adulthood and late adulthood) was formed by computing the sum of the mean score on each item (mean ± SD = 52.22±10.30). Subjects completed each item several times according to their age (Figure 2). There were no missing values on any items of the CRS. The CRS generated scores varying from 0 to 96, with higher scores indicating more frequent participation. Thus, having a more active lifestyle was interpreted as having more CR. The Kolmogorov–Smirnov test gave a non-significant result (p = 0.201), suggesting normality of the distribution. The distribution of the CRS scores and the percentile breakdown for all participants are presented in Figure 3 and Table 3, respectively. The analysis revealed that there were no significant differences in the CRS score between men and women (t = 0.51, p = 0.611) and that age (adults versus elderly adults) did not affect the CRS (t = 0.87, p = 0.384).
Figure 3. Distribution of Cognitive Reserve Scale (CRS) scores for all participants.
Table 3. Percentiles for the total Cognitive Reserve Scale (CRS) score.
| Percentile | Total CRS score |
| 95 | 67.03 |
| 90 | 63.50 |
| 75 | 59.50 |
| 50 | 53.00 |
| 25 | 46.58 |
| 10 | 37.00 |
| 5 | 33.97 |
Internal consistency
The total score on the CRS showed high internal consistency (Cronbach's α = 0.77), supporting the idea that a composite measure could adequately summarize the frequency of participation in activities throughout the lifetime [41]. This alpha value was similar to that obtained in the pilot study (α = 0.81).
Validity evidence
Relationships between the total CRS score and other CR proxies commonly used in studies on CR, such as years of education, occupational attainment and IQ score, were assessed. It was hypothesized that education and occupational attainment could be related to the CRS score, but no relation to IQ was expected [45]. The results showed that educational attainment affected the CRS scores (t = −2.98, p = 0.004, partial h2 = 0.07), whereas occupational attainment was not related to the CRS (F2,116 = 0.11, p = 0.898). As expected, no significant relationship was found between the total CRS score and IQ (Vocabulary subtest) (r = 0.09, p = 0.33). The distribution of participants according to educational attainment and occupational attainment is presented in Table 1. The observed effect size was medium (partial h2≥0.05).
Since the literature shows heterogeneity with regard to relationships among proxies of CR based on activities across the lifespan and cognitive results, it was hypothesized that the relationships between the CRS and the neuropsychological variables would show heterogeneity and a lack of negative relationships. It can be observed in Table 4 that: (i) there were positive correlations between the CRS score and TAVEC last trial, TAVEC sum, and TAVEC short-term and long-term memory (verbal memory); (ii) there was a positive correlation between the CRS score and the Matrix Reasoning subtest (executive functions); (iii) the CRS score and the Block Design subtest were also significantly associated (visuoconstruction–visuoperception); (iv) the CRS score was not correlated with any test of working memory, attention and processing speed; and (v) no significant relationships were demonstrated between the CRS score and TAVEC first trial, TAVEC recognition, ROCF, FAS and Animals. As expected, all of the significant correlations were positive, and the sizes of the correlations were medium to low.
Table 4. Correlations between the Cognitive Reserve Scale (CRS) score and each cognitive variable classified into six cognitive domains.
| Cognitive domain | CRS score | ||||
| n | Mean | SD | r | p | |
| Working memory | |||||
| Digit Span (backward) | 117 | 10.68 | 2.53 | 0.077 | 0.409 |
| Corsi's Block-Tapping (backward) | 117 | 15.19 | 2.66 | −0.071 | 0.450 |
| Memory | |||||
| TAVEC – first trial | 117 | 0.02 | 0.95 | 0.102 | 0.273 |
| TAVEC – last trial | 117 | 0.67 | 0.98 | 0.241 | 0.009 |
| TAVEC sum | 117 | 0.46 | 0.88 | 0.320 | 0.000 |
| TAVEC short-term | 117 | 0.45 | 0.98 | 0.286 | 0.002 |
| TAVEC long-term | 117 | 0.39 | 1.13 | 0.219 | 0.018 |
| TAVEC recognition | 117 | 0.20 | 0.93 | 0.098 | 0.291 |
| ROCF short-term | 116 | 9.36 | 4.93 | 0.066 | 0.485 |
| ROCF long-term | 116 | 8.78 | 2.91 | 0.060 | 0.525 |
| Attention | |||||
| Digit Span (forward) | 117 | 9.97 | 2.71 | −0.035 | 0.708 |
| Corsi's Block-Tapping (forward) | 117 | 14.6 | 2.93 | −0.109 | 0.244 |
| Stroop: word–color score | 117 | 9.76 | 2.75 | 0.135 | 0.147 |
| Executive functions | |||||
| FAS sum | 117 | 47.66 | 28.31 | 0.095 | 0.309 |
| Animals | 117 | 10.56 | 2.85 | 0.145 | 0.118 |
| Matrix Reasoning | 117 | 11.96 | 2.94 | 0.204 | 0.027 |
| Visuoconstruction-visuoperception | |||||
| Block Design | 117 | 11.21 | 2.80 | 0.202 | 0.029 |
| ROCF copy | 116 | 7.23 | 2.64 | −0.067 | 0.477 |
| Processing speed | |||||
| Time to copy ROCF (s) | 116 | 9.13 | 2.59 | 0.018 | 0.847 |
| TMT-A | 117 | 10.68 | 2.59 | −0.091 | 0.328 |
| TMT-B | 116 | 9.28 | 2.63 | 0.091 | 0.330 |
TAVEC: Test de Aprendizaje Verbal España–Complutense (Verbal Learning Spanish–Complutense Test); ROCF: Rey–Osterrieth Complex Figure; TMT: Trail Making Test.
