Abstract
Preexisting lymphocytic infiltration of tumors is associated with superior prognostic outcomes in a variety of cancers. Recent studies also suggest that lymphocytic responses may identify patients more likely to benefit from therapies targeting immune checkpoints, suggesting that therapeutic efficacy of immune checkpoint blockade can be enhanced through strategies that induce tumor inflammation. To achieve this effect, here we explored the immunotherapeutic potential of oncolytic Newcastle Disease Virus (NDV). We find that localized intratumoral therapy of B16 melanoma with NDV induces inflammatory responses leading to lymphocytic infiltrates and anti-tumor effect in distant (non-virally injected) tumors without distant virus spread. The inflammatory effect coincided with distant tumor infiltration with tumor-specific CD4+ and CD8+ T cells, which was dependent on the identity of the virus-injected tumor. Combination therapy with localized NDV and systemic CTLA-4 blockade led to rejection of pre-established distant tumors and protection from tumor re-challenge in poorly-immunogenic tumor models, irrespective of tumor cell line sensitivity to NDV-mediated lysis. Therapeutic effect was associated with marked distant tumor infiltration with activated CD8+ and CD4+ effector but not regulatory T cells, and was dependent on CD8+ cells, NK cells and type I interferon. Our findings demonstrate that localized therapy with oncolytic NDV induces inflammatory immune infiltrates in distant tumors, making them susceptible to systemic therapy with immunomodulatory antibodies, which provides a strong rationale for investigation of such combination therapies in clinic.
Introduction
The discovery of T cell regulatory receptors provided targets for immunotherapies aiming to enhance activation of anti-tumor immune responses or to reverse immunosuppressive mechanisms governing tumor resistance to immune surveillance and destruction(1). Targeting of the latter with antibodies to immunologic checkpoints such as CTLA-4 and PD-1 demonstrated durable tumor regressions, though the therapeutic efficacy in patients and in poorly-immunogenic animal models has not been universal(2–5). These findings call for identification of biomarkers predictive of response and development of combinatorial strategies that could make therapy beneficial for a larger patient population and a broader range of tumor types.
Data from clinical trials identified pre-existing tumor infiltrating lymphocytes (TILs) and an immune-active tumor transcriptional profile as strong predictors of response to immunotherapy(6, 7), with type I interferon (IFN) emerging as an important pathway in CD8-mediated tumor rejection(8, 9). These findings provide a strong incentive to explore strategies that could activate the type I IFN pathway and enhance tumor immune infiltration as a means to render tumors sensitive to therapy with immune checkpoint blockade.
Oncolytic viruses (OVs) represent another class of promising emerging cancer therapeutics, with viruses from several families currently being evaluated in clinical trials(10). While in many studies OVs appeared to be effective anti-tumor agents with locoregional administration, very few studies have demonstrated therapeutic efficacy or characterized immune responses in established distant or metastatic lesions(11–13), which presents an obvious impediment to clinical investigation.
To address the limitations of these two therapeutic approaches, we explored whether the inflammatory responses generated by OVs with local administration could be harnessed to improve therapeutic efficacy of agents targeting immunologic checkpoints, which would, in turn, eliminate the need for viral delivery to all tumor sites. To this end, we utilized the nonpathogenic Newcastle Disease Virus (NDV), an avian paramyxovirus with robust type I IFN-inducing and oncolytic properties and strong clinical safety record(14–18). We initially set out to characterize the effects of NDV on the microenvironment of the virus-injected tumors and distant tumors, modeling metastatic disease. Unexpectedly, we find that intratumoral administration of NDV results in distant (non-virally injected) tumor infiltration with activated lymphocytes in the absence of distant viral spread. Conversion of distant tumors to an inflammatory phenotype made them susceptible to therapy with systemic CTLA-4 blockade, leading to tumor rejection and long-term survival in the majority of mice treated with the combination approach. These findings demonstrate an attractive strategy to enhance therapeutic efficacy of immunotherapeutic antibodies and to overcome the limitations of oncolytic virotherapy, providing a strong rationale for exploration of such combination strategies in a clinical setting.
Results
NDV replication is restricted to the injected tumor site
In an attempt to utilize NDV for therapy that would be active against metastatic disease, we initially characterized the viral distribution kinetics with intratumoral and systemic administration. Intratumoral injection of recombinant NDV expressing firefly luciferase reporter (NDV-Fluc) resulted in sustained luciferase signal in the injected flank tumor, while systemic administration of the virus resulted in no detectable luciferase signal in the tumor (Fig. S1A). As limited systemic virus delivery was unlikely to induce sufficient tumor lysis and immune response, we explored the intratumoral NDV injection as a means to elicit an anti-tumor immune response that could potentially overcome the limitations of systemic OV therapy. As such, for our further studies we modeled metastatic disease by using the bilateral flank B16-F10 tumor model (Fig. 1A). NDV-Fluc administration into the right flank tumor resulted in viral replication within the injected tumor, with the luciferase signal detectable for up to 96 hours (Fig. S1B–D). No virus was detected in the contralateral (left flank) tumor by luminescent imaging (Fig. S1B–D), by passage in embryonated eggs, or RT-PCR. This system thus allowed us to characterize the immune responses in both virus-injected and distant tumors, which were not directly affected by NDV.
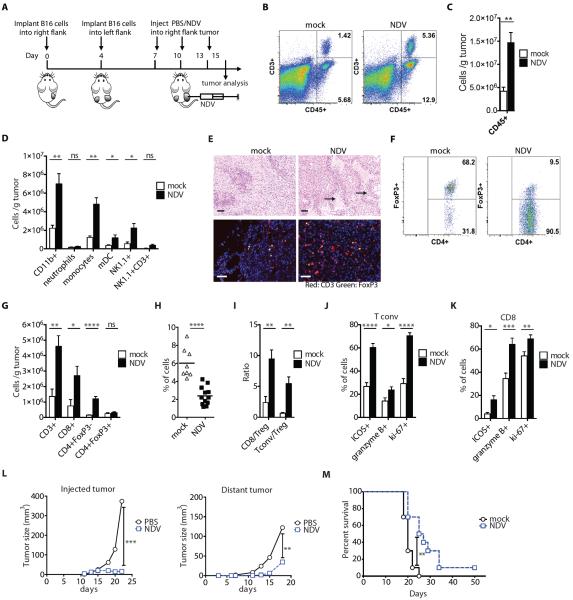
Figure 1. NDV increases distant tumor lymphocyte infiltration and delays tumor growth.
