Abstract
Background
A periodic electrical activity, termed “slow waves”, coordinates gastrointestinal contractions. Slow wave dysrhythmias are thought to contribute to dysmotility syndromes, such as post-operative gastroparesis, but the clinical significance of these dysrhythmias remains poorly defined. Electrogastrography (EGG) has been unable to characterise dsyrhythmic activity reliabily, and the most accurate method of evaluating slow waves is to record directly from the surface of the target organ. This study presents a novel laparoscopic device for recording serosal slow wave activity, together with its validation.
Methods
The device consists of a shaft (diameter, 4 mm; length, 300 mm) and a flexible connecting cable. It contains four individual electrodes and is fully shielded. Validation was performed by comparing slow wave recordings from the laparoscopic device with those from a standard electrode platform in an open-abdomen porcine model. An intraoperative human trial of the device was also performed by recording activity from the gastric antrum of a patient undergoing a laparoscopic cholecystectomy.
Results
Slow wave amplitudes were similar between the laparoscopic device and the standard recording platform (mean 0.38 ± 0.03 mV vs range 0.36 – 0.38 ± 0.03 mV) (p=0.94). The signal to noise ratio (SNR) was also similar between the two types of electrodes (13.7 dB vs 12.6 dB). High quality antral slow wave recordings were achieved in the intraoperative human trial (amplitude, 0.41 ± 0.04 mV; SNR, 12.6 dB), and an activation map was constructed showing normal aboral slow wave propagation at a velocity of 6.3 ± 0.9 mm s−1.
Conclusions
The novel laparoscopic device achieves high-quality serosal slow wave recordings. It is easily deployable and is atraumatic. It is anticipated that this device will aid in the clinical investigation of normal and dsyrhythmic slow wave activity. In particular, it offers new potential to investigate the effect of surgical procedures on slow wave activity.
Keywords: Gastric electrical activity, GEA, smooth muscle, slow wave, laparoscopy, minimally-invasive
Introduction
Slow waves are generated and propagated by the interstitial cells of Cajal (ICCs) in the gastrointestinal (GI) tract wall. This activity confers a fundamental level of control over GI motility by serving to induce and organise phasic muscle contractions (1),(2). In the stomach, slow waves originate at the site of an intrinsic pacemaker high on the greater curvature and travel to the antrum at a normal frequency of approximately 3 cycles per minute (cpm) (2). Slow wave dysrhythmias are thought to play an important role in a number of dysmotility syndromes, such as gastroparesis and functional dyspepsia (3),(4),(5), and may also play an important role in the acute or chronic delayed gastric emptying seen after various types of GI surgery (6).
To date, the major tool for investigating the nature and significance of slow wave dysrhythmias clinically has been electrogastrography (EGG), which involves placing cutaneous electrodes over the abdomen in a manner analogous to electrocardiography (ECG). A non-invasive approach, EGG can provide basic information on the frequency, rhythmicity and amplitude of slow wave activity (7). However, EGG as currently practiced cannot reliably define the spatiotemporal properties of slow wave activation such as the direction and velocity of propagation, details that are required for accurate description of both normal and abnormal slow wave activity (8),(9).
For recording the spatiotemporal properties of slow wave activation reliably, it is currently necessary to record the electrical potentials directly from the surface of the target organ (2). To date, only a relatively small number of such recordings have been performed in clinical studies. As a result, the clinical significance of slow wave dysrhythmias remains poorly defined (8).
The adoption of minimally invasive techniques for the treatment of gastrointestinal disease has been striking. Laparoscopic techniques offer a minimally invasive way to record electrical activity directly from the serosal surface of the GI tract, but this has rarely been attempted or validated. In this study, we present the design and validation of a novel device for laparoscopically recording high-quality slow wave activity.
Materials and Methods
Design and Fabrication
The novel laparoscopic device consists of a shaft (diameter, 4 mm; length, 300 mm), containing a 2 × 2 array of electrodes at its recording tip and a flexible connecting cable (length, 1.7 m). The device was designed for atraumatic placement on the GI serosal surface, without the need for electrode penetration into the seromuscular layer or for clipping or suturing the electrodes into a fixed position.
