Abstract
Over the last decade, significant progress has been made in the field of drug delivery. The advent of engineered nanoparticles has allowed us to circumvent the initial limitations to drug delivery such as pharmacokinetics and solubility. However, in spite of significant advances to tumor targeting, an effective treatment strategy for malignant tumors still remains elusive. Tumors possess distinct physiological features which allow them to resist traditional treatment approaches. This combined with the complexity of the biological system presents significant hurdles to the site-specific delivery of therapeutic drugs. One of the key features of engineered nanoparticles is that these can be tailored to execute specific functions. With this review, we hope to provide the reader with a clear understanding and knowledge of biological barriers and the methods to exploit these characteristics to design multifunctional nanocarriers, effect useful dosing regimens and subsequently improve therapeutic outcomes in the clinic.
Keywords: MPS, nanoparticle, drug delivery, biological barriers, cancer, tumors, EPR, multifunctional nanoparticles
Introduction
Cancer accounts for the deaths of millions of people worldwide. It occurs when normal cells acquire a series of critical mutations leading to their uncontrolled cell growth.1 The fact that cancer originates from an organisms own cells makes it harder to selectively treat. Current clinical approaches are based on the systemic administration of chemotherapeutics drugs. These therapies are limited by solubility and pharmacokinetic factors on account of their physio-chemical properties, as well as fraught with toxicity issues as they generally target any rapidly dividing cells in the body such as those of the hair, skin, spleen and liver among others. Therefore, delivery of these anti-cancer agents with the use of nanoparticles (NPs) helps overcome some of these disastrous side-effects. But it was found that only a small fraction of the administered dose ends up reaching the target site to have its intended effect. This may result in further complications as tumors when exposed to limiting amounts of drug, develop resistance. As a consequence, subsequent dosing regimens will then need to be significantly higher in order to elicit any therapeutic response. It is thus evident that current treatment modalities have significant scope for improvement. In order to arrive at the appropriate solutions we must first seek to identify and understand the problems. The complexity of the in vivo system thus inflicts multiple biological barriers which impedes NP drug delivery to solid tumors.2 In current anti-cancer therapies, NPs are generally administered intravenously (IV). This route is fast, reliable and allows complete distribution via the systemic circulation. Once in circulation, the NPs face a number of challenges. They may be opsonized by blood proteins following which they can be recognized by the cells of the mononuclear phagocyte system (MPS) and cleared from circulation. The NP population which has evaded clearance by the MPS now needs to extravasate out of circulation effectively past the endothelial lining toward the tumor microspace. Effective extravasation thus represents the second barrier followed by the penultimate barrier, the tumor interstitium. Here the NP encounters the smooth muscle cells, extra-cellular matrix, pericytes, cancer associated fibroblasts etc. in addition to various physiological factors such as low pH, low oxygenation and high interstitial fluid pressure.3 Once the NPs have extravasated out of systemic circulation, past the tumor microspace etc. the tumor cell membrane and intracellular machinery represents the final barrier the NPs have to get past for the effective intracellular delivery of drug cargo. The design of multifunctional NPs layered with specific attributes in order to sequentially execute functions to cross these biological barriers one at a time is thus imperative.4 This review presents in detail not only the various biological barriers but also the latest advancements in biomedical nanotechnology and the strategies used by the scientific community to overcome them.
Biological Barriers
The mononuclear phagocyte system
In order for a NP or drug vehicle to reach its target and have its intended effect, it first needs to be stable in systemic circulation. The blood contains a variety of proteins like albumin, fibrinogen and globulin as well as other complement system proteins. Once the NP enters the systemic circulation, these serum proteins can adsorb onto their surface forming a ‘protein corona’.5 The formation of this particle-protein corona is dynamic and is controlled by a number of biological, physical and chemical interactions at a molecular level. The NP-protein complex is a key determinant of the subsequent fate of the NP in vivo and therefore understanding the extent of their interactions is crucial to their effective design.6 Cedervall et al. have very elegantly demonstrated a number of methods to study these NP-protein interactions and how these translate to responses in vivo.7