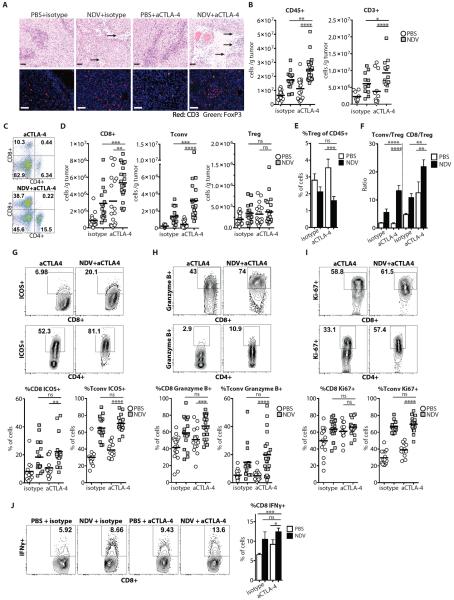
(A) Treatment scheme. (B) Representative flow cytometry plots of percentages of tumor-infiltrating CD45+ and CD3+ cells. (C) Absolute numbers of CD45+ cells/g tumor. (D) Absolute numbers of innate immune cells/g tumor. (E) Tumor sections from distant tumors were stained with H&E (upper panels) or labeled for CD3 and FoxP3 (bottom panels) and analyzed by microscopy. Areas denoted by arrows indicate areas of necrosis and inflammatory infiltrates. Scale bars represent 200 μm. (F) Representative flow cytometry plots of percentages of CD4+FoxP3+ (Treg) and CD4+FoxP3− (Tconv) cells. (G) Absolute numbers of conventional and regulatory CD4+ cells and CD8+ cells/g tumor calculated from flow cytometry. (H) Relative percentages of Tregs out of CD45+ cells. (I) Calculated Tconv/Treg and CD8+/Treg ratios. (J,K) Upregulation of ICOS, Granzyme B, and Ki-67 on tumor-infiltrating Tconv (J) and CD8+ cells (K). (L) Growth of NDV-injected and distant tumors. (M) Overall animal survival. Data represent cumulative results from 3 (B–K) or 2 (L–M) independent experiments with n=3–5 per group. Mean +/− SEM is shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
NDV therapy increases local and distant tumor lymphocyte infiltration and delays tumor growth
Analysis of the virus-injected tumors revealed an inflammatory response as evidenced by increased infiltration with cells expressing leukocyte common antigen CD45 (Supplementary Figs. 2A,B). The immune infiltrates were characterized by increase in innate immune compartment, including myeloid cells, NK cells, and NKT cells (Fig. S2C), and the adaptive compartment, including CD8+ and conventional CD4+FoxP3− (Tconv) T cells, leading to significant increase of CD8 and Tconv to regulatory (Treg) T cell ratios (p=0.0131 and p=0.0006, respectively) (Supplementary Figs. 2D–2F). Remarkably, analysis of the contralateral tumors revealed a similar increase in the inflammatory infiltrates (Fig. 1B,C and table S1), characterized by increased numbers of both innate immune cells (Fig. 1D and table S1) and effector T cells (Fig. 1E,G and table S1). Notably, although there were no major changes in the absolute number of Tregs (Fig. 1G), there was a substantial decrease in their relative percentages (Fig. 1E,F,H and table S1), with significant enhancement of the CD8 and Tconv to Treg ratios (p=0.002 and p=0.0021, respectively) (Fig. 1I and table S1). Effector T cells isolated from the distal tumors expressed increased activation, proliferation, and lytic markers ICOS, Ki-67, and Granzyme B, respectively (Figure 1J,K and table S1). As previously, we were unable to isolate the virus or viral RNA from the distant tumors, suggesting that the observed changes in the distant tumor microenvironment were not due to direct viral infection. In order to further exclude the possibility of undetectable local viral spread, tumors were implanted at other distant sites, such as bilateral posterior footpads, which generated similar findings (Fig. S3).
Consistent with the observed inflammatory effect, intratumoral administration of NDV resulted in growth delay not only of the injected, but also of the contralateral tumors, resulting in prolonged animal survival (Figure 1L,M and table S2). To determine whether this effect was transient and whether durable anti-tumor protection was possible, we intratumorally treated single-flank B16-F10 tumor-bearing mice with NDV, and injected long-term survivors with B16-F10 cells on the opposite flank. The majority of the animals demonstrated tumor growth delay, and 30% of the animals completely rejected rechallenged cells, suggesting that intratumoral therapy with NDV can indeed induce protective anti-tumor memory responses (Fig. S4).
NDV induces tumor infiltration and expansion of tumor-specific lymphocytes
To determine whether the anti-tumor immune response was dependent on the NDV-injected tumor type or a result of nonspecific inflammation generated by NDV infection, we performed the experiment with heterologous tumors (MC38 colon carcinoma and B16-F10 melanoma) implanted at the opposite flanks (Fig. 2A and table S3). To track tumor-specific lymphocytes, we adoptively transferred T cell receptor-transgenic congenically-marked CD8+ (Pmel) cells or luciferase-marked CD4+ (Trp1) cells recognizing the melanoma differentiation antigens gp100 (Pmel) and Trp1 (Trp1) (19, 20). Bioluminescent imaging was used to measure the distribution and expansion kinetics of the adoptively transferred Trp1 cells. Transfer of Trp1 cells into PBS-treated tumor-bearing animals failed to result in Trp1 accumulation in the tumors, highlighting the highly immunosuppressive nature of the tumor microenvironment in this model (Fig. 2B–D and table S3). NDV injection into B16-F10 tumors resulted in significant increase in the luciferase signal within the injected tumors (Fig. 2B–D and table S3), indicating Trp1 T cell expansion (area under the curve (AUC) p=0.0084). Remarkably, similar expansion was seen in the contralateral tumor, albeit at a delay (p=0.0009) (Fig. 2B–D and table S3). In contrast, NDV injection into MC38 tumors failed to induce substantial Trp1 infiltration into the injected MC38 tumors or distant B16-F10 tumors (Fig. 2B–D and table S3), suggesting that the distant tumor-specific lymphocyte infiltration is likely dependent on the antigen identity of the injected tumor. Similarly, intratumoral injection of NDV resulted in increased infiltration of Pmel cells in distant tumors, which was more pronounced when the injected tumor was B16-F10 rather than MC38 (Fig. 2E and table S4).