The design of the device is demonstrated in Figure 1. The four electrodes were fabricated from Teflon-coated silver wires with a diameter of 0.3 mm. The choice of this electrode size was based on previous experience regarding the optimal size for slow wave recording (10). The inter-electrode distance was limited to approximately 2 mm to allow the complete device to fit through a 5 mm surgical trochar. The electrode wires were soldered individually to Teflon-coated stainless-steel connecting wires, and together these were sheathed in a heat-shrink tube. A stainless-steel shield was pulled over the shrink tubing, and the whole bundle was inserted into a larger diameter (4 mm) Teflon tube. The Teflon tube was then filled with enough epoxy-resin to fill the contained space, and make the shaft stiff enough to allow straightforward direction of the electrodes toward the target organ through a laparoscopic trochar.
Figure 1. The novel laparoscopic device.
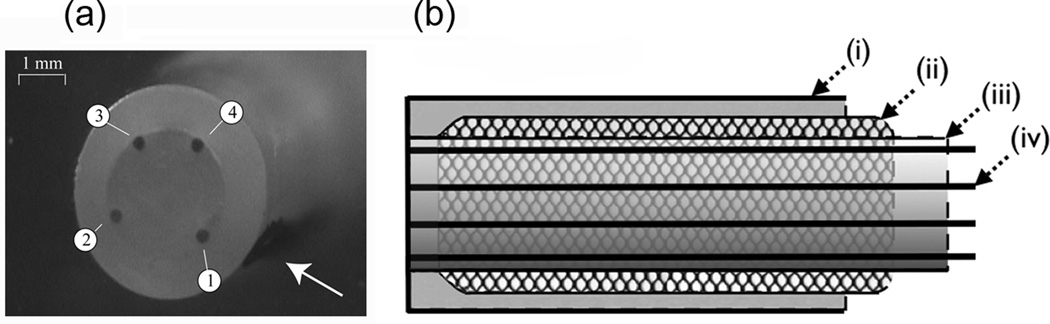
(a): Photograph of recording tip; the electrodes are labelled 1–4 and an arrow points to a reference marker denoting the position of electrode 1; (b): Shaft design: (i) 4 mm Teflon sleeve; (ii) stainless steel shield; (iii) heat shrink tube; (iv) 0.3 mm Teflon-coated silver wires. The sleeve was filled with epoxy resin.
At the end of the shaft, the connecting wires were run through a flexible connecting cable of adequate length (1.7 m) to allow positioning of the recording equipment clear of the sterile operating field. This cable was fabricated from a sheath of 6 mm silicone tubing, which was placed over stainless-steel shielding in continuity with that of the shaft. At the end of the cable, the connecting wires were connected to a bayonet jack and socket connector set, designed to connect with the slow wave acquisition device as described in the next section.
Methods of the Porcine In-vivo Validation Trial
Validation of the device was performed in a porcine model by comparing gastric slow wave recordings from the novel laparoscopic device with those from a 32-channel (4 × 8) epoxy-resin platform (E32). The E32 is a standard rigid multi-electrode platform similar to those used in many previous studies involving the serosal mapping of slow wave activity, and was custom built in our laboratory ((8),(9),(10)). Both the novel device and the E32 platform were connected via a 1.5 m 68-way ribbon cable to an ActiveTwo signal acquisition system (Biosemi, Amsterdam, The Netherlands). The ActiveTwo is a direct current (DC) amplifier, performing 24-bit sampling at 31 nV resolution, through as many as 256 electrode channels.
Unipolar recordings were taken, with a reference electrode positioned on the lower abdomen. The ActiveTwo system was in turn connected to a notebook computer via a fibre-optic cable. This setup allows the acquisition system, which runs on battery power, to be electrically decoupled from the external current source. Acquisition software was written in Labview 8.2 (National Instruments, Texas). The recording frequency was set at 512 Hz. The on-screen display signals were filtered by a low-pass filter with a cut-off frequency of 10 Hz.
After each recording, signals were filtered, slow waves characterised, and activation times mapped according to our previously described methods (9),(11). Recorded noise was quantified by the amplitude of the highest frequency component in a power spectrum of a representative 20 s recording window, and the signal-to-noise ratio (SNR) expressed in decibels (dB).