The process of protein adsorption onto the particle surface is termed as opsonization and is usually followed by phagocytosis by the cells of the MPS like circulating as well as residual macrophages.8 Together, these two processes form the main mechanism for the elimination of NPs from the blood. The process of opsonization mainly depends on the physio-chemical characteristics of the NP like size,9 shape,10 charge11 and surface heterogeneity.12 Recently, Lunov et al. showed that the carboxy-functionalization of the NP surface enhanced its phagocytosis by macrophages while the amino-functionalization allowed for enhanced dynamin-dependent endocytosis by the PMA-differentiated monocytic THP-1 cells.13 Using apolipoproteinE (apoE) knockout mouse models, Yan et al. were able to demonstrate that the uptake of neutrally-charged liposomes was almost exclusively apoE-mediated while that of the negatively charged ones was not.14 But cationic or neutrally charged NPs are not the only ones susceptible to enhanced serum protein interactions. To specifically study the effect of surface charge on MPS uptake, Xiao et al. were able to demonstrate that NPs with high negative or positive charge were taken up by murine macrophages in vitro as well as in vivo, leading to higher accumulation of NPs in the liver.15 More recently, it was shown that the cellular binding of a variety of anionic NPs like quantum dots, citrate-modified gold NPs and low-density lipo-protein particles were significantly inhibited by extracellular serum proteins.16 It was suggested that this was due to the fact that the protein-NP complex would compete for the same receptors as the free extracellular proteins present thereby reducing their binding efficiencies. Similarly, Caracciolo et al. had showed that though substitution of cationic lipids like DOTAP with neutral lipids like dioleoylphosphatidylethanolamine (DOPE) and cholesterol helped reduce the binding of fibrinogens, it increased the surface adsorption of other extracellular proteins like albumin and apolipoproteins by DOPE and immunoglobulins as well as complement proteins by cholesterol.17 In order to circumvent this MPS barrier, a variety of approaches have been used. One strategy has been to modify the NP surface with polymers in order to effectively increase its blood circulation time.18 One of the most common approaches involves the use of polyethylene glycol (PEG).19,20 Over the past decade, our group has repeatedly demonstrated the advantages of using PEGylated NPs.21-23 Recently, Parveen et al. showed that coating paclitaxel-loaded poly(lactic-co-glycolic acid) (PLGA) NPs with an optimal combination of chitosan and PEG dramatically prolonged blood circulation times while reducing the sequestration of NPs in the liver.24 Surface-coating of lipid emulsions with monoleate-modified PEG was also shown to significantly enhance the plasma concentration of the loaded breviscapine.25 The coating of PLGA NPs with poloxamer 188 was also shown to evade uptake by macrophages.26
Nanoparticle extravasation
Once past the MPS barrier, the NP still has multiple hurdles to overcome before it is able to reach its target site. The vascular endothelial layer is one of its most significant barriers and represents a semi-permeable layer of cells which lines the inner walls of the blood vessels27 and along with the glycocalyx, a proteoglycan layer, serves to control the permeability of solutes and macromolecules across blood vessels.28 The glycocalyx ‘coat’ imparts a negative charge to the endothelial cell membrane and has been implicated in increased interactions with cationic particles.29 This could potentially sequester NPs thus preventing them from further extravasation into the tumor microspace.
Hemodynamics
The hemodynamics of nanoparticles is also an important parameter that determines their effective extravasation (See Figure 1). More than a decade ago, Aarts et al. had shown that red blood cells tend to flow in the center of blood vessels forcing the platelets out radially causing them to concentrate near the vessel wall.30 When applied to the field of nanoparticulate drug delivery, this could lead to better extravasation past the endothelia. However, this has not been studied well enough and there is only a handful of pertaining literature available.31-33
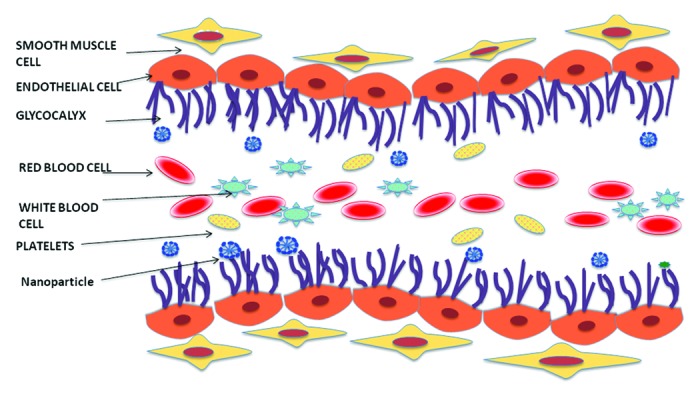
Figure 1. Hemodynamics of blood flow
Enhanced permeability and retention effect
Although ‘leakage’ of molecules through the vascular endothelium may occur through trans- and para-cellular pathways,34 NPs generally bigger than 5–6 nm would not be able to cross healthy vessels characterized by a continuous endothelium. However, under pathological states like inflammation, infarcts and tumors, the endothelial lining tends to become more permeable leading to ‘gaps’ in the lining. Matsumura and Maeda were the first to show that nanoparticles are able to extravasate through these gaps to reach the tumor space and stay there due to the poor lymphatic drainage of tumors.35 This phenomenon was later termed as the enhanced permeability and retention (EPR) effect and paved the way for the passive targeting of tumors using NPs. Our group has successfully exploited this strategy in order to deliver a wide range of PEGylated nanoparticles like micelles,36-38 liposomes39-41 and dendrimers42 among others. However, a number of limitations still exist, linked to the heterogeneity of tumors which can prevent the efficient extravasation of NPs.43 There seem to be significant differences in the endothelial pore sizes in primary and metastatic tumors as well as within the same tumor type. Targeting and manipulation of the tumor vasculature have emerged as useful strategies to overcome some of these limitations and have been discussed in greater detail below.