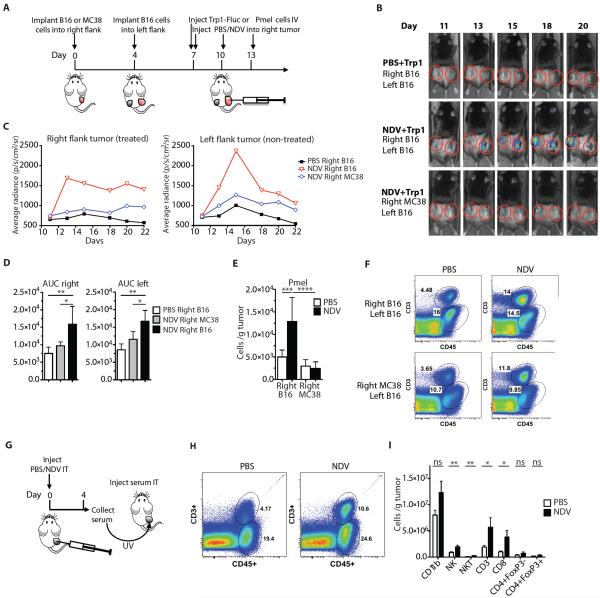
Figure 2. NDV induces infiltration of tumor-specific lymphocytes and facilitates tumor inflammation.
(A) Treatment scheme. (B) Representative luminescence images from animals treated with NDV and adoptively-transferred Trp1-Fluc lymphocytes. (C) Quantification of average luminescence from the tumor sites. (D) The area under the curve (AUC) calculated from the data in panel (C). (E) Absolute number of Pmel lymphocytes from distant tumors calculated from flow cytometry. (F) Representative flow cytometry plots of percentages of CD45+ and CD3+ cells infiltrating distant tumors of animals treated per treatment scheme in panel (A). (G) Experimental scheme for serum transfer from animals treated intratumorally with single injection of NDV or PBS. (H) Representative flow cytometry plots of percentages of CD45+ and CD3+ cells infiltrating serum-injected tumors. (I) Absolute numbers of the indicated cell subsets in serum-injected tumors calculated from flow cytometry. Data for panels B–E represent 1 of 3 experiments with n=4–5 per group. Data for panels G–I represent pooled data from 2 independent experiments with n=5 per group. Mean +/− SEM is shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Interestingly, although infiltration of distant B16-F10 tumors with adoptively-transferred lymphocytes was dependent on the injected tumor identity, distant tumors did demonstrate increased immune infiltration even when the primary injected tumor was MC38 (Fig. 2F), suggesting that a nonspecific inflammatory response component may also play a role. Indeed, serum from NDV-treated animals, treated with UV irradiation to inactivate any potential virus, induced tumor leukocyte infiltration when injected intratumorally into naïve B16-F10 tumor-bearing mice (Fig. 2G,H and table S4), with the majority of the increase seen in the NK and CD8+ compartments (p=0.0089 and p=0.0443, respectively) (Fig. 2I and table S4).
NDV and CTLA-4 blockade synergize to reject local and distant tumors
Despite the prominent inflammatory response and growth delay seen in distant tumors, complete contralateral tumor rejection with long-term survival was only seen in approximately 10% of animals (Fig. 1M and table S2), suggestive of active immunosuppressive mechanisms in the tumor microenvironment. Characterization of NDV-injected and distant tumors revealed upregulation of CTLA-4 on tumor-infiltrating T cells (Fig. S5). We thus hypothesized that NDV-induced tumor inflammation would make the tumors sensitive to systemic therapy with CTLA-4 blockade, as was previously suggested in patients with pre-existing tumor inflammatory infiltrates(6, 7). Remarkably, combination therapy of NDV with anti-CTLA-4 antibody (Fig. 3A and table S5) resulted in rejection of bilateral tumors and long-term survival in the majority of the animals, an effect that was not seen with either treatment alone (Fig. 3B–D and table S5). To determine the durability of the observed protection, we injected the surviving animals in the right flank on day 90 with B16-F10 cells without any further therapy. Animals treated with NDV and anti-CTLA-4 combination therapy demonstrated over 80% protection against tumor re-challenge, compared with 40% protection in the animals treated with single agent anti-CTLA-4 antibody (Fig. 3E and table S5).
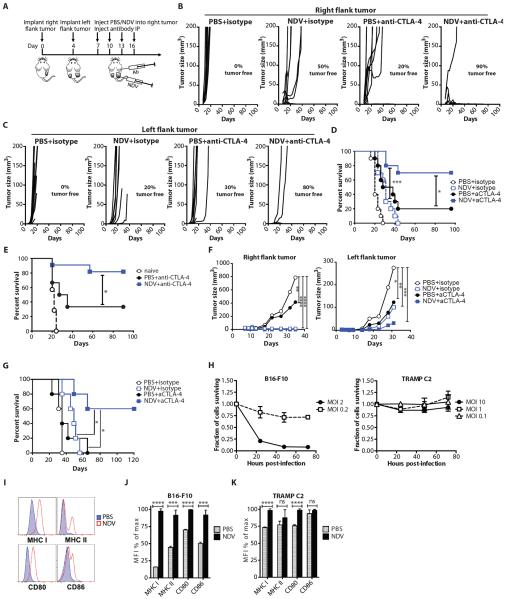
Figure 3. NDV and CTLA-4 blockade synergize to reject local and distant tumors.
