Abstract
Background
In premature ovarian failure (POF), cessation of menstruation occurs before the expected age of menopause. Approximately 1% of women are affected. FMR1 premutation was reported to be responsible for up to 3.3%–6.7% of sporadic POF and 13% of familial cases in Caucasians, while the data was absent in Chinese population. Therefore, the impact of FMR1 CGG repeat on ovarian reserve is needed to be investigated in large Chinese cohort.
Methods
The number of FMR1 CGG repeat was determined in 379 Han Chinese women with well-defined 46, XX non-syndromic sporadic POF and 402 controls. The age of menopause onset in respect to CGG repeats was further analyzed.
Results
The frequency of FMR1 premutation in Han Chinese POF was only 0.5% (2/379), although it was higher than that in matched controls (0%, 0/402), it was much lower than that reported in Caucasian with POF (3.3%–6.7%). The prevalence of intermediate FMR1 (41–54) was not increased significantly in sporadic POF than that in controls (2.9% vs. 1.7%, P = 0.343). However, POF patients more often carried a single additional CGG repeat in a single allele than did fertile women (allele-1: 29.7 vs. 28.8, P<0.001; allele-2: 32.6 vs. 31.5, P<0.001). POF patients with both alleles of CGG repeats outside (below or above) the normal range (26–34) showed an earlier age of cessation of menses than those with two alleles within normal range (hom-high/high vs. norm: 20.4±4.8 vs. 24.7±6.4, p<0.01; hom-low/high vs. norm: 18.7±1.7 vs. 24.7±6.4, p<0.01).
Conclusions
FMR1 premutation seems to be an uncommon explanation for POF in Han Chinese. However, having both alleles with CGG repeats outside the normal range might still adversely affect ovarian aging.
Introduction
Fragile X mental retardation 1 (FMR1) is an X-linked gene carrying dynamic triplet CGG repeats in its 5′ untranslated region [1]. Alleles with ≤40 CGG repeats are traditionally considered normal [2]. The full mutation, in which alleles have >200 CGG repeats, causes transcriptional silence of FMR1 gene, absence of the fragile X mental retardation protein (FMRP) and well characterized features of the fragile X syndrome, which was the most common cause of inherited mental retardation and autism [3]. Between the normal (29–31) and full mutation (>200) range, there are intermediate and premutation range, both unstable and capable of expanding to full mutation over several generations [2]. The premutation range starts with 55 repeats and ends at 199 repeats; the intermediate range remains controversial, but generally is considered 41 or 45 to 54 repeats [2], [4].
FMR1 premutation (55–199) is accepted as a relatively common explanation for altered ovarian function and loss of fertility [5]. In mouse model carrying FMR1 premutation, reduced number of growing follicles and impaired female fertility was observed and a reduced phosphorylation of Akt and mTOR proteins was elucidated [6]. In Caucasians, the prevalence of premature ovarian failure (POF) in FMR1 premutation carriers is significantly higher than that in general population (13%–26% vs. 1%) [2]. FMR1 premutation occurs more frequently in POF patients having a positive family history compared to sporadic ones (13%–16% vs. 1.6%–3.0% in British; 12.1% vs.10% in Italian) [7]–[11]. However, in POF patients from Hong Kong (China) and Japan, the frequency of FMR1 premutation was only 0.9% (1/116) and 1.6% (2/128), respectively [4], [12]. Another two studies in Indian, including 80 and 289 POF patients respectively, revealed none of them carrying FMR1 premutation [13], [14]. These data indicated that fewer patients with POF from Asian than Caucasian carried FMR1 premutation. Barasoain et.al found intermediate FMR1 (35–54 CGG repeats) associated with diminished ovarian reserve [15]. Gleicher and colleagues believe that having less than intermediate (41–54) but greater than the accepted normal (29–31) CGG repeats deleteriously affects ovarian ageing [16], [17], perhaps influencing the ovarian response [18] and adversely affecting outcome during IVF-ET treatment [19]. In contrast, Lledo [20], Bennett [21] and Murray [8] found that in Spanish and British populations neither ovarian reserve nor ovarian response was adversely affected by intermediate or normal high sized CGG repeat. These contradictions led us to wonder how the FMR1 mutations in Chinese women with POF, and furthermore the clinical significance of FMR1 tests.