Targeting to facilitate extravasation
Though the addition of targeting moieties to NPs has been used extensively to improve intracellular delivery, it can also be used to enhance extravasation across endothelia characterized by tight junctions as is the case with the blood-brain barrier (BBB). Most of these approaches are based on the fact that the brain endothelium as well as glioma cells, overexpress certain cell-surface receptors like the transferrin, glucose transporter and low-density lipoprotein (LDL) receptors. Transferrin-targeted PLGA nanoparticles co-loaded with doxorubicin and paclitaxel have been used to demonstrate enhanced anti-glioma activity.44 The targeting with transferrin allowed for an 8-fold decrease in IC50 values in vitro while in an in vivo murine tumor model, the targeted drug combination showed a 47-fold reduction in tumor volume compared with a 1.3-fold reduction observed with the un-targeted combo. Similarly, Miao et al. demonstrated the use of paclitaxel-loaded NPs targeted with lactoferrin and a tumor-specific peptide as a novel approach for anti-glioma therapy.45 Kuang et al. have also employed the use of transferrin receptor-specific T7 peptide to deliver RNA46 showing that this strategy is not just specific to the delivery of small molecule drugs.47,48 Apart from transferrin-based strategies, various groups have also shown that targeting the LDL49,50 as well as glucose transporter receptors51 with NPs are equally viable approaches representing high therapeutic potentials.
Role of tumor vasculature
Initially, tumors are dependent on the vasculature of the surrounding host tissues for their blood supply. But as they grow further, they switch into an angiogenic state in order to meet their increasing metabolic demands.52 These changes in the tumor vasculature have been quantified in a study by Liotta et al.53 Tumors also show increased levels of growth factors like vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) among others.54,55 These growth factors significantly increase permeability of macromolecules by modulating the sub-endothelial structures.56 But the vascular permeability of tumors is also very heterogeneous in its distribution due to this abnormal angiogenesis and can negate the effects of EPR. Therefore, a number of groups have attempted to manipulate the tumor vasculature by the use of hyperthermia, growth factors, tumor necrosis factor (TNF) etc. in order to facilitate extravasation of NPs into the tumor microspace. Li et al. have shown that local hyperthermia was able to increase the vascular permeability upto 10 µm in a variety of tumor models.57 This allowed for increased liposomal extravasation not seen with normothermia. Similarly, Liu et al. were able to demonstrate that the thermally induced extravasation of liposomes led to their increased accumulation in murine mammary carcinomas.58 Application of exogenous VEGF was found to increase pore size of human colon carcinoma xenografts allowing for the enhanced extravasation of albumin (7nm) as well as PEGylated liposomes (100–400nm).59 Interestingly, no significant difference in pore size was seen on the application of other growth factors like placental growth factor (PIGF) and basic fibroblast growth factor (bFGF). The short half-life and acute toxicity resulting from high dose administration makes systemic treatment with free TNF-α difficult. To circumvent these issues, PEGylated polycyanoacrylate NPs were developed and successfully evaluated as a delivery vehicle for TNF-α.60 In order to further minimize TNF-α associated toxicity, Corti et al. had coupled this with a tumor vasculature-specific cyclic CNGRC peptide.61 This followed by combination therapy with targeted doxorubicin liposomes, demonstrated a marked uptake in neuroblastoma tumors leading to enhanced therapeutic effects. The same group was also able to show that combinatorial treatment of TNF-α with a variety of chemotherapeutic drugs like paclitaxel, cisplatin, gemcitabine and melphalan led to better therapeutic outcomes.62,63 Interestingly, combination treatments did not show a marked increase in cytotoxicity in vitro whereas in vivo an almost synergistic effect was seen which indicates that TNF may be acting on the stromal compartment rather than directly on the tumor itself.
Tumor Microenvironment
After successfully crossing the vascular-endothelial barrier by extravasation, the NP still has to get through the complicated maze that is the tumor microenvironment. The main features of the microenvironment have been investigated in detail here, but their heterogeneous distribution throughout the microspace remains the biggest challenge.