(A) Treatment scheme. (B) Growth of virus-treated (right flank) B16-F10 tumors. (C) Growth of distant (left flank) B16-F10 tumors. (D) Long-term survival in the B16-F10 model. (E) Surviving animals were injected with 1×105 B16-F10 cells in right flank on day 90 and followed for survival. Data represent cumulative results from 3 experiments with n=6–11 per group. (F) Growth of virus-treated (right flank) and distant (left flank) TRAMP C2 tumors. (G) Long-term survival in the TRAMP C2 model. (H) In vitro sensitivity of B16-F10 and TRAMP C2 cells to NDV-mediated lysis at different multiplicities of infection (MOI's). (I–K) Upregulation of MHC I, MHC II, CD80, and CD86 in B16-F10 and TRAMP C2 cells infected with NDV. Representative flow cytometry plots from B16-F10 cells (I) and calculated average median fluorescent intensities (MFI) for B16-F10 (J) and TRAMP C2 (K) cells are shown. Mean +/− SEM is shown. Data represent results from 1 of 3 (B–E), or 1 of 2 (F,G) independent experiments with n=5–10 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Combination therapy with NDV and CTLA-4 blockade is effective against virus non-permissive tumors
To determine whether this treatment strategy could be extended to other tumor types, we evaluated it in the poorly-immunogenic TRAMP C2 prostate adenocarcinoma model. Similarly to the B16-F10 model, combination therapy caused regression of the injected tumors (Fig. 3F and table S6), and either delayed the outgrowth of distant tumors or led to complete distant tumor regression with prolonged long-term survival (Fig. 3F,G and table S6). Interestingly, whereas B16-F10 cells were susceptible to NDV-mediated lysis in vitro, TRAMP C2 cells were strongly resistant, with low cytotoxicity observed at a multiplicity of infection (MOI) of up to 10 (Fig. 3H). In both cell lines, NDV infection in vitro resulted in surface upregulation of MHC and co-stimulatory molecules (Fig. 3I–K). MHC class I was upregulated uniformly in all cells, even though not all cells get infected with NDV at the MOI of 1 In our previous studies we demonstrated that NDV induces type I IFN expression in B16-F10 cells (14). Both type I IFN (21) and IFNγ (22) are known to upregulate MHC class I on B16-F10 cells, suggesting that within the context of the infected tumors these mechanisms may play an additional role in enhancement of tumor immunogenicity. These results thus suggest that in vitro sensitivity to virus-mediated lysis is not necessary for sensitivity to NDV therapy in vivo and further highlight the importance of a virus-generated inflammatory response, rather than direct oncolysis, in the observed anti-tumor efficacy. A similar therapeutic effect was observed in the CT26 colon carcinoma model, which also showed poor in vitro sensitivity to NDV-mediated lysis (Fig. S6).
Systemic anti-tumor effect is antigen-restricted to the injected tumor type
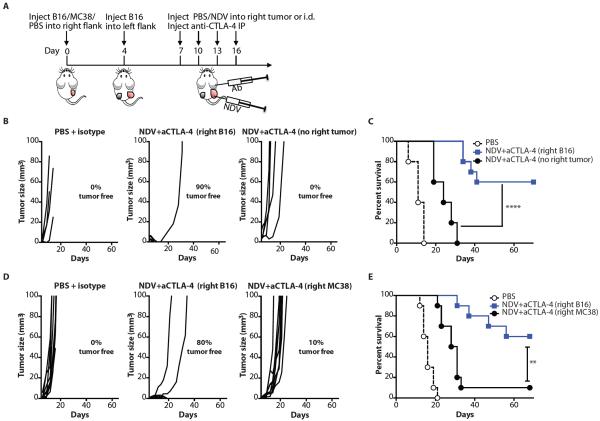
To determine whether the observed anti-tumor effect in the distant tumor was specific to the injected tumor type, we evaluated the combination therapy in animals bearing a unilateral distant B16-F10 tumor and in animals with heterologous tumor types (MC38 colon carcinoma and B16-F10 melanoma) implanted at the opposite flanks (Fig. 4A). Although administration of the virus intradermally into the non-tumor-bearing right flank resulted in delayed left flank tumor outgrowth, it failed to result in long-term protection and tumor rejection seen in the animals bearing bilateral B16-F10 tumors (Fig. 4B,C and table S7). Similarly, injection of NDV into the right flank MC38 tumors of the animals bearing left flank B16-F10 tumors failed to induce B16-F10 tumor rejection (Fig. 4D,E and table S7), suggesting that the NDV-induced anti-tumor immune response is likely antigen-restricted to the injected tumor.
Figure 4. Systemic anti-tumor effect is restricted to the injected tumor type.
(A) Animals were injected i.d. in right flank with B16-F10 melanoma, MC38 colon carcinoma, or PBS, and in the left flank with B16-F10 cells and treated as outlined in the scheme. (B,C) Growth of distant tumors (B) and overall survival (C) of animals that received right B16-F10 or no right flank tumors. Data show representative results from 1 out of 2 independent experiments with 5–10 mice/group. (D,E) Growth of distant tumors (D) and overall survival (E) of animals that received right B16-F10 or MC38 tumors. Data represent results from 1 out of 2 independent experiments with n=10 per group. **p<0.01, ****p<0.0001.