Materials and Methods
Study population
In this control cohort study, we investigated 379 sporadic POF patients and 402 matched controls for the number of triple CGG repeats in both alleles of FMR1 gene. Inclusion criteria for POF consisted of cessation of menstrual cycles before 40 years of age, with at least twice serum follicle stimulating hormone (FSH) concentrations exceeding 40 IU/L. Women with known chromosomal abnormalities, previous ovarian surgery, chemotherapy, radiotherapy or familial member diagnosed with POF were excluded. Information was sought on associated somatic anomalies, specifically adult onset neurologic disorder, referred to as fragile X–associated tremor/ataxia syndrome, or mental retardation. The 402 controls were recruited from a cohort for health checkup. They were known to be menstruating regularly, had normal FSH levels and normal pelvic ultrasound imaging. Clinical features of POF patients and controls were shown in Table 1.
Table 1. Clinical characteristics of POF patients and controls.
| Characteristics | POF | Control | |
| <40 yrs | 40–44 yrs | ||
| No. | 379 | 378 | 24 |
| Age (yrs) | 31.7±4.2 | 30.5±4.4 | 41.1±1.4 |
| Age at menarche (yrs) | 14.5±1.8 | 14.9±1.5 | 15.2±1.5 |
| Age at onset of menstrual dysfunction (yrs) | 23.3±6.5 | - | - |
| Age of amenorrhea (yrs) | 25.1±5.6 | - | - |
| Age at diagnosis (yrs) | 30.1±4.8 | - | - |
| FSH (IU/L) | 77.0±27.4 | 7.4±1.8 | 8.2±2.6 |
Note: All of the normal control women did not have amenorrhea.
Informed consents were signed by all subjects. This study was approved by the Institutional Review Board of Reproductive Medicine of Shandong University.
Genotype analysis
With the genomic DNA extracted from peripheral blood lymphocytes, the FMR1 gene was amplified by PCR with fluorescently labeled primers [1], [22], and the CGG repeats of two alleles were counted by capillary electrophoresis (CE) on an ABI 3730 instrument (Applied Biosystems, Madrid, Spain). If only one allele was identified, a rich CGG repeat primed (RP) PCR was performed using AmplideX FMR1 PCR Kit (Asuragen, USA), and the PCR products detected by CE were analyzed using Gene Mapper 4.0 software (Applied Biosystems).
Referring to the nomenclature of Gleicher, et al., the allele with lower number of CGG repeats was defined as allele-1, whereas the one with a higher number was defined as allele-2 [16]. The normal range was defined as 26–34 CGG repeats and the designations of FMR1 genotypes under discussion here referred to the classification rules raised by Gleicher, et al [23]: normal (norm)– both alleles within normal range; heterozygous (het)– one allele within normal range and the other one outside, further stratified as het-norm/low and het-norm/high, according to the abnormal allele being below or above normal range; homozygous (hom)– both alleles outside normal range, further stratified as hom-low/low, hom-high/high and hom-low/high according to the rules took above (Table 2).
Table 2. Distribution of FMR1 genotype and sub-genotype in sporadic POF patients.
| Allele-1 | Allele-2 | POF (n = 379) | ||
| norm | 26–34 | 26–34 | 256 (67.5%) | |
| het | het-norm/low | <26 | 26–34 | 20 (5.3%) |
| het-norm/high | 26–34 | >34 | 90 (23.7%) | |
| Total | 110 (29.0%) | |||
| hom | hom-low/low | <26 | <26 | 1 (0.3%) |
| hom-high/high | >34 | >34 | 9 (2.4%) | |
| hom-low/high | <26 | >34 | 3 (0.8%) | |
| Total | 13 (3.5%) |
Note: By definition allele 1 has the lowest number of repeats. norm: both alleles within normal range; het: one allele within normal range and the other allele outside; hom: both alleles outside normal range.
Statistical analysis
SPSS 19.0 computer software (IBM, Armonk, NY, USA) was used for data analysis. The continuous data were checked for normality and described as mean ± SD or mean (95% confidence interval for mean). Numerical data were analyzed by independent sample T-tests or Mann-Whitney Test, and Pearson chi-square test or Fisher's exact test was used to test categorical data. All the P values were two-sided, and P<0.05 was considered statistically significant.