Extracellular matrix
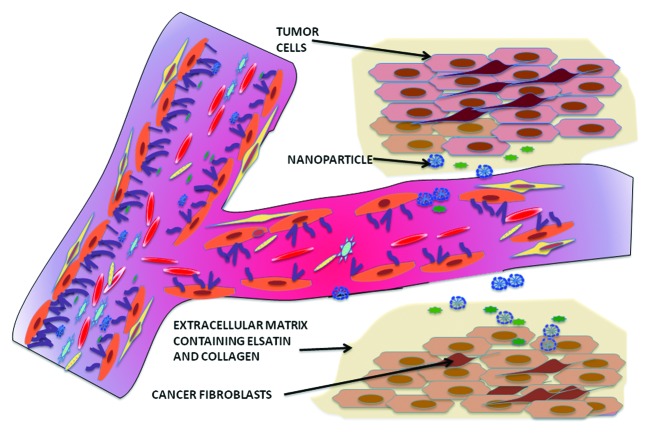
One of the first challenges is to cross the tumor interstitium or extra-cellular matrix (ECM) (See Figure 2). This consists of a cross-linked network of collagen and elastin fibers, proteoglycans and hyaluronic acid. It not only provides structural integrity, but also helps to transport important nutrients as well as oxygen to support cell growth. A highly developed matrix may result in significant resistance to the diffusion of therapeutic particles through the interstitium causing the drug-cargo to be released too far from the tumor space to have its intended effect.64 Netti and coworkers have studied the impeding role of the ECM to the passive diffusion of macromolecules like ImmunoglobulinG (IgG) through the interstitium.65 For their studies, they used four different tumor lines: human colon adenocarcinoma (LS174T), human glioblastoma (U87), human soft tissue sarcoma (HSTS26T) and a murine mammary carcinoma (MCaIV). They found that IgG had more resistance to diffusion in the U87 and HSTS26T than in the MCaIV and LS174T lines. In lieu of this observation, they found that collagenase treatment improved the diffusivity of IgG almost 2-fold. Subsequent histological studies showed that this difference in diffusivity correlates with the fact the U87 and HSTS26T lines had well-organized collagen-proteoglycan-linked matrices, which were lacking in the other two tumor lines. Studies by Graff et al.66 as well as Pun et al.67 have shown that in many cases NPs are not able to efficiently penetrate and are localized in the peripheral regions of the tumor microspace. Using a multicellular spheroid model, Goodman and coworkers were able to demonstrate the efficiency of collagenase pre-treatment in improving the penetration of NPs.68 In a novel approach by Kuhn et al., superparamagnetic NPs were cross-linked with collagenase.69 Application of a magnetic field allowed for increased mobility of the NP while the collagenase served to keep the NP clear of any collagen barriers. Another approach has been to dilate the pores of the ECM by co-infusion of NPs with hyperosmolar mannitol solution or hypertonic buffer solution.70 Pre-treatment with hyaluronidase was also able to significant enhance NP penetration.
Figure 2. Extravasation of nanoparticles from systemic circulation into the tumor interstitium
Matrix metalloproteinase
However aggressive a tumor maybe, their growth cannot be indefinite and will be limited depending on a number of factors such as the vascular supply, supply of nutrients, hypoxia, physical boundaries of the surrounding space etc. They are able to circumvent these limitations by the process of metastasis where they migrate and colonize distant organs or tissues.71 The presence of the ECM impedes the migration of metastatic cells. The destruction or partial degradation of this matrix by a battery of enzymes allows the tumor cell to circumvent this barrier.72 The presence of the ECM can be perceived as a hurdle or as an opportunity to be exploited. Almost two decades ago, Jones and coworkers showed that a variety of metastatic human tumor cell lines demonstrated both elastolytic and collagenolytic activity as well as a plasmin-induced degradation of the other matrix glycoproteins.73 The proteolysis of the extracellular matrices thus fuels the process of angiogenesis and is mediated by a family of proteases known as matrix metalloproteinases (MMPs).74,75 MMPs have been implicated in a variety of late stage metastatic cancers and have been evaluated as diagnostic biomarkers for a variety of malignant tumors.76 Their specific inhibition therefore, is of significant therapeutic value.77,78 Of the MMP family, MMP-2 and MMP-9 are thought to play a more prominent role in tumorigenesis. One of the most common strategies to target MMPs has been the use of tissue inhibitor of metalloproteinases (TIMP).79 Chetty and coworkers have showed that downregulating MMP-2 in a murine lung cancer model was able to significantly inhibit angiogenesis by expression of tissue inhibitor of matrix protease-3 (TIMP-3) which prevents endothelial cell proliferation as well as VEGF expression.80 In a similar study, forced expression of TIMP-3 by retroviral gene delivery led to significant anti-angiogenic effects by the inhibition of capillary morphogenesis in a murine tumor model.81 Song et al. were also able to show that in a cervical cancer model, histone deacetylase (HDAC) inhibitors downregulate MMP-2 and MMP-9 levels and could subsequently prevent cancer metastasis.82 Zarrabi and coworkers showed that peptides targeting a PEX domain on MMP-14, a membrane anchored MMP, were able to significantly inhibit tumor dissemination.83 Administration of pigment-epithelium-derived factor has been shown to significantly reduce MMP-9 expression levels allowing for effective inhibition of gliomas.84 For more information on the early clinical studies, drawbacks and current approaches to MMP inhibition, see references.85-87 Another approach to exploiting MMPs has been the development of MMP-sensitive drug-release systems. Our group had recently demonstrated the use of a novel MMP-2 sensitive multifunctional liposome system.23 Presence of MMP triggers the shedding of the PEG coat exposing the underlying targeting moieties, a cancer-specific 2C5 antibody as well as a TATp cell-penetrating peptide, thus allowing for enhanced targetability and internalization. More recently, a TATp-targeted micellar carrier containing a MMP-2-sensitive paclitaxel prodrug was developed.88 This ‘smart’ nanocarrier was able to demonstrate enhanced anti-tumor activity both in vitro and in vivo in an A549 lung cancer model. For more information on such stimulus-sensitive preparations see reference.89