Discussion
The CRS is a new CR proxy based on a person's participation in cognitively stimulating activities throughout his or her lifetime [46]. The psychometric results in the current study suggest that the CRS is an adequate tool for assessing CR in the Spanish population. With regard to sociodemographic characteristics, it was observed that the CRS score was not affected by gender, and the possible effect of age was corrected by averaging the CRS score. Thus, age did not influence the CRS score and was controlled for so that it did not bias the results.
Regarding the study of validity evidence, it should be noted that the development of the CRS was based on the frequency of participation in brain-stimulating activities across the lifespan and that different analyses were performed to study the relationships between the CRS score and other frequent proxies of CR (education, occupational attainment and IQ), as well as the associations between the CRS and cognitive performance.
Differences in CRS were found between participants who had attained a high level of education and those who had not. The finding that educational attainment has an effect on the CRS score was expected. For example, Wilson et al. [40] found that a composite measure of cognitive activity frequency correlated with education, and Nucci et al. [45] reported a correlation between leisure time and education subscores on the Cognitive Reserve Index Questionnaire. However, in both studies, the values of the correlations were low (r≤0.30) and different measures of education were applied. Likewise, it was hypothesized that occupational attainment (high, medium and low) would affect the CRS score, although no relationship was found between these two variables. Occupation may provide an indication of experiences, but it is unrealistic to expect a connection with the activities included in the CRS (activities of daily living, training–information, hobbies and social life), as was hypothesized. Furthermore, the association between cognitively stimulating activities across the lifetime and occupational attainment has not been studied in depth.
In this investigation, as in many previous CR studies [20], [30], [64], verbal IQ was estimated using the Vocabulary subtest [29]. This measure of IQ was not included as part of the total CRS score [45], [46]. Although IQ is a very common measure of CR, the approach taken in this study was based on the construct of CR as the variety of brain-stimulating activities that people took part in during their lifetime. Therefore, the two measures, IQ and CRS score, did not share the same definition of CR and it was reasonable to expect a lack of association between these variables. Thus, further psychometric research is needed on the operational and relational definitions of CR, and the properties of the instruments used to measure it.
In agreement with previous CR studies focusing on active cognitive lifestyles, heterogeneity was observed in the relationships between the CRS and cognitive performance. As hypothesized, there were significant relationships between the CRS score and memory tests, and specifically verbal memory tests. However, no associations were found with non-verbal memory tasks. In addition, according to the hypothesis, significant correlations were found between the CRS and abstract tests such as the Matrix Reasoning and Block Design subtests. No other neuropsychological variable was related to the CRS score.
These results are in line with the active model of CR, which postulates that high CR allows subjects to solve more successfully cognitive tasks in general, but not necessarily in a specific cognitive domain [23]. High levels of reserve are associated with more flexible and effective networks that promote the capacity to cope with brain pathology and lead to better performance in healthy subjects [1], [65].