Ethical approval for validation was obtained from the local institutional committee (The University of Auckland Animal Ethics Committee) and The International Guiding Principles for Biomedical Research Involving Animals were followed. Validation was performed in two female weaner cross-breed pigs weighing, respectively, 37.6 and 33.4 kg. Induction anaesthesia was achieved with tiletamine HCl 50 mg/ml and zolazepam HCl 50 mg/ml, followed by intubation and maintenance anaesthesia with isoflurane.
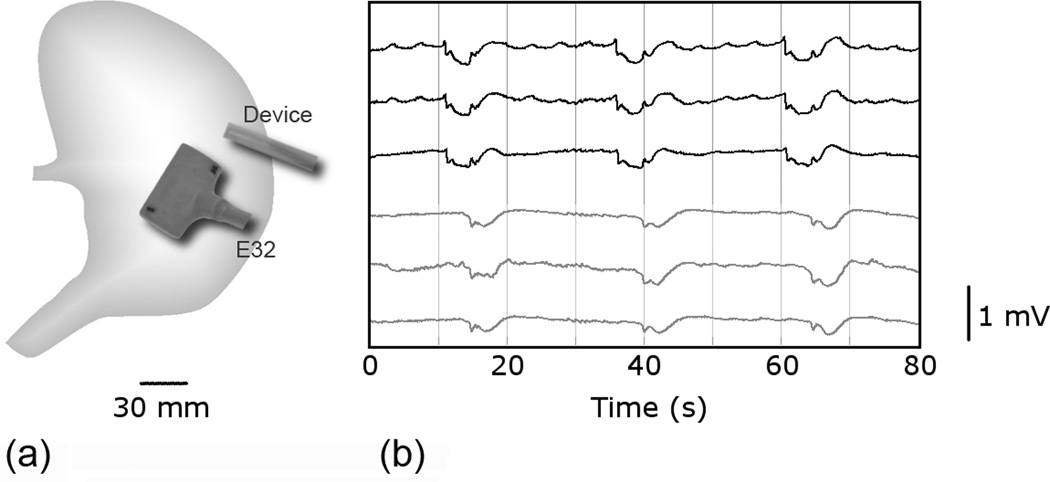
The pigs were placed supine on a heating pad, with continuous monitoring undertaken to ensure that arterial blood pressure, temperature and oxygen saturations were maintained within normal physiological ranges. A bilateral subcostal laparotomy incision was performed and the anterior gastric serosal surface exposed. The E32 platform and laparoscopic device were positioned on the gastric corpus of each pig, separated by a 45 mm gap, in the positions shown in Figure 2a. The electrode positions were the same in both pigs. The platforms were secured into position with test tube clamps. The wound edges were approximated as much as possible, and a 10 minute period of stabilisation was allowed before a 15 minute recording period.
Figure 2. Porcine in-vivo validation trial.
(a) Position of the novel laparoscopic device and standard electrode platform (E32) on the anterior serosal surface of the porcine stomach. The devices were separated by a 45 mm gap. (b) A typical 80 s segment of slow wave recordings from the positions shown in (a). The top three channels are from the laparoscopic device and the bottom three channels are from the standard platform. A time lag of approximately 6.5 s is noted in the detection of slow wave events between the two devices.
Slow wave amplitudes were statistically compared over 20 consecutive representative slow wave events from each pig in the laparoscopic device and E32 platform recording channels using Student’s t-test. A p-value less than 0.05 was considered significant for a difference in recording amplitudes between the platforms.
Methods for the Human Intraoperative Validation Trial
After validation in the porcine model, an additional trial was conducted with a separate prototype for additional validation the device in the human intraoperative environment. Ethical approval was obtained from the New Zealand Northern X Regional Human Ethics Committee. Informed consent for participation was obtained from a 28-year old female undergoing a routine laparoscopic cholecystectomy for mild acute cholecystitis. This patient had no significant additional medical history, no personal or family history of gastric symptoms or diseases, and was receiving only intravenous antibiotics and analgesia.
After routine anaesthesia, abdominal insufflation, and laparoscopic trochar placement for cholecystectomy, but before commencement of further dissection, the novel laparoscopic device was inserted via a 10 mm epigastric port under direct vision. The device tip was placed on the serosal surface of the mid antrum (Figure 3a). The device was hand held, with the recording hand held steady against the anterior abdominal wall and the trocar itself. The recording setup was otherwise as described earlier. A 10 minute period of recording was obtained, and the quality of the recorded signals was assessed at the completion of the experiment by determining the frequency, amplitude and velocity of slow wave events and the SNR.