Interstitial fluid pressure
Interstitial blood flow is one of the major effectors of nanoparticle distribution in the tumor microspace as the drug is effluxed from the vasculature through the interstitium and finally to the target cells. Drugs and various NPs move through the interstitium by diffusion based on a concentration gradient or by convection based on a pressure gradient.90 Uneven vascularization typical of tumors causes considerable heterogeneity in vascular blood flow. This combined with a lack of proper lymphatic drainage due to uneven lymphatic vessel distribution results in increased interstitial fluid pressure (IFP).91 In a recent study, Lunt and coworkers had experimentally determined the IFP in murine fibrocarcinoma as well as human cervical carcinoma models.92 They were not only able to find substantial variations in IFP values between the different models, but also between tumors growing in the same mouse thus demonstrating the extent of IFP heterogeneity. High IFP has also been implicated in increased metastatic frequency as wells as reduced sensitivity to radiation.93 As it is evident that IFP is a direct consequence of angiogenesis, targeting the latter represents a simple approach to circumvent this barrier.94 Paclitaxel treatment has been shown to be effective in reducing IFP values in the clinic.95 VEGF blockade to inhibit angiogenesis is another promising strategy to aid in drug penetration against the pressure gradient.96 Treatment with Imatinib, a PDGF receptor-β inhibitor, led to decreased VEGF expression and subsequently decreased IFP.97 Similarly, Dickson et al. have shown that pretreatment with Bevacizumab, an anti-VEGF monoclonal antibody, helped to improve the anti-tumor efficacy of systemically administered topotecan in a murine neuroblastoma model.98 Vascular disrupting agents such as combretastatin and ZD6126, a tubulin-binding agent, have also successfully been used to reduce IFP.99,100
Hypoxic core and extracellular pH of tumors
Due to the Warburg effect, the extracellular pH and oxygen concentration decreases as we move away from the vasculature into the tumor space.101 Acidic pH and low oxygen levels have been shown to impart resistance to certain anti-cancer therapies such as radiation and a variety of chemotherapeutic drugs.102 Hypoxic tumors demonstrate increased expression of chemokine ligand 28 (CCL28) which is implicated in angiogenesis and evasion of immune cell detection.103 Hypoxia can also be used as a diagnostic marker for late-stage tumors as they are associated with more malignant phenotypes.104 Hypoxia-specific targeting of drugs could thus significantly improve therapeutic efficacy. Harada et al. have shown the development of a hypoxia imaging system using a hypoxia-specific luciferase reporter.105 They subsequently showed that treatment with a hypoxia-sensitive prodrug TOP3, significantly enhanced hypoxic cell-death. Cairns and coworkers have shown that further increasing its hypoxic environment using inhibition of hypoxia inducible factor allows increased mitochondrial metabolism to facilitate enhanced targeting of hypoxia-specific cytotoxins.106 Targeting 4T1 mammary as well as MDAMB-231 lung metastatic tumor’s hypoxia machinery with the use carboxic anhydrase inhibitors represents another novel approach.107 More recently, our group developed a novel hypoxia-sensitive NP for the tumoral delivery of siRNA which demonstrated enhanced downregulation of GFP-expressing tumors.108 In another very interesting study, Bettegowda and coworkers have used anaerobic bacteria to overcome this hypoxic barrier and re-sensitize the cells to radiation therapy.109 The lower extracellular pH characteristic of tumors, may also affect the permeability of drugs.110 The change in pH may cause the drug to be more polar or charged thus preventing it from crossing biological membranes and exert its cytotoxic effects.111 A study by Vukovic et al. demonstrated the weakened cytotoxic effects of paclitaxel, mitoxantrone and topotecan at an extracellular pH of 6.5.112 Similar studies were performed on other chemotherapeutic agents like anthracyclines.113 Encapsulation of drugs into pH-sensitive NPs represents a novel approach to overcome this barrier. Some examples include 2C5-targeted pH-sensitive liposomes for doxorubicin delivery,114 pH-sensitive liposomes for gene delivery,115 micelles116 as well as polyethyleneimine-based NPs for DNA delivery.117 For more information see reviews.89,118
Cellular Barriers
Cell membrane as a barrier