Results
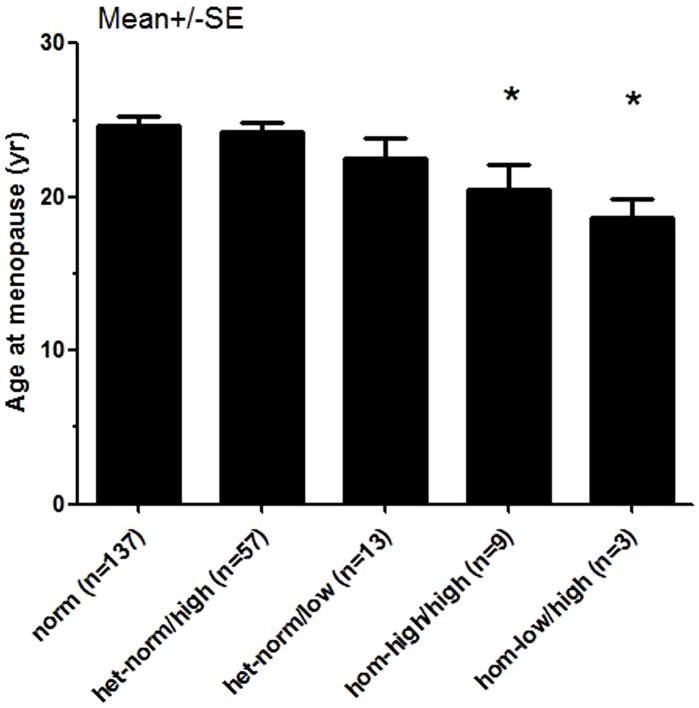
Compared with controls, both allele-1 and allele-2 in POF cases demonstrated a small but significant shift towards a single additional CGG repeat (29.7 vs. 28.8, P<0.001; 32.6 vs. 31.5, P<0.001, respectively) (Table 3). FMR1 premutation (55–199) was observed in only two POF patients (2/379, 0.5%) and none of normal matched controls. The prevalence of intermediate FMR1 (41–54) in POF cases was not increased significantly compared with controls (2.9% vs. 1.7%, P = 0.343) (Table 3). POF patients with sub-genotype hom-high/high and hom-low/high showed earlier age at menopause compared with norm (20.4±4.8 vs. 24.7±6.4, p<0.01; 18.7±1.7 vs. 24.7±6.4, p<0.01, respectively) (Figure 1).
Table 3. Comparation of the CGG repeats on FMR1 gene between sporadic POF patients and normal control women.
| POF (n = 379) | Control (n = 402) | P-value | |
| Allele-1 | 29.7 (29.5–30.0) | 28.8 (28.6–29.0) | <0.001a |
| Allele-2 | 32.6 (32.1–33.1) | 31.5 (31.1–31.8) | <0.001a |
| Unaffected(≤40; n%) | 366(96.6) | 395(98.3) | 0.174b |
| Intermediate (41–54; n%) | 11(2.9) | 7(1.7) | 0.343b |
| Premutation (55–200; n%) | 2(0.5) | 0 | - |
| Full mutation (>200; n%) | 0 | 0 | - |
Note: P-value was obtained by Mann-Whitney Testa or Pearson chi-square testb between POF and Control.
Figure 1. POF patients with sub-genotype hom-high/high and hom-low/high showed earlier age at menopause compared with norm (20.4±4.8 vs. 24.7±6.4, p<0.01; 18.7±1.7 vs. 24.7±6.4, p<0.01, respectively).
Discussion
Investigating the largest POF cohort of Han Chinese females accumulated to date, we initially determined the frequency of FMR1 premutation to be only 0.5% (2/379), much lower than that previously reported in Caucasian cohorts: British (1.6–3%) [7], [8], [10], Italian (3.3–10%) [9], [11], Polish (7.9%) [24], Turkish (4.9%) [25] and Slovenian samples (4.8%) [26]. The premutation frequency was 1.6% in the only reported Japanese study [4], and 0.9% in the smaller Chinese study [12]. Therefore, FMR1 premutation seems to be an uncommon explanation for POF in Han Chinese. Furthermore, there was no significant difference with respect to intermediate FMR1 between POF cases and controls (2.9% vs. 1.7%, P = 0.343). Similar prevalence has been drawn from Japanese POI cohorts (3.9%) [4], indicating that intermediate FMR1 does not contribute to POF in Chinese either [8], [21]. However, in some Caucasian studies the effect of intermediate FMR1 on ovarian function was controversial. Barasoain and Gleicher et al. found normal high sized and intermediate FMR1 (35–54 CGG repeats) associated with diminished ovarian reserve [15]. In contrast, Lledo [20], Bennett [21] and Murray [8] found that in Spanish and British populations neither ovarian reserve nor ovarian response was adversely affected by intermediate or normal high sized CGG repeat. The contradiction might be attributed to ethnic differences and the bias of recruited participators in each study.