Combination therapy with NDV and anti-CTLA-4 induces tumor infiltration with activated lymphocytes
To examine the B16-F10 tumor microenvironment in the treated animals, bilateral tumors were collected and processed for analysis of infiltrating cells. Analysis of the injected and distant tumors from the treated animals revealed prominent inflammatory infiltrates and large areas of tumor necrosis in the animals treated with combination therapy (Fig. 5A, Fig. S7, and table S8). This correlated with increased numbers of CD45+ cells and T cells in the combination therapy group (Fig. 5A–C, Fig. S7A–C, and table S8). As previously, the observed increase in TILs was primarily due to infiltration of CD8+ and Tconv, but not Treg cells, leading to enhanced effector to Treg ratios (Fig. 5D–F, Fig. S7C–E, and table S8). Phenotypic characterization of CD4+ and CD8+ TILs from animals receiving the combination treatment demonstrated upregulation of ICOS, Granzyme B, and Ki-67 over the untreated and anti-CTLA-4 treated animals (Fig. 5G–I and table S8) and a larger percentage of IFNγ-expressing CD8+ cells in response to re-stimulation with dendritic cells (DCs) pulsed with B16-F10 tumor lysates (Fig. 5J and table S8).
Figure 5. Combination therapy with NDV and CTLA-4 blockade induces inflammatory changes in distant tumors.
Animals were treated per schema in Fig. 3A. Tumors were harvested on day 15 and analyzed for infiltrating immune cells. (A) Tumor sections from distant tumors were stained with H&E (upper panels) or for CD3 and FoxP3 (lower panels) and analyzed by light and fluorescence microscopy, respectively. Areas denoted by arrows indicate necrosis and inflammatory infiltrates. Scale bars represent 200 μm. (B) Absolute number of tumor-infiltrating CD45+ and CD3+ cells/g tumor calculated from flow cytometry. (C) Representative flow cytometry plots of percent of tumor-infiltrating CD4+ and CD8+ cells gated on CD45+ population. (D) Absolute numbers of Tconv, Treg, and CD8+ cells per gram of tumor. (E) Relative percentages of tumor-infiltrating Tregs out of CD45+ cells. (F) Calculated Tconv/Treg and CD8+/Treg ratios. (G–I) Upregulation of ICOS, Granzyme B, and Ki-67 on tumor-infiltrating CD8+ and Tconv lymphocytes. Representative flow cytometry plots (upper panels) and cumulative results (bottom panels) are shown. (J) TILs were restimulated with DC's pulsed with B16-F10 tumor lysates, and IFNγ production was determined by intracellular cytokine staining. Representative flow cytometry plots (left panel) and cumulative results (right panel) are shown. Data represent cumulative results from 5 (A–I) or 2 (J) independent experiments with n=3–5 per group. Mean +/− SEM is shown. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Anti-tumor activity of NDV combination therapy depends on CD8+ cells, NK cells and type I and II interferons
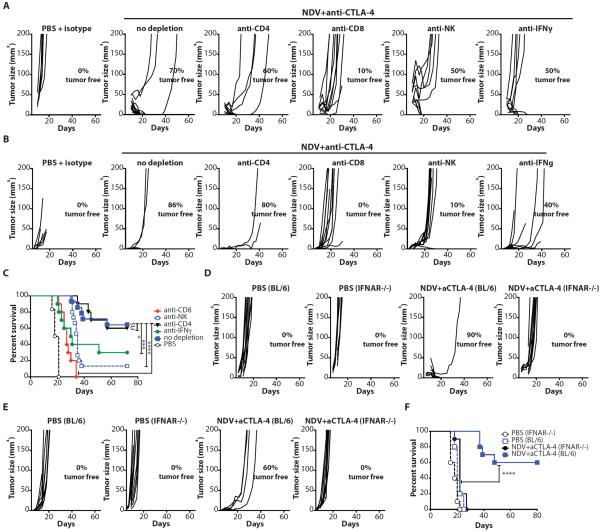
To determine which components of cellular immunity were responsible for the observed therapeutic effect, we repeated the treatment in the presence of depleting antibodies for CD4+, CD8+, or NK cells. Adequate cell depletion of each cell subset was confirmed by flow cytometry of peripheral blood (Fig. S8). Depletion of either CD8+ or NK cells resulted in abrogation of therapeutic effect in both virus-injected and distant tumors (Fig. 6A,B and table S9), with significant reduction in long-term survival (p<0.0001 for CD8 and p=0.0011 for NK depletion) (Fig. 6C and table S9). Consistent with these findings, treatment of the animals with an anti-IFNγ neutralizing antibody also decreased therapeutic efficacy. In contrast, depletion of CD4+ cells did not result in appreciable change in anti-tumor effect, though these results must be interpreted with caution since anti-CD4+ depletion also results in concurrent depletion of Tregs.
Figure 6. Anti-tumor activity of NDV combination therapy depends on CD8+ and NK cells and type I and type II interferons.
(A–C) Animals were treated as described in Fig. 3A with or without depleting antibodies for CD4+, CD8+, NK cells, or IFNγ. (A) Growth of injected tumors. (B) Growth of distant tumors. (C) Long-term survival. (D–F) IFNAR−/− or age-matched C57BL/6 mice (BL/6) were treated as described in Fig. 3A and monitored for tumor growth. (D) Growth of injected tumors. (E) Growth of distant tumors. (F) Long-term survival. Data for all panels represent cumulative results from 2 independent experiments with n=3–10 per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Type I IFN has been previously demonstrated to play an important role in priming of CD8+ cells for anti-tumor immune response(8, 9). To investigate the role of type I IFN in tumor rejection by NDV, we repeated the experiments in the type I IFN receptor knockout (IFNAR−/−) mice. The IFNAR−/− mice demonstrated rapid progression of both injected and contralateral tumors and were completely resistant to the combination therapy (Fig. 6D–F and table S9). Overall, these findings highlight the important role of both innate and adaptive immune responses for the systemic therapeutic efficacy of the virus observed in this study.
Discussion
The presence of TILs has been shown to be a favorable prognostic indicator in a number of cancers, and gene expression profiling demonstrated that patients with high baseline tumor expression of genes related to both innate and adaptive immune response were more likely to favorably respond to immunotherapy (7, 23, 24). Presence of TILs in tumors has been shown to be associated with type I IFN transcriptional profile, and additional studies demonstrated the critical role for type I IFN in CD8a+ DC-mediated antigen cross-presentation and priming of tumor-specific CD8+ T cells(8, 9). These findings provide a strong rationale to explore tumor therapeutic strategies that activate the type I IFN pathway. Indeed, combination therapy using intratumoral CpG oligonucleotides with antibodies targeting immune checkpoints has been shown to be an effective therapeutic strategy resulting in depletion of Tregs at the injected tumor site and in regression of distant tumors (25).