Most macromolecular drugs and genes exert their effects intracellularly. It is therefore imperative that their carrier be able to traverse the outer membrane of the cell. This internalization of the NP depends solely on its interaction with the cell membrane. Various models have been used to study these interactions and it has been seen that surface charge, hydrophobicity and size play prominent roles.119 It is well known that charged particles in general have increased interactions with the membrane while uncharged ones like PEGylated NPs have reduced interactions by virtue of their steric hindrance.120 This may cause the NP to ‘cluster’ around the membrane preventing further entry of the other NPs. As mentioned earlier in this review, proteins may adsorb onto the NP surface depending on their charge and this may well dictate their entry into the cell by receptor-mediated endocytosis.16 The effect of size on intracellular entry was studied in detail by Rejman and coworkers.121 It was seen that particles lesser than 200 nm were preferentially internalized by clathrin-mediated endocytosis, while with increasing particle sizes beyond 200 nm, a shift toward caveolin-mediated endocytosis was observed. It was also noticed that internalization was an energy-dependant process and depleting cholesterol seemed to inhibit particle uptake. Cancer cells overexpress a variety of cell-surface receptors like transferrin, folic acid, glucose transporters, integrins, and LDL among others and the active-targeting of NPs is a promising approach to facilitate their internalization.122 This concept has been around for a long time now and as numerous reviews have been published to keep abreast of advances, these will not be discussed here.122-124 However, just increasing the density of targeting ligands does not necessarily translate to better internalization. Sometimes, the targeting ligands may bind to the peripheral cancer cells with high affinity causing a ‘binding barrier’ which prevents the subsequent NPs from penetrating further. This was also noticed when the use of cell-penetrating peptides (CPPs) targeting heparan sulfate chains resulted in significant clusters which did not correlate with increased NP uptake.125 This could also be due to the fact that only a finite amount of energy is devoted by the cell for particle uptake. The kinetics of receptor recycling is thus an important parameter to study when designing ligand density and NP dosing regimens so as to not over-saturate the receptors. For more information see references.126-128
Vesicular and organellar barriers
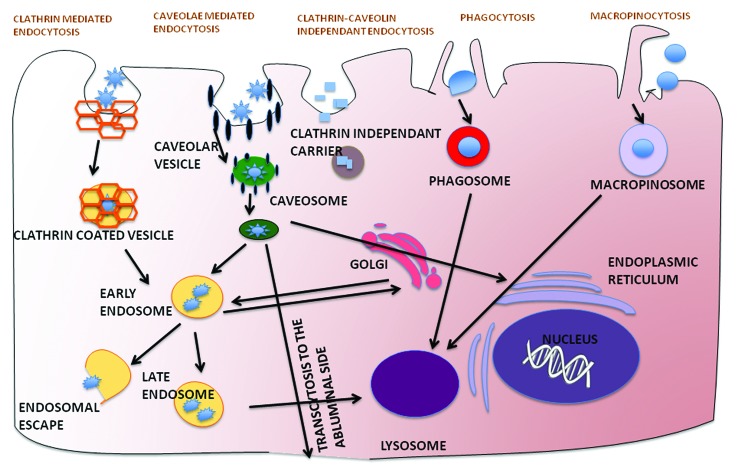
Once internalized, the NP needs to reach its intracellular target to unload its cargo. Endosomal vesicles are responsible for the trafficking of macromolecules to various intracellular sites like golgi, endoplastic reticulum, nucleus, mitochondria and lysosome among others. This pre-determined trafficking of cargo has been shown to be signal-dependant and forms the basis of intracellular drug delivery.129 Endocytosis is mediated by a number of pathways and processes such as clathrin-dependant endocytosis, caveolin-dependant endocytosis, macropinocytosis, phagocytosis etc. (See Figure 3) and different NPs are internalized by different pathways.130 However a majority of these pathways may result in trafficking to a non-target organelle site as well as the lysosome where the NP is subsequently degraded. This is especially important for the delivery of more labile drugs like genes and peptides. The use of cationic lipids and polymers for intracellular drug delivery by facilitating endosomal escape is well documented.131 Lipids such as DOTMA and DOTAP as well as branched polymers like polyethyleneimine and PAMAM dendrimers have been used extensively for their ability to fuse with the endosomal membrane thus dispelling their contents into the cytoplasm.132 As the endosome matures from early to late and subsequently lysososome, it acidifies internally by the action of ATPases. This property has been exploited by a variety of NPs to facilitate endosomal escape into the cytoplasm. Use of fusogenic lipids like DOPE has also proven popular, as these undergo transition from bilayer to hexagonal phase based on pH change facilitating the fusion with endosomal membrane.133,134 DOPE was also conjugated to low molecular weight polyethyleneimine to enhance endosomal escape and subsequent delivery of DNA as well as siRNA.135,136 For more information on these, see references.137,138 Another approach to improve intracellular drug delivery is receptor-mediated endocytosis by active targeting of NPs. Transferrin-targeting of liposomal ceramide allowed for enhanced lysosomal compartmentalization of ceramide.139 Once in the lysososome the ceramide is able to permeabilize the membrane and induce caspase-dependant apoptosis. Similarly, as folic acid is essential at sites of nucleotide synthesis, its targeting of liposomal doxorubicin allows for nuclear accumulation of the drug as it subsequently intercalates with DNA and induces apoptosis.140
Figure 3. The various mechanisms of cellular internalization of nanoparticles via clathrin-mediated endocytosis, caveolin-mediated endocytosis, clathrin-caveolin independent endocytosis, phagocytosis and macropinocytosis and their subsequent intracellular trafficking
Drug efflux transporters