Previous research that focused on CR as the frequency of participation in cognitively stimulating activities throughout life showed diverse associations between reserve and neuropsychological performance. Valenzuela and Sachdev [42] found that, after a follow-up period of 18 months, the relationship between the total score on the Lifetime of Experiences Questionnaire and neuropsychological change was only significant for the attentional domain (r = 0.32, p = 0.01). Another longitudinal study revealed that cognitive activities were associated with a decrease in cognitive deficit in general, working memory and perceptual speed [40]. Ramí et al. found that the Cognitive Reserve Questionnaire was associated mainly with executive tasks in subjects with Alzheimer's disease and healthy subjects [44]. Sánchez et al. [66] developed a model that included the frequency of participation in a variety of activities and demonstrated that people with high CR have better neuropsychological performance than those with low CR.
It is important to note that, in these studies, (i) the cognitive domains did not include the same tests, (ii) different frequency accounts of cognitive activities were used, (iii) different numbers of activities were considered, and (iv) different life stages were considered. Thus, there is a need to continue carrying out studies in this vein [45], [47]. The present study follows this line of research. The CRS was influenced by previous studies based on the frequency of participation in leisure activities [9], [40], in which different periods of life were considered [42]: young adulthood (18–35 years), adulthood (36–64 years) and late adulthood (over the age of 65 years).
Proxies of CR that may be strongly influenced by culture or education (reading level, IQ or years of education) could explain their associations with specific cognitive tasks [21], [35]. However, these CR proxies also correlate with abstract and reasoning tasks that are initially less influenced by education [19], [21]. Zahodne et al. [67], using a large sample (1014 participants), demonstrated that education influenced cognitive performance. The authors suggested that education was associated with different cognitive domains (processing speed, working memory, verbal fluency and verbal episodic memory); in contrast, education had no influence on cognitive decline with age [68]. Thus, some observations must be stressed: (i) heterogeneous results were observed in CR studies; (ii) CR is a hypothetical construct that is not directly measurable; and (iii) the most appropriate CR proxy has not been defined [28].
The well-known benefits of an enriched environment may be comparable with those gained from an active lifestyle. These benefits could contribute toward delaying the risk of dementia or cognitive impairment associated with aging [69]–[71], and may promote brain changes through neuroplasticity and neuroprotection [72], [73]. Lee [74] suggested that to comprehend CR complex networks, studies need to include biomarkers and neuroimaging techniques. For example, Yaffe et al. [75] demonstrated that CR modifies the association between beta-amyloid in the blood and cognitive impairment in elderly people. In short, environmental and genetic factors could contribute to the development of neurodegenerative disorders, such as Alzheimer's disease [76], and it is apparent that studies on CR can be very complex.
Some limitations of this study should be noted. First, the sample size was small and recruitment to the study was not randomized; therefore, further research should include more participants and control recruitment to confirm the present results. Second, a longitudinal study could have gathered information to complete analyses on both the CRS score and potential cognitive change. Finally, although diverse cognitive tasks were included in the cognitive domains, other tasks could be considered to analyze the relation between CRS scores and neuropsychological performance.
In conclusion, the psychometric analyses in this study suggest that the CRS is an adequate test for assessing CR in the Spanish population. Educational attainment influenced the CRS score, and significant relationships were found between the CRS and the memory and abstract reasoning domains. The CRS could be a powerful tool for use in clinical contexts.
Acknowledgments
The authors thank the participants in the study, and Charlotte Pover for her assistance in copy-editing.
Funding Statement
This work was supported by the Ministry of Economy and Competitiveness (Spain) [PSI2011-26985] and Plan Propio de Investigacion Grant to IL (University of Almeria). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stern Y (2009) Cognitive reserve. Neuropsychologia 47: 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scarmeas N, Levy G, Tang MX, Manly J, Stern Y (2001) Influence of leisure activity on the incidence of Alzheimer's disease. Neurology 57: 2236–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valenzuela M, Sachdev P (2006) Brain reserve and cognitive decline: a nonparametric systematic review. Psychol Med 36: 441–454. [DOI] [PubMed] [Google Scholar]