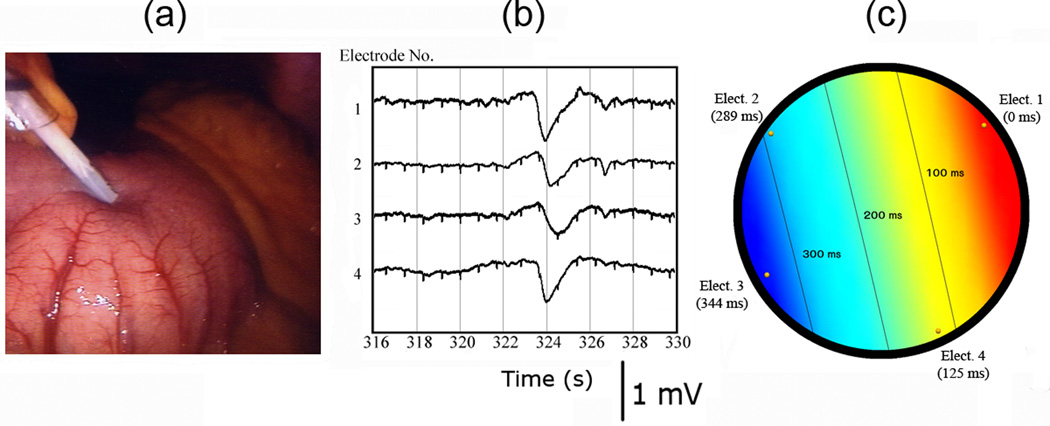
Figure 3. Human intraoperative validation trial.
(a) The device tip is shown in position against the serosa of the gastric antrum. (b) A typical slow wave event recorded by the device is shown at t = 324 s. A slight lag is noted between channels and distant ECG signals are recognisable as small notched deflections. (c) Activation map of the event in (b), showing aboral slow wave propagation at a velocity of 6.3 ± 0.9 mm s−1.
Results
Porcine In-vivo Validation Trial
Slow wave activity was immediately and continuously recorded via both the novel laparoscopic device and standard E32 platform in the two porcine trials. A typical 80 s recording window is shown in Figure 2b comparing slow wave events from three laparoscopic device channels and three E32 channels. Clean, high-quality signals are demonstrated in both recordings. Regular slow wave activity was recorded in all channels at 2.35 ± 0.05 cycles per minute (cpm) in the first trial, and 2.37 ± 0.21 cpm in the second trial.
Figure 2b also demonstrates a time lag of approximately 6.5s in the registration of slow wave events between the E32 platform and laparoscopic device. Because the two types of electrode platforms were placed 45 mm apart, this time lag implies that the velocity of the slow waves was approximately 6.9 mm s−1 between the two platforms. This velocity is within the anticipated range for the porcine corpus (9). This anticipated lag confirms that the recorded events were true slow wave activity and not extraneous noise such as movement artifacts, which would register in all channels simultaneously.
Slow wave amplitudes were found to be comparable between the laparoscopic device and the E32 platform. In the first trial, the recorded amplitudes were 0.38 ± 0.02 mV in the laparoscopic device and 0.36 ± 0.01 mV in the E32 platform (p=0.45), and in the second trial they were 0.38 ± 0.03 mV in the laparoscopic device and 0.38 ± 0.03 mV in the E32 (p=0.94). The SNR was comparable between the laparoscopic device (13.7 dB) and the E32 platform (12.6 dB) for the evaluated period.
Human Intraoperative Validation Trial
The novel laparoscopic device was easily inserted through the surgical trochar (Figure 3a). Slow wave activity was immediately and continuously recorded by the device upon contact with the gastric antrum and was found to be regular at 3.1 ± 0.2 cpm for the duration of the recording. The slow wave amplitude over 10 consecutive cycles was 0.41 ± 0.04 mV, with a SNR (12.6 dB) similar to that noted in the in-vivo porcine validation study. A typical slow wave event from the intraoperative trial is shown in Figure 3b. A small lag may be identified between the device’s individual recording channels, again serving to confirm that the recorded event represents true slow wave activity, and not extraneous noise, which would register in all channels simultaneously.