As the journey of the NP nears its end, only a small fraction of the administered dose is available to exert its cytotoxic effects intracellularly. It is due to this that a variety of solid tumors, equip themselves to be able to efflux out these drugs with specialized machinery known as drug-efflux pumps. Overexpression of the drug efflux transporter, P-glycoprotein (P-gp) is one such mechanism and clinical refractoriness to chemotherapy has been extensively correlated with P-gp expression.141 It has been demonstrated that certain targeted NPs serve to circumvent these membrane bound drug-efflux pumps. Paclitaxel NPs targeted with a tumor vasculature-specific peptide were shown to be a valid strategy.142 Various small molecule P-gp inhibitors such as tariquidar, benzyl dihydropyridines as well as bacterial-derived compounds like H6 have been used in conjunction with anti-cancer drugs like paclitaxel to effectively combat drug-resistant cancers.143-146 As P-gp is also expressed in the BBB, Patil et al. have demonstrated the use of targeted tariquidar and paclitaxel using NPs to minimize drug toxicity.147 Recent work shows that this P-gp expression is regulated by the MDR gene family and induced by a variety of transcription factors such as NF-κB.148 Kovalchuk and coworkers have also showed that transfection of cancer cells with a microRNA was able to re-sensitize the cells to the primary treatment modality.149 Co-delivery of anti-survivin siRNA with doxorubicin was also shown to successfully overcome resistance to doxorubicin in human breast cancer cells.150
Conclusion
It is thus evident that the tremendous amount of research that has been performed in the last few decades has allowed us to effectively identify the specific barriers that exist in vivo to drug delivery. A lot of progress has also been made in the field of nanoparticulate delivery in order to overcome each of these barriers and a number of novel approaches have been brought to the forefront. It is now clearly evident that the active targeting of long-circulating NPs is not sufficient to translate into clinical success. A review of the recent literature suggests that along with combinatorial treatment regimens, stimulus-sensitive functions need to be accorded to these NPs as well. With the increasing number of multi-functional NPs now entering clinical studies, the development of the ideal ‘smart’ nanocarrier that will be able to tranverse all these hurdles is fast becoming a distinct possibility.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- NP
Nanoparticle
- PEG
Polyethyleneglycol
- IV
Intravenously
- MPS
Mononuclear phagocyte system
- apoE
Apolipoprotein E
- PLGA
poly(lactic-co-glycolic acid)
- Red blood cells
RBCs
- White blood cells
WBCs
- LDL
Low-density lipoprotein
- IC
Inhibitory concentration
- EPR
Enhanced permeability and retention effect
- VEGF
Vascular endothelial growth factor
- PDGF
Platelet-derived growth factor
- TNF
Tumor necrosis factor
- PIGF
Placental growth factor
- bGFG
Basic fibroblast growth factor
- ECM
Extra-cellular matrix
- MMP
Matrix metalloproteinase’s
- TIMP
Tissue inhibitor of metalloproteinase’s
- IFP
Interstitial fluid pressure
- CPP
Cell-penetrating peptide
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Chrastina A, Massey KA, Schnitzer JE. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:421–37. doi: 10.1002/wnan.143. [DOI] [PubMed] [Google Scholar]
- 3.Rabanel JM, Aoun V, Elkin I, Mokhtar M, Hildgen P. Drug-loaded nanocarriers: passive targeting and crossing of biological barriers. Curr Med Chem. 2012;19:3070–102. doi: 10.2174/092986712800784702. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2012;64:302–15. doi: 10.1016/j.addr.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–37. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A. 2007;104:2050–5. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Harashima H, Sakata K, Funato K, Kiwada H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm Res. 1994;11:402–6. doi: 10.1023/A:1018965121222. [DOI] [PubMed] [Google Scholar]
- 10.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010 Feb 15;141(3):320-7 [DOI] [PubMed]
- 11.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–66. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni SA, Feng SS. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30:2512–22. doi: 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- 13.Lunov O, Syrovets T, Loos C, Beil J, Delacher M, Tron K, Nienhaus GU, Musyanovych A, Mailänder V, Landfester K, et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano. 2011;5:1657–69. doi: 10.1021/nn2000756. [DOI] [PubMed] [Google Scholar]
- 14.Yan X, Kuipers F, Havekes LM, Havinga R, Dontje B, Poelstra K, Scherphof GL, Kamps JA. The role of apolipoprotein E in the elimination of liposomes from blood by hepatocytes in the mouse. Biochem Biophys Res Commun. 2005;328:57–62. doi: 10.1016/j.bbrc.2004.12.137. [DOI] [PubMed] [Google Scholar]
- 15.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–46. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischer CC, Kumar U, Payne CK. Cellular Binding of Anionic Nanoparticles is Inhibited by Serum Proteins Independent of Nanoparticle Composition. Biomater Sci. 2013;1:975–82. doi: 10.1039/c3bm60121h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Laganà A. Effect of DOPE and cholesterol on the protein adsorption onto lipid nanoparticles. J Nanopart Res. 2013;•••:15.3:1-11. [Google Scholar]
- 18.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48. doi: 10.1016/0169-409X(95)00039-A. [DOI] [Google Scholar]
- 19.Torchilin V, Papisov M. Why do polyethylene glycol-coated liposomes circulate so long?: Molecular mechanism of liposome steric protection with polyethylene glycol: Role of polymer chain flexibility. J Liposome Res. 1994;4:725–39. doi: 10.3109/08982109409037068. [DOI] [Google Scholar]