- 4. Valenzuela M, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychol Med 52: 195–201. [DOI] [PubMed] [Google Scholar]
- 5. Wang HX, Karp A, Winblad B, Fratiglioni L (2002) Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol 155: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 6. Meng X, D'Arcy C (2012) Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7: e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brayne C, Ince PG, Keage HAD, McKeith IG, Matthews FE, et al. (2010) Education, the brain and dementia: neuroprotection or compensation? Brain 133: 2210–2216. [DOI] [PubMed] [Google Scholar]
- 8. Stern Y, Albert S, Tang MX, Tsai WY (1999) Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology 53: 1942–1947. [DOI] [PubMed] [Google Scholar]
- 9. Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, et al. (2003) Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol 60: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Carret N, Lafont S, Letenneur L, Dartigues JF, Mayo F, et al. (2003) The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Dev Neuropsychol 23: 317–337. [DOI] [PubMed] [Google Scholar]
- 11. Roe CM, Xiong C, Miller JP, Morris JC (2007) Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology 68: 223–228. [DOI] [PubMed] [Google Scholar]
- 12. Perneczky R, Drzezga A, Boecker H, Ceballos-Baumann AO, Granert O, et al. (2008) Activities of daily living, cerebral glucose metabolism, and cognitive reserve in Lewy body and Parkinson's disease. Dement Geriatr Cogn Disord 26: 475–481. [DOI] [PubMed] [Google Scholar]
- 13. Solé-Padullés C, Bartrés-Faz D, Junqué C, Vendrell P, Ramí L, et al. (2009) Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 30: 1114–1124. [DOI] [PubMed] [Google Scholar]
- 14. Roldán-Tapia L, García J, Cánovas R, León I (2012) Cognitive reserve, age, and their relation to attentional and executive functions. Appl Neuropsychol Adult 19: 2–8. [DOI] [PubMed] [Google Scholar]
- 15. García-Molina A, Enseñat-Cantallops A, Sánchez-Carrión R, Rodríguez P, Tormos JM, et al. (2013) Interindividual variability in recovery after traumatic brain injury: effect of cognitive reserve. Med Clin (Barc) 140: 527–531. [DOI] [PubMed] [Google Scholar]
- 16. Carnero-Pardo C, Del Ser T (2007) Education provides cognitive reserve in cognitive deterioration and dementia. Neurologia 22: 78–85. [PubMed] [Google Scholar]
- 17. Bastin C, Yakushev I, Bahri MA, Fellgiebel A, Eustache F, et al. (2012) Cognitive reserve impacts on inter-individual variability in resting-state cerebral metabolism in normal aging. Neuroimage 63: 713–722. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz CE, Snook E, Quaranto B, Benedict RH, Vollmer T (2013) Cognitive reserve and patient-reported outcomes in multiple sclerosis. Mult Scler 19: 87–105. [DOI] [PubMed] [Google Scholar]
- 19. Liu Y, Cai ZL, Xue S, Zhou X, Wu F (2013) Proxies of cognitive reserve and their effects on neuropsychological performance in patients with mild cognitive impairment. J Clin Neurosci 20: 548–553. [DOI] [PubMed] [Google Scholar]
- 20. De la Serna E, Andrés-Perpiñá S, Puig O, Baez I, Bombin I, et al. (2013) Cognitive reserve as a predictor of two year neuropsychological performance in early onset first-episode schizophrenia. Schizophr Res 143: 125–131. [DOI] [PubMed] [Google Scholar]
- 21. Alosco ML, Spitznagel MB, Raz N, Cohen R, Sweet LH, et al. (2012) Cognitive reserve moderates the association between heart failure and cognitive impairment. J Clin Exp Neuropsychol 34: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satz P (1993) Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 7: 273–295. [Google Scholar]
- 23. Stern Y, Zarahn E, Hilton J, Flynn J, DeLaPaz R, et al. (2003) Exploring the neural basis of cognitive reserve. J Clin Exp Neuropsychol 25: 691–701. [DOI] [PubMed] [Google Scholar]
- 24. Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, et al. (2012) Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging 33(2): 423.e15–25. [DOI] [PubMed] [Google Scholar]
- 25. Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF (1999) Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology 53: 189–196. [DOI] [PubMed] [Google Scholar]
- 26. Christensen H, Batterham PJ, Mackinnon AJ, Anstey KJ, Wen W, et al. (2009) Education, atrophy, and cognitive change in an epidemiological sample in early old age. Am J Geriatr Psychiatry 17: 218–226. [DOI] [PubMed] [Google Scholar]
- 27. Kesler SR, Adams HF, Blasey CM, Bigler ED (2003) Premorbid intellectual functioning, education, and brain size in traumatic brain injury: an investigation of the cognitive reserve hypothesis. Appl Neuropsychol 10: 153–162. [DOI] [PubMed] [Google Scholar]
- 28. Bartrés-Faz D, Arenaza-Urquijo EM (2011) Structural and functional images correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topogr 24: 340–357. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D (1999) Escala de Inteligencia para Adultos – III. Madrid: TEA Ediciones.