The slow wave activation times of each of the device’s four individual electrodes were calculated from the event demonstrated in Figure 3b. Figure 3c displays these activation times, together with an activation map that was constructed by interpolation over a projection of the laparoscopic device’s recording tip. This activation map demonstrates normal aboral slow wave propagation at a velocity of 6.3 ± 0.9 mm s−1.
Discussion
This report has detailed the design of a novel laparoscopic device for recording gastrointestinal slow wave activity. Validation of this device was achieved in an in-vivo porcine trial, with recordings found to be on-par with those of a standard multi-electrode platform of the type used in many previous serosal slow wave mapping studies. The device was successfully tested in a human intraoperative trial in which it was also found to achieve high-quality slow wave recordings with a good SNR.
This novel device incorporates a number of important design features that make it a valuable and practical reseach tool. The recording head contains four separate electrodes. This duplication that translates to a stronger assurance of achieving a quality recording in one or more channels if the other channels are affected by signal artefact or poor contact. The comprehensive stainless steel shielding is shown to minimise signal interference successfully, resulting in the excellent SNR. A major strength of the device is ease of use. It is readily deployed through a 5 or 10 mm trochar and handheld in the desired position against the serosa, without the need for technically-demanding manipulations such as suture fixation, external devices or retractor arms to support it.
Although the device has a small intra-electrode distance, this distance is large enough to discern an appreciable time lag in the detection of activities between the individual channels. This time lag eliminates the possibility of confusing slow wave events with signal noise, such as respiration or movement artefacts, which would occur in all channels simultaneously. This time lag also allows the determination of the slow wave activation sequence across the device’s recording tip, as was demonstrated during the human trial in this study (Figure 3c). The estimation of the slow wave velocity by this method has a relatively large error due to the close proximity of the electrodes. However, the activation sequence nevertheless provides useful spatiotemporal knowledge regarding the direction and velocity of slow wave propagation at the point of measurement.
To our knowledge, only one technique of recording slow waves via a laparoscopic approach has been previously described (12). With that technique, two stainless-steel cardiac pacing wires were inserted into the seromuscular layers of the gastric antrum and small bowel.
The novel device presented in this report offers a number of advantages over the pacing wire method including the increased number of electrodes, effective shielding, high signal quality, ease of use, and the extra spatiotemporal detail conveyed. Our novel device is also atraumatic, eliminating the risk of peritoneal contamination with GI contents from the puncture sites that are created during insertion of penetrating wires.
Recordings of slow wave activity are considered to be stable to the influence of anaesthesia on the basis of previous studies, and this device is therefore expected to achieve reliable interoperative recordings (13),(14),(15). However, slow wave dysrhythmias have been characterised previously under the influence of opiates, including fentanyl, and recordings should therefore be interpreted with an awareness for confounding effects when opiates are in use (8).
The described device provides another method for recording slow wave activity. No current method is ideal because all represent a compromise between invasiveness, spatiotemporal detail, and recording quality. The non-invasive technique of EGG has been the most widely employed method, but EGG as currently practiced cannot provide the spatiotemporal detail necessary to quantify the direction and velocity of slow wave propagation, or to discriminate reliably between normal and abnormal activity. For these reasons, EGG does not correlate strongly with symptoms or clinical markers of dysmotility, and remains of limited diagnostic and research value (16),(17).
A more recently developed non-invasive method for recording slow wave activity is magnetogastrography (MGG), which records the magnetic field accompanying slow wave propagation, via the use of a Superconducting QUantum Interference Device (SQUID) (18). However, the SQUID is an expensive and specialised tool currently only available to a small number of researchers, and MGG continues to be an experimental technique (2).
The most accurate method for recording the detailed spatiotemporal properties of slow wave activation is by high-resolution mapping. High-resolution mapping involves placing a grid of recording electrodes (typically >100) over the serosal surface of target organs, ensuring precise knowledge of the sequence of electrical activation over an organ surface (8),(9). Although ideal, this technique requires an open abdomen and therefore is only applicable to operations requiring a large incision. Recordings can be achieved via a less invasive approach at the mucosal surface, (e.g., by hemoclipping or suctioning electrodes into position (19),(20). Although sparse detail regarding slow wave propagation direction and velocity can be gained via these mucosal recording techniques, the high electrical impedence of the mucosa significantly reduces the signal quality of recordings, and the mucosal electrodes are prone to dislodging (20).