In our study, the distribution of FMR1 genotypes in sporadic POF patients was similar to that previously reported in Asian cohort [27]. POF patients with sub-genotype hom-high/high and hom-low/high showed an earlier age of onset of menopause compared with those with norm (20.4±4.8 vs. 24.7±6.4, p<0.01; 18.7±1.7 vs. 24.7±6.4, p<0.01, respectively) (Figure 1). That consist with Gleicher's conclusion [19], [28], which suggested that not only abnormally higher CGG repeat numbers have deleterious effect on ovarian aging but also abnormally lower numbers. Our results indicated that having both alleles, rather than a single allele, with CGG repeats outside (above or below) the normal range might adversely affects ovarian function and presumably ovarian reserve in Han Chinese. Considering the relatively small sample size of the sub-genotype hom-high/high (n = 9) and hom-low/high (n = 3), more studies with larger sample size are needed.
The seemingly contradictory findings of a low sporadic FMR1 premutation carrier rate (0.5%) and no increased prevalence of intermediate FMR1 in Han Chinese POF, the deleterious effect of a single additional CGG repeat are not well explained. However in other dynamic mutations (e.g. Huntington disease, myotonic dystrophy, spino-bulbar muscular atrophy), the number of amplified repeats needed to exert a phenotypic effect is not much larger than that considered normal. For example, in Huntington disease, deleterious effects are manifested with more than 36 CAG repeats, whereas 8–35 repeats is normal [29]. In myotonic dystrophy type 1, >50 CTG repeats is abnormal, while 5–37 repeats normal [30]. In spino-bulbar muscular atrophy, >40 CAG repeats is abnormal, but 14–32 repeats normal [31]. In Fragile X syndrome the wide dichotomy between modal number of CGG repeats (29–31) and the >200 required for mental retardation is the exception. By contrast, considering POF cases having CGG repeats outside normal but below intermediate range showed relatively earlier age at menopause, ovarian disturbance may be sensitive to small changes in CGG repeats, more analogous to observations in Huntington disease and myotonic dystrophy type 1.
In summary, FMR1 premutation was not a common explanation for sporadic POF in Han Chinese. But in some familial POF patients, male or female relative with mental retardation could be observed; thus, POF or fragile X tremor/ataxia syndrome could arise in the next generation of familial POF patients. Therefore, from a clinical perspective our results do not obviate the need for FMR1 testing in a woman having POF, even Chinese. Yet, pause may be in order before embarking on general population screening programs in Chinese or Asian populations in the absence of a positive family history.
Acknowledgments
We are grateful to all of the participants involved in this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the National Basic Research Program of China (973 program-2012CB944700); the National Natural Science Foundation of China (81270662); Foundation for the Author of National Excellent Doctoral Dissertation of China (201078); and Independent Innovation Foundation of Shandong University, IIFSDU (2012TS130). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, et al. (1991) Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 67: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 2. Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, et al. (2007) The FMR1 premutation and reproduction. Fertil Steril 87: 456–465. [DOI] [PubMed] [Google Scholar]
- 3. Broadie K, Pan L (2005) Translational complexity of the fragile x mental retardation protein: insights from the fly. Mol Cell 17: 757–759. [DOI] [PubMed] [Google Scholar]
- 4. Ishizuka B, Okamoto N, Hamada N, Sugishita Y, Saito J, et al. (2011) Number of CGG repeats in the FMR1 gene of Japanese patients with primary ovarian insufficiency. Fertil Steril 96: 1170–1174. [DOI] [PubMed] [Google Scholar]
- 5. Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, et al. (2005) Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 20: 402–412. [DOI] [PubMed] [Google Scholar]
- 6. Lu C, Lin L, Tan H, Wu H, Sherman SL, et al. (2012) Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet 21: 5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murray A, Webb J, Grimley S, Conway G, Jacobs P (1998) Studies of FRAXA and FRAXE in women with premature ovarian failure. J Med Genet 35: 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, et al. (2014) Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med 16: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodega B, Bione S, Dalpra L, Toniolo D, Ornaghi F, et al. (2006) Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod 21: 952–957. [DOI] [PubMed] [Google Scholar]