- 30. Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, et al. (2005) Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 15: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santamarina-Pérez P, Corral M (2009) Influencia de la reserva cognitiva en el rendimiento neuropsicológico de los pacientes con epilepsia. Med Clin (Barc) 132: 459–462. [DOI] [PubMed] [Google Scholar]
- 32.Nelson HE (1982) National Adult Reading Test (NART). Windsor: NFER-Nelson.
- 33. Richards M, Sacker A (2003) Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol 25: 614–624. [DOI] [PubMed] [Google Scholar]
- 34. Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, et al. (2011) Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain 134: 1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soto-Añari M, Flores-Valdivia G, Fernández-Guinea S (2013) Level of reading skills as a measure of cognitive reserve in elderly adults. Rev Neurol 56: 79–85. [PubMed] [Google Scholar]
- 36. Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, et al. (2008) Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol 63: 112–118. [DOI] [PubMed] [Google Scholar]
- 37. Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, et al. (2011) White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 32: 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghaffar O, Fiati M, Feinstein A (2012) Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PLoS One 7: e47206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fratiglioni L, Wang HX (2007) Brain reserve hypothesis in dementia. J Alzheimers Dis 12: 11–22. [DOI] [PubMed] [Google Scholar]
- 40. Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, et al. (2002) Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 287: 742–748. [DOI] [PubMed] [Google Scholar]
- 41. Wilson R, Barnes L, Bennett D (2003) Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol 25: 634–642. [DOI] [PubMed] [Google Scholar]
- 42. Valenzuela M, Sachdev P (2007) Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ). Psychol Med 37: 1015–1025. [DOI] [PubMed] [Google Scholar]
- 43. Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, et al. (2003) Leisure activities and the risk of dementia in the elderly. N Engl J Med 348: 2508–2516. [DOI] [PubMed] [Google Scholar]
- 44. Ramí L, Valls-Pedret C, Bartrés-Faz D, Caprile C, Solé-Padullés C, et al. (2011) Cognitive reserve questionnaire. Scores obtained in a healthy elderly population and in one with Alzheimer's disease. Rev Neurol 52: 195–201. [PubMed] [Google Scholar]
- 45. Nucci M, Mapelli D, Mondini S (2012) Cognitive reserve index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res 24: 218–226. [DOI] [PubMed] [Google Scholar]
- 46. León I, García J, Roldán-Tapia L (2011) Development of the scale of cognitive reserve in Spanish population: a pilot study. Rev Neurol 52: 653–660. [PubMed] [Google Scholar]
- 47. Apolinario D, Brucki SMD, Ferretti REL, Farfel JM, Magaldi RM, et al. (2013) Estimating premorbid cognitive abilities in low-educated populations. PLoS One 8: e60084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, et al. (2011) Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc 17: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Folstein M, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 50. Lobo A, Esquerra J, Gomez-Burgada F, Sala JM, Seva A (1979) El Mini-Examen Cognoscitivo: un test sencillo y práctico para detectar alteraciones intelectuales en pacientes médicos. Actas Luso Esp Neurol Psiquiatr 3: 189–202. [PubMed] [Google Scholar]
- 51.Instituto Nacional de Estadística (INE) (2011). Clasificación Nacional de Ocupaciones (CNO). Available: http://www.ine.es/jaxi/menu.do?type=pcaxis&path=/t40/cno11&file=inebase. Accessed 3 February 2012.
- 52.Benedet M, Alejandre M (1998) Test de aprendizaje verbal España–Complutense (TAVEC). Madrid: TEA. [PubMed]
- 53.Rey A (2009) Test de copia de una figura compleja. Madrid: TEA.
- 54. Palomo R, Casals-Coll M, Sánchez-Benavides G, Quintana M, Manero RM, et al. (2013) Estudios normativos españoles en población adulta joven (proyecto NEURONORMA jóvenes): normas para las pruebas Rey–Osterrieth complex figure (copia y memoria) y free and cued selective reminding test. Neurologia 28: 226–235. [DOI] [PubMed] [Google Scholar]
- 55. Peña-Casanova J, Gramunt-Fombuena N, Quiñones-Úbeda S, Sánchez-Benavides G, Aguilar M, et al. (2009) Spanish multicenter normative studies (NEURONORMA Project): norms for the Rey–Osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol 24: 371–393. [DOI] [PubMed] [Google Scholar]
- 56. Peña-Casanova J, Quiñones-Úbeda S, Quintana-Aparicio M, Aguilar M, Badenes D, et al. (2009) Spanish multicenter normative studies (NEURONORMA Project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch Clin Neuropsychol 24: 321–341. [DOI] [PubMed] [Google Scholar]
- 57. Tamayo F, Casals-Coll M, Sánchez-Benavides G, Quintana M, Manero RM, et al. (2012) Estudios normativos españoles en población adulta joven (Proyecto NEURONORMA Jóvenes): normas para las pruebas span verbal, span visuoespacial, letter–number sequencing, trail making test y symbol digit modalities Test. Neurologia 27: 319–329. [DOI] [PubMed] [Google Scholar]
- 58.Golden CJ (2010) Stoop: test de colores y palabras. Madrid: TEA.