Here, to trigger immunogenic tumor cell death and an inflammatory response, we employed nonpathogenic NDV, which, despite its relatively weak lytic activity, has been demonstrated to be a potent inducer of type I IFN and DC maturation(26, 27). For our studies we chose a bilateral flank melanoma model with staggered implantation of tumors at a schedule that was previously demonstrated not to be affected by concomitant immunity (28). We find that intratumoral injection of NDV results in distant tumor immune infiltration in the absence of distant virus spread. Notably, this effect was associated with relative reduction in the number of Tregs and marked enhancement of CD4 and CD8 effector to Treg ratios, which has been previously demonstrated to be a marker of a favorable immunological response to immunotherapy(29, 30). At present it is unclear what contributes to the relative reduction in the number of Tregs, although NDV-induced inflammatory cytokines have been previously suggested to have functions that could counter Treg activity (31). There is also a possibility that not all of the infiltrating FoxP3- CD4+ cells exhibit effector functions, as FoxP3- regulatory T cells have been previously reported (32).
We further demonstrated that NDV enhances tumor infiltration with tumor-specific lymphocytes, an effect that was dependent on the identity of the virus-injected tumor. The enhanced tumor infiltration and expansion of adoptively-transferred lymphocytes further suggest that there may be potential synergy between OV therapy and therapeutic approaches utilizing adoptive T cell transfer. It is plausible that the tumor-specific lymphocytes undergo activation and expansion at the site of the initial viral infection, followed by their migration to other tumor sites, which is likely dependent on chemokines and lymphocyte homing receptors (33). We also observed that distant tumor immune infiltration was in part non-specific and could be induced by NDV infection of a heterologous tumor or by transfer of serum from treated animals to naïve tumor-bearing mice. We speculate that increased vascular permeability induced by inflammatory cytokines such as IL-6 may strongly contribute to activation of tumor vasculature and lymphocyte recruitment into the tumors(34). While it would be useful to identify the cytokines and other factors that mediate this inflammatory effect, it is unlikely that they alone will provide the same degree of therapeutic efficacy as that seen with intratumoral NDV injection. Indeed, while injection of MC38 tumor induced inflammatory infiltrates in the contralateral B16-F10 tumors, it failed to induce tumor infiltration with adoptively-transferred T cells or increase therapeutic efficacy in combination with CTLA-4 blockade.
Despite the pronounced increase in TILs, therapeutic effect in distant tumors was rather modest with NDV monotherapy, highlighting the immunosuppressive nature of the microenvironment of these tumors(6). Remarkably, combination of systemic anti-CTLA-4 antibody with intratumoral NDV led to rejection of distant B16-F10 tumors with long-term animal survival. The animals were also protected against further tumor rechallenge, suggestive of establishment of long-term memory, though we did not specifically assess whether the memory lymphocytes resided in the lymphoid organs or the bone marrow (35). Interestingly, therapeutic efficacy was also seen with TRAMP C2 and CT26 tumor models, which exhibit poor sensitivity to NDV-mediated cell lysis in vitro. These findings highlight the importance of the NDV-induced anti-tumor immune/inflammatory response, rather than direct lysis, as the primary mechanism driving the anti-tumor efficacy in this model. Indeed, analysis of NDV-injected and distant tumors treated with combination therapy demonstrated prominent infiltration with innate immune cells and activated CD8+ and CD4+ effector cells, while depletion of CD8+ and NK cells abrogated the therapeutic efficacy. Furthermore, the combination strategy was completely ineffective in IFNAR−/− mice, which support the role of the type I IFN pathway in the induction of anti-tumor immunity in this system(8, 9, 36).
Our study model does present several limitations. First of all, we start therapy on day 3 after contralateral tumor challenge, where CTLA-4 blockade is ineffective as monotherapy. Although we are optimistic that the current combination approach will translate into improved clinical efficacy of CTLA-4 blockade, the development of approaches effective against larger B16 tumors is certainly warranted. Secondly, in our studies we demonstrate increased immune infiltration and regression of distant soft tissue tumor sites with long-term survival without evidence of disease recurrence in the lungs, despite the known propensity for B16-F10 melanomas for early spontaneous metastases(37). Similarly, prior studies demonstrated that therapy of flank 3LL-D122 lung carcinoma with NDV protected the animals from spontaneous lung metastases(38). It would, however, be important to evaluate whether a similar effect is present in animal models with pre-established visceral metastases. Thirdly, whereas our study focused primarily on characterization of the adaptive immune responses, it would be interesting to determine the role of NK cells and monocytes, which are also increased in the distant tumors of NDV-treated animals. Interestingly, although depletion of NK cells resulted in accelerated early tumor outgrowth, the established tumors grew at a slower rate than was seen with CD8 depletion. This finding suggests that NK cells may play an early role in the anti-tumor effect of NDV, but the CD8 cells are required for long-term tumor control. Further experiments will be needed to elaborate on this observation.
In our studies we find that systemic administration of NDV-Fluc fails to reach the tumors, although evidence of viral infection in the lungs can be seen by presence of luciferase signal. Prior studies demonstrated that although NDV is capable of infection and protein expression in normal lung epithelial cells, virus progeny produced from such infection is minimal (38). This suggests that the virus likely undergoes a single-cycle replication with persistence of the luciferase reporter expression for several days. Of note, in clinical trials with systemically administered virulent NDV, which possesses a higher replicative capacity, no pulmonary toxicities were observed with the exception of patients with large pulmonary tumor burden who developed inflammatory responses in the lung tumors (18). Nevertheless, a single-cycle infection could still potentially generate an inflammatory response in normal tissues, which could be amplified by immune checkpoint blockade. Since our strategy utilizes intratumoral administration of NDV, this might not be as relevant, but the potential for systemic toxicity certainly needs to be taken into account when designing clinical trials utilizing combinations of immune checkpoint blocking agents with systemically-administered oncolytic viruses.