Similar to the mucosal recording methods, our novel laparoscopic device can record GI electrical activity in sparse spatiotemporal detail. However, as shown in this study, it does allow construction of a small activation map, and two or more of the devices can also be used simultaneously, and placed in a succession of positions, to generate a more detailed evaluation of electrical activation.
It is likely that the laparoscopic approach would be considered too invasive for the routine investigation of dysmotility disorders in humans, although this might prove useful in severe cases. However, the device does open up the opportunity to investigate and define normal and altered electrical activity in patients undergoing routine laparoscopic surgery.
It is known that gastric incisions can significantly alter the frequency, rhythm, and propagation patterns of slow wave activity in the stomach (21). Although a number of routine operations irreversibly alter the anatomy of significant portions of the gastric conduction system, the myoelectrical effects of these procedures have not yet been adequately documented or used to predict functional outcome. In particular, only very few studies to date have evaluated whether altered electrophysiology is a factor contributing significantly to the acute or chronic delays in gastric emptying seen after operations such as fundoplication for gastro-oesophageal reflux, proximal gastrectomy for cancer, pancreatocoduodenectomy, sleeve gastrectomy, and gastric bypass surgery for morbid obesity (6),(22),(23).
We anticipate that the new laparoscopic device and HR mapping tools suited to open surgery (9) will be used to compare slow wave activity before and after surgical manipulations in these and other operations to define the precise electrophysiological consequences of the surgery. Correlations between the slow wave outcomes measured (frequency, rhythm, amplitude, direction and velocity of propagation), and the patients’ dysmotility and symptom indices will thereafter allow a clear determination of the role of electrical disturbances in post-operative dysmotility.
In addition, a recent study has described the complex events underlying gastric dysrhythmias for the first time, showing complex focal activities and re-entrant patterns similar to pathological electrical activities well known to occur in the heart (8). The presence, prevalence and effect of these complex dysrhythmic behaviours in clinical and post-operative dysmotility disorders needs to be determined, and this novel laparoscopic device should provide a useful investigative tool, at least for severe cases. Gastric electrical stimulation (GES) has been proposed previously as a potential means to treat gastric dysrhythmias and dysmotility problems, including in post-surgical patients (24). This device may therefore also assist in clarifying whether there is a real potential benefit to be gained from using GES to manipulate slow wave behaviour to enhance recovery after surgery, and to help in the development of clinically effective GES devices for human use.
Because this tool is limited to the intraoperative setting, it would also be of future value if an implantable device were developed to allow chronic recordings in selected patients. Prototype implant devices have been developed previously and tested in animal models, but further work is needed before such devices are deemed suitable for human use (25).
In conclusion, the novel laparoscopic device presented in this report, together with its in-vivo validation, will provide new opportunities to recording slow wave activity during surgery. The device will be a valuable tool to facilitate new clinical studies of normal and dsyrhythmic slow wave activity. In particular, it will help to define the effects of various GI surgical procedures on slow wave activity.
Acknowledgements
This work is partially supported by grants from the NIH (R01 DK64775), NZ Society of Gastroenterology, the NZ Health Research Council, and the Auckland Medical Research Foundation, and the University of Auckland. We thank Linley Nisbet for her assistance with the in-vivo validation study.