- 59. Peña-Casanova J, Quiñones-Úbeda S, Gramunt-Fombuena N, Quintana M, Aguilar M, et al. (2009) Spanish multicenter normative studies (NEURONORMA Project): norms for the Stroop color–word interference test and the Tower of London–Drexel. Arch Clin Neuropsychol 24: 413–429. [DOI] [PubMed] [Google Scholar]
- 60. Rognoni T, Casals-Coll M, Sánchez-Benavides G, Quintana M, Manero RM, et al. (2013) Estudios normativos españoles en población adulta joven (proyecto NEURONORMA jóvenes): normas para las pruebas Stroop color–word interference test y Tower of London–Drexel University tests. Neurologia 28: 73–80. [DOI] [PubMed] [Google Scholar]
- 61.Mitrushina M, Boone KB, Razani J, D'Elia LF (2005) Handbook of normative data for neuropsychological assessment (2nd ed.). New York: Oxford University Press.
- 62. Casals-Coll M, Sánchez-Benavides G, Quintana M, Manero RM, Rognoni T, et al. (2013) Estudios normativos españoles en población adulta joven (proyecto NEURONORMA jóvenes): normas para los test de fluencia verbal. Neurologia 28: 33–40.22652141 [Google Scholar]
- 63. Peña-Casanova J, Quiñones-Úbeda S, Gramunt-Fombuena N, Quintana-Aparicio M, Aguilar M, et al. (2009) Spanish multicenter normative studies (NEURONORMA Project): norms for verbal fluency tests. Arch Clin Neuropsychol 24: 395–411. [DOI] [PubMed] [Google Scholar]
- 64. Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, et al. (2008) A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cereb Cortex 18: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scarmeas N (2007) Lifestyle patterns and cognitive reserve. In: Stern Y, editor. Cognitive reserve. Theory and applications. New York: Taylor & Francis. pp. 187–206.
- 66. Sánchez JL, Torrellas C, Martín J, Barrera I (2011) Study of sociodemographic variables linked to lifestyle and their possible influence on cognitive reserve. J Clin Exp Neuropsychol 33: 874–891. [DOI] [PubMed] [Google Scholar]
- 67. Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, et al. (2011) Education does not slow cognitive decline with aging: 12-year evidence from the Victoria longitudinal study. J Int Neuropsychol Soc 17: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Van Dijk KR, Van Gerven PW, Van Boxtel MP, Van der Elst W, Jolles J (2008) No protective effects of education during normal cognitive aging: results from the 6-year follow-up of the Maastricht Aging Study. Psychol Aging 23: 119–130. [DOI] [PubMed] [Google Scholar]
- 69. Marioni RE, Van den Hout A, Valenzuela MJ, Brayne C (2012) Active cognitive lifestyle associates with cognitive recovery and a reduced risk of cognitive decline. J Alzheimers Dis 28: 223–230. [DOI] [PubMed] [Google Scholar]
- 70. Reed BR, Dowling M, Farias ST, Sonnen J, Strauss M, et al. (2011) Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc 17: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, et al. (2009) Leisure activities and the risk of dementia in the elderly: results from the three-city study. Neurology 73: 854–861. [DOI] [PubMed] [Google Scholar]
- 72. Chao S, León I, Brodaty H, Trollor J, Wen W, et al. (2012) Supervisory experience at work is linked to low rate hippocampal atrophy in late life. Neuroimage 63: 1542–1551. [DOI] [PubMed] [Google Scholar]
- 73. Steffener J, Stern Y (2012) Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta 1822: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JH (2007) Understanding cognitive reserve through genetics and genetic epidemiology. In: Stern Y, editor. Cognitive reserve. Theory and applications. New York: Taylor & Francis. pp. 187–206.
- 75. Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, et al. (2011) Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 305: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Borenstein AR, Copenhaver CI, Mortimer JA (2006) Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord 20: 63–72. [DOI] [PubMed] [Google Scholar]