In summary, here we have characterized the systemic tumor inflammatory effects induced by localized oncolytic NDV therapy and demonstrated that such inflammatory responses could be harnessed to enhance therapeutic efficacy of CTLA-4 blockade. Several distinguishing features of NDV, such as lack of pre-existing immunity in humans, lack of recombination and genomic integration, strong induction of type I IFN, strong clinical safety record, and the ubiquitous nature of the NDV receptor (sialic acid), define potential advantages of this virus over some other OVs(39), though it is unknown whether combination of other OVs with CTLA-4 blockade would elicit similar therapeutic responses. Indeed, recent clinical studies with engineered HSV-1 expressing GM-CSF (talimogene laherparepvec) demonstrated response in distal tumors, with evidence of enhanced TILs(13, 40), while preclinical studies with systemically-administered oncolytic VSV expressing a library of tumor antigens demonstrated rejection of established melanomas (41). These findings suggest that the polyclonal anti-tumor immune response generated during the localized oncolytic virus infection can be used to drive systemic anti-tumor immunity and provide a strong rationale for clinical exploration of combinations of immunoregulatory antibodies with NDV or other OVs. Studies utilizing combination of talimogene laherparepvec with ipilimumab are currently underway and will provide a clinical proof of concept for the efficacy and safety of such combinations in humans (42).
Materials and Methods
Study Design
The primary research objective was to characterize the anti-tumor immune responses induced by oncolytic NDV and to evaluate combinatorial strategies of NDV with CTLA-4 blockade. Our pre-specified hypothesis suggested that therapy with NDV would result in tumor inflammation, increasing tumor sensitivity to immunotherapy with anti-CTLA-4. The overall study design was a series of controlled laboratory experiments in mice, as described in the sections below. In all experiments, animals were assigned to various experimental groups in random. For survival studies, sample sizes of 10–15 mice per group were used. With 15 mice per group, 90% power, and a 5% significance level, we could detect differences in tumor-free survival from 20% to 80%. Survival analyses were performed using the log-rank test. The experiments were replicated 2–3 times as noted and the final analysis included either pooled data or representative experiments where indicated. For the experiments reporting isolation of TILs, 5 mice per group were used for each experiment, with 3–5 replicates. All outliers were included in the data analysis.
In vitro infection experiments
For cell surface labeling, cells were infected in 6-well dishes at MOI 1 (B16-F10) or MOI 5 (TRAMP C2) in triplicate. Twenty-four hours later, the cells were harvested by scraping and processed for surface labeling and quantification by flow cytometry. For in vitro cytotoxicity experiments, cells were infected at the indicated MOI's and incubated at 37°C in serum-free media in presence of 250 ng/ml TPCK trypsin. At 24, 48, 72, and 96 hours after infection, the cells were washed and incubated with 1% Triton X-100 at 37°C for 30 minutes. LDH activity in the lysates was determined using the Promega CytoTox 96 assay kit, according to the manufacturer's instructions.
Tumor challenge survival experiments
All mouse procedures and experiments for this study were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee. Treatment schedules and cell doses were established for each tumor model to achieve 10–20% tumor clearance by NDV or anti-CTLA-4 as single agents. For the B16-F10 model, tumors were implanted by injection of 1×105 cells in the right flank intradermally (i.d.) on day 0 and 5×104 cells in the left flank on day 4. On days 7, 10, 13, and 16, the mice were treated with intratumoral injections of 1×107 pfu of NDV in PBS in a total volume of 100 μl and intraperitoneally (i.p.) with anti-CTLA-4 antibody (100 μg in 100 μl). Control groups received a corresponding dose of isotype antibody i.p. and intratumoral injection of PBS. The animals were euthanized for signs of distress or when the total tumor volume reached 1000 mm3. For depletion of immune cells, mice were injected i.p. with 500 μg of monoclonal antibodies to CD8+, CD4+, NK1.1 or IFNγ one day before and two days after tumor challenge, followed by injection of 250 μg every 5 days throughout the experiment. For the TRAMP-C2 model, animals received 1×106 cells in the right flank and 5×105 cells in the left flank on day 0 and day 4, respectively. Treatment was performed on days 7, 10, 13, and 16 in a similar fashion to above. For the CT26 model, animals received 1×106 cells in the right flank and 1×106 cells in the left flank on day 0 and day 2, respectively. Treatment was performed on days 6, 9, 12, and 15 in a similar fashion to above.
Serum transfer experiments
Groups of tumor-bearing mice were treated intratumorally with single injection of NDV or PBS. On day 4, blood was collected by terminal bleeding and serum was isolated by centrifugation. Sera were pooled from each group and UV-treated in Stratalinker 1800 with six pulses of 300 mJ/cm2 UV light to inactivate any virus that could be potentially present. Undiluted 100 μl of serum was injected intratumorally into naïve B16-F10 tumor-bearing mice for a total of 3 injections given every other day. Tumors were removed 3 days after the last injection and processed for isolation of tumor-infiltrating lymphocytes.
Isolation of tumor-infiltrating lymphocytes
B16-F10 tumors were implanted by injection of 2×105 B16-F10 cells in the right flank i.d. on day 0 and 2×105 cells in the left flank on day 4. On days 7, 10, and 13, the mice were treated with intratumoral injections of 2×107 pfu of NDV, and 100 μg of i.p. anti-CTLA-4 antibody where specified. The rare animals that died from tumor burden (always in untreated control groups) or animals that completely cleared the tumors (always in treatment groups) were not used for the analysis. On day 15, mice were sacrificed and tumors were processed as discussed in the supplementary materials and methods.