- 10. Conway GS, Payne NN, Webb J, Murray A, Jacobs PA (1998) Fragile X premutation screening in women with premature ovarian failure. Hum Reprod 13: 1184–1187. [DOI] [PubMed] [Google Scholar]
- 11. Marozzi A, Vegetti W, Manfredini E, Tibiletti MG, Testa G, et al. (2000) Association between idiopathic premature ovarian failure and fragile X premutation. Hum Reprod 15: 197–202. [DOI] [PubMed] [Google Scholar]
- 12. Lo TK, Lo IF, Chan WK, Tong TM, Lam ST (2005) Chromosomal abnormalities and FMR1 gene premutation in Chinese women with premature menopause. Hong Kong Med J 11: 243–250. [PubMed] [Google Scholar]
- 13. Chatterjee S, Maitra A, Kadam S, Patel Z, Gokral J, et al. (2009) CGG repeat sizing in the FMR1 gene in Indian women with premature ovarian failure. Reprod Biomed Online 19: 281–286. [DOI] [PubMed] [Google Scholar]
- 14. Tosh D, Rao KL, Rani HS, Deenadayal DA, Murty US, et al. (2014) Association between fragile X premutation and premature ovarian failure: a case-control study and meta-analysis. Arch Gynecol Obstet 289: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 15. Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, et al. (2013) Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque Country. Gene 521: 145–149. [DOI] [PubMed] [Google Scholar]
- 16. Gleicher N, Weghofer A, Barad DH (2009) A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Mullerian hormone. Fertil Steril 91: 1700–1706. [DOI] [PubMed] [Google Scholar]
- 17. Gleicher N, Weghofer A, Oktay K, Barad D (2009) Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online 19: 385–390. [DOI] [PubMed] [Google Scholar]
- 18. Gleicher N, Weghofer A, Oktay K, Barad DH (2009) Can the FMR1 (fragile X) gene serve as predictor of response to ovarian stimulation? Reprod Sci 16: 462–467. [DOI] [PubMed] [Google Scholar]
- 19. Gleicher N, Weghofer A, Lee IH, Barad DH (2011) Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS One 6: e18781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lledo B, Guerrero J, Ortiz JA, Morales R, Ten J, et al. (2012) Intermediate and normal sized CGG repeat on the FMR1 gene does not negatively affect donor ovarian response. Hum Reprod 27: 609–614. [DOI] [PubMed] [Google Scholar]
- 21. Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A (2010) Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod 25: 1335–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khaniani MS, Kalitsis P, Burgess T, Slater HR (2008) An improved Diagnostic PCR Assay for identification of Cryptic Heterozygosity for CGG Triplet Repeat Alleles in the Fragile X Gene (FMR1). Mol Cytogenet 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gleicher N, Weghofer A, Barad DH (2010) Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online 20: 768–775. [DOI] [PubMed] [Google Scholar]
- 24. Rajkiewicz M, Szlendak-Sauer K, Sulek A, Gawlik-Zawislak S, Krysa W, et al. (2011) A molecular and cytogenetic investigation of FMR1 gene premutations in Polish patients with primary ovarian insufficiency. Eur J Obstet Gynecol Reprod Biol 155: 176–179. [DOI] [PubMed] [Google Scholar]
- 25. Ceylaner G, Altinkaya SO, Mollamahmutoglu L, Ceylaner S (2010) Genetic abnormalities in Turkish women with premature ovarian failure. Int J Gynaecol Obstet 110: 122–124. [DOI] [PubMed] [Google Scholar]
- 26. Gersak K, Meden-Vrtovec H, Peterlin B (2003) Fragile X premutation in women with sporadic premature ovarian failure in Slovenia. Hum Reprod 18: 1637–1640. [DOI] [PubMed] [Google Scholar]
- 27. Gleicher N, Weghofer A, Barad DH (2010) Effects of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. Reprod Biomed Online 20: 485–491. [DOI] [PubMed] [Google Scholar]
- 28. Gleicher N, Kim A, Barad DH, Shohat-Tal A, Lazzaroni E, et al. (2013) FMR1-dependent variability of ovarian aging patterns is already apparent in young oocyte donors. Reprod Biol Endocrinol 11: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dorsey E (2012) Characterization of a large group of individuals with huntington disease and their relatives enrolled in the COHORT study. PLoS One 7: e29522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Machuca-Tzili L, Brook D, Hilton-Jones D (2005) Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve 32: 1–18. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka F, Doyu M, Ito Y, Matsumoto M, Mitsuma T, et al. (1996) Founder effect in spinal and bulbar muscular atrophy (SBMA). Hum Mol Genet 5: 1253–1257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.