References
- 1.Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–G8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 2.Cheng LK, O'Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. Gastrointestinal System. Wiley Interdiscip Rev Syst Biol Med. 2010 Jan-Feb;2(1):65–79. doi: 10.1002/wsbm.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978 Sep;75(3):497–501. [PubMed] [Google Scholar]
- 4.Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90–G98. doi: 10.1152/ajpgi.1994.266.1.G90. [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Lin HH, Huang LC, Huang SC, Liu TT. Electrogastrography differentiates reflux disease with or without dyspeptic symptoms. Dig Dis Sci. 2004;49:715–719. doi: 10.1023/b:ddas.0000030079.20501.62. [DOI] [PubMed] [Google Scholar]
- 6.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–1816. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 7.Koch KL, Stern RM. Handbook of electrogastrography. Oxford: Oxford University Press; 2004. [Google Scholar]
- 8.Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008;135:1601–1611. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Du P, O'Grady G, Egbuji J, Lammers WJ, Budgett D, Nielsen P, Windsor JA, Pullan AJ, Cheng LK. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: methodology and validation. [Feb 18, 2009];Ann Biomed Eng. 2009 doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lammers WJ, Stephen B, Arafat K, Manefield GW. High resolution electrical mapping in the gastrointestinal system: initial results. Neurogastroenterol Motil. 1996;8:207–216. doi: 10.1111/j.1365-2982.1996.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Lammers WJ, Ver Donck L, Schuurkes JA, Stephen B. Peripheral pacemakers and patterns of slow wave propagation in the canine small intestine in vivo. Can J Physiol Pharmacol. 2005;83:1031–1043. doi: 10.1139/y05-084. [DOI] [PubMed] [Google Scholar]
- 12.Familoni BO, Abell TL, Voeller G. Measurement of gastric and small bowel electrical activity at laparoscopy. J Laparoendosc Surg. 1994;4:325–332. doi: 10.1089/lps.1994.4.325. [DOI] [PubMed] [Google Scholar]
- 13.Lammers WJ, Abazer FA, Ver Donck L, Smets D, Schuurkes JA, Coulie B. Electrical activity in the rectum of anaesthetized dogs. Neurogastroenterol Motil. 2006 Jul;18(7):569–577. doi: 10.1111/j.1365-2982.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 14.Lammers WJ, Donck LV, Schuurkes JA, Stephen B. Longitudinal and circumferential spike patches in the canine small intestine in vivo. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1014–G1027. doi: 10.1152/ajpgi.00138.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 16.Abid S, Lindberg G. Electrogastrography: poor correlation with antro-duodenal manometry and doubtful clinical usefulness in adults. World J Gastroenterol. 2007;13:5101–5107. doi: 10.3748/wjg.v13.i38.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buist ML, Cheng LK, Sanders KM, Pullan AJ. Multiscale modelling of human gastric electric activity: can the electrogastrogram detect functional electrical uncoupling? Exp Physiol. 2006;91:383–390. doi: 10.1113/expphysiol.2005.031021. [DOI] [PubMed] [Google Scholar]
- 18.Bradshaw LA, Irimia A, Sims JA, Gallucci MR, Palmer PL, Richards WO. Biomagnetic characterization of spatiotemporal parameters of the gastric slow wave. Neurogastroenterol Motil. 2006;18:619–631. doi: 10.1111/j.1365-2982.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwong NK, Brown BH, Whittaker GE, Duthie HL. Electrical activity of the gastric antrum in man. Br J Surg. 1970;57:913–916. doi: 10.1002/bjs.1800571211. [DOI] [PubMed] [Google Scholar]
- 20.Coleski R, Hasler WL. Directed endoscopic mucosal mapping of normal and dysrhythmic gastric slow waves in healthy humans. Neurogastroenterol Motil. 2004;16:557–565. doi: 10.1111/j.1365-2982.2004.00542.x. [DOI] [PubMed] [Google Scholar]
- 21.Weber J, Jr, Koatsu S. Pacemaker localization and electrical conduction patterns in the canine stomach. Gastroenterology. 1970 Nov;59(5):717–726. [PubMed] [Google Scholar]
- 22.Hocking MP, Harrison WD, Sninsky CA. Gastric dysrhythmias following pylorus-preserving pancreaticoduodenectomy. Possible mechanism for early delayed gastric emptying. Dig Dis Sci. 1990;35:1226–1230. doi: 10.1007/BF01536411. [DOI] [PubMed] [Google Scholar]
- 23.Hocking MP. Postoperative gastroparesis and tachygastria--response to electric stimulation and erythromycin. Surgery. 1993;114:538–542. [PubMed] [Google Scholar]
- 24.Hasler WL. Methods of gastric electrical stimulation and pacing: a review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009;21:229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 25.Ver Donck L, Lammers WJ, Moreaux B, Smets D, Voeten J, Vekemans J, Schuurkes JA, Coulie B. Mapping slow waves and spikes in chronically instrumented conscious dogs: implantation techniques and recordings. Med Biol Eng Comput. 2006;44:170–178. doi: 10.1007/s11517-005-0018-9. [DOI] [PubMed] [Google Scholar]