Statistics
Data were analyzed by 2-tailed unpaired Student's t test (for comparisons of 2 groups in studies comparing NDV to control) and ANOVA (for comparison of multiple groups in studies where combinations of NDV with anti-CTLA-4 were used). Data for survival were analyzed by Log-Rank (Mantel-Cox) Test. Two-sided p < 0.05 was considered statistically significant (P ≤ 0.05 (*), P ≤ 0.01 (**), P<0.001 (***), P<0.0001 (****)). The numbers of animals included in the study are discussed in each figure.
Supplementary Material
Acknowledgements
We thank Ingrid Leiner and Dr. Eric Pamer for providing IFNAR−/− mice, Dr. Patrick Hwu for providing CD2-luciferase mice, Dr. Nicholas Restifo for Trp1 and Pmel mice, Dr. Isaiah Fidler for the original B16-F10 cells, and Dr. David Schaer for help with illustrations.
Funding This work was supported by the Howard Hughes Medical Institute (J.P.A.), the National Institutes of Health (CA056821 to J.D.W.), Swim Across America (J.D.W.), a fellowship from the Danish Cancer Society (R.B.H.) and the NIH T32 training grant CA009207 (D.Z) and CA078207 (M.M.). D.Z. is the Bart A. Kamen Fellow of the Damon Runyon Cancer Research Foundation and a recipient of the Young Investigator Award from the ASCO Conquer Cancer Foundation.
Footnotes
Author contributions D.Z. designed and performed the experiments, analyzed the data, and prepared the manuscript. R.B.H, S.S., J.P., and M.M. performed the experiments and assisted in manuscript preparation.
P.P., T.M., J.D.W, and J.P.A. assisted in experimental design, data interpretation, and manuscript preparation.
References
- 1.Peggs KS, Quezada SA, Allison JP. Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists. Clin Exp Immunol. 2009;157:9. doi: 10.1111/j.1365-2249.2009.03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006504. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, Pelusio A, Le Boeuf F, Burns J, Evgin L, De Silva N, Cvancic S, Robertson T, Je JE, Lee YS, Parato K, Diallo JS, Fenster A, Daneshmand M, Bell JC, Kirn DH. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 12.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, Moon A, Lee YS, Kim MK, Daneshmand M, Dubois K, Longpre L, Ngo M, Rooney C, Bell JC, Rhee BG, Patt R, Hwang TH, Kirn DH. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andtbacka RH, Collichio FA, Amatruda T, Senzer NN, Chesney J, Delman KA, Spitler LE, Puzanov I, Doleman S, Ye Y, Vanderwalde AM, Coffin R, Kaufman H. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. presented at the 2013 ASCO Annual Meeting; Chicago, IL. 2013. [Google Scholar]
- 14.Zamarin D, Martinez-Sobrido L, Kelly K, Mansour M, Sheng G, Vigil A, Garcia-Sastre A, Palese P, Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol Ther. 2009;17:697. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elankumaran S, Chavan V, Qiao D, Shobana R, Moorkanat G, Biswas M, Samal SK. Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy. J Virol. 2010;84:3835. doi: 10.1128/JVI.01553-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bart RS, Kopf AW, Vilcek JT, Lam S. Role of interferon in the anti-melanoma effects of poly (I).poly (C) and Newcastle disease virus. Nat New Biol. 1973;245:229. doi: 10.1038/newbio245229a0. [DOI] [PubMed] [Google Scholar]
- 18.Pecora AL, Rizvi N, Cohen GI, Meropol NJ, Sterman D, Marshall JL, Goldberg S, Gross P, O'Neil JD, Groene WS, Roberts MS, Rabin H, Bamat MK, Lorence RM. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J Clin Oncol. 2002;20:2251. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dezfouli S, Hatzinisiriou I, Ralph SJ. Enhancing CTL responses to melanoma cell vaccines in vivo: synergistic increases obtained using IFNgamma primed and IFNbeta treated B7-1+ B16-F10 melanoma cells. Immunol Cell Biol. 2003;81:459. doi: 10.1046/j.0818-9641.2003.01189.x. [DOI] [PubMed] [Google Scholar]
- 22.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer research. 2001;61:1095. [PubMed] [Google Scholar]
- 23.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, Chasalow SD, Berman D. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 25.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, Luong R, Rosenblum MD, Steinman L, Levitsky HI, Tse V, Levy R. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilden H, Fournier P, Zawatzky R, Schirrmacher V. Expression of RIG-I, IRF3, IFN-beta and IRF7 determines resistance or susceptibility of cells to infection by Newcastle Disease Virus. Int J Oncol. 2009;34:971. doi: 10.3892/ijo_00000223. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fournier P, Arnold A, Wilden H, Schirrmacher V. Newcastle disease virus induces pro-inflammatory conditions and type I interferon for counter-acting Treg activity. Int J Oncol. 2012;40:840. doi: 10.3892/ijo.2011.1265. [DOI] [PubMed] [Google Scholar]
- 32.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franciszkiewicz K, Boissonnas A, Boutet M, Combadiere C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012;72:6325. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 34.Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, Vardam TD, Weis EL, Passanese J, Wang WC, Gollnick SO, Dewhirst MW, Rose-John S, Repasky EA, Baumann H, Evans SS. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. The Journal of clinical investigation. 2011;121:3846. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schirrmacher V, Feuerer M, Fournier P, Ahlert T, Umansky V, Beckhove P. T-cell priming in bone marrow: the potential for long-lasting protective anti-tumor immunity. Trends Mol Med. 2003;9:526. doi: 10.1016/j.molmed.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 37.Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218. [PubMed] [Google Scholar]
- 38.Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, Panet A, Zakay-Rones Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15:795. doi: 10.1038/cgt.2008.31. [DOI] [PubMed] [Google Scholar]
- 39.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 41.Pulido J, Kottke T, Thompson J, Galivo F, Wongthida P, Diaz RM, Rommelfanger D, Ilett E, Pease L, Pandha H, Harrington K, Selby P, Melcher A, Vile R. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol. 2012;30:337. doi: 10.1038/nbt.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ipilimumab with or without talimogene laherparepvec in unresected melanoma. ClinicalTrials.gov; NCT01740297. 2013 ClinicalTrials.gov
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.