- 20.Vonarbourg A, Passirani C, Saulnier P, Simard P, Leroux JC, Benoit JP. Evaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptake. J Biomed Mater Res A. 2006;78A:620–8. doi: 10.1002/jbm.a.30711. [DOI] [PubMed] [Google Scholar]
- 21.Apte A, Koren E, Koshkaryev A, Torchilin VP. Doxorubicin in TAT peptide-modified multifunctional immunoliposomes demonstrates increased activity against both drug-sensitive and drug-resistant ovarian cancer models. Cancer Biol Ther. 2014;15:69–80. doi: 10.4161/cbt.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etzerodt A, Maniecki MB, Graversen JH, Moller HJ, Torchilin VP, Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Control Release. 2012 May 30;160(1):72-80 [DOI] [PubMed]
- 23.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–8. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parveen S, Sahoo SK. Long circulating chitosan/PEG blended PLGA nanoparticle for tumor drug delivery. Eur J Pharmacol. 2011;670:372–83. doi: 10.1016/j.ejphar.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Xiong F, Xiong C, Yao J, Chen X, Gu N. Preparation, characterization and evaluation of breviscapine lipid emulsions coated with monooleate-PEG-COOH. Int J Pharm. 2011;421:275–82. doi: 10.1016/j.ijpharm.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Jain D, Athawale R, Bajaj A, Shrikhande S, Goel PN, Gude RP. Studies on stabilization mechanism and stealth effect of poloxamer 188 onto PLGA nanoparticles. Colloids Surf B Biointerfaces. 2013;109:59–67. doi: 10.1016/j.colsurfb.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Malik AB, Lynch JJ, Cooper JA. Endothelial barrier function. J Invest Dermatol. 1989;93(Suppl):62S–7S. doi: 10.1111/1523-1747.ep12581072. [DOI] [PubMed] [Google Scholar]
- 28.Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–23. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Dull RO, Dinavahi R, Schwartz L, Humphries DE, Berry D, Sasisekharan R, Garcia JG. Lung endothelial heparan sulfates mediate cationic peptide-induced barrier dysfunction: a new role for the glycocalyx. Am J Physiol Lung Cell Mol Physiol. 2003;285:L986–95. doi: 10.1152/ajplung.00022.2003. [DOI] [PubMed] [Google Scholar]
- 30.Aarts PA, van den Broek SA, Prins GW, Kuiken GD, Sixma JJ, Heethaar RM. Blood platelets are concentrated near the wall and red blood cells, in the center in flowing blood. Arteriosclerosis. 1988;8:819–24. doi: 10.1161/01.ATV.8.6.819. [DOI] [PubMed] [Google Scholar]
- 31.Charoenphol P, Onyskiw PJ, Carrasco-Teja M, Eniola-Adefeso O. Particle-cell dynamics in human blood flow: implications for vascular-targeted drug delivery. J Biomech. 2012;45:2822–8. doi: 10.1016/j.jbiomech.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31:1392–402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Charoenphol P, Mocherla S, Bouis D, Namdee K, Pinsky DJ, Eniola-Adefeso O. Targeting therapeutics to the vascular wall in atherosclerosis--carrier size matters. Atherosclerosis. 2011;217:364–70. doi: 10.1016/j.atherosclerosis.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong SM, Khajoee V, Wang C, Wang T, Tigdi J, Yin J, Kuebler WM, Gillrie M, Davis SP, Ho M, et al. Co-regulation of transcellular and paracellular leak across microvascular endothelium by dynamin and Rac. Am J Pathol. 2012;180:1308–23. doi: 10.1016/j.ajpath.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 36.Riehle RD, Cornea S, Degterev A, Torchilin V. Micellar formulations of pro-apoptotic DM-PIT-1 analogs and TRAIL in vitro and in vivo. Drug Deliv. 2013;20:78–85. doi: 10.3109/10717544.2013.766780. [DOI] [PubMed] [Google Scholar]
- 37.Sawant RR, Jhaveri AM, Koshkaryev A, Qureshi F, Torchilin VP. The effect of dual ligand-targeted micelles on the delivery and efficacy of poorly soluble drug for cancer therapy. J Drug Target. 2013;21:630–8. doi: 10.3109/1061186X.2013.789032. [DOI] [PubMed] [Google Scholar]
- 38.Sawant RR, Torchilin VP. Enhanced cytotoxicity of TATp-bearing paclitaxel-loaded micelles in vitro and in vivo. Int J Pharm. 2009;374:114–8. doi: 10.1016/j.ijpharm.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas S, Deshpande PP, Perche F, Dodwadkar NS, Sane SD, Torchilin VP. Octa-arginine-modified pegylated liposomal doxorubicin: an effective treatment strategy for non-small cell lung cancer. Cancer Lett. 2013;335:191–200. doi: 10.1016/j.canlet.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elbayoumi TA, Torchilin VP. Tumor-specific antibody-mediated targeted delivery of Doxil reduces the manifestation of auricular erythema side effect in mice. Int J Pharm. 2008;357:272–9. doi: 10.1016/j.ijpharm.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sawant RR, Vaze OS, Wang T, D’Souza GG, Rockwell K, Gada K, Khaw BA, Torchilin VP. Palmitoyl ascorbate liposomes and free ascorbic acid: comparison of anticancer therapeutic effects upon parenteral administration. Pharm Res. 2012;29:375–83. doi: 10.1007/s11095-011-0557-8. [DOI] [PubMed] [Google Scholar]
- 42.Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34:1289–301. doi: 10.1016/j.biomaterials.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–7. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–20. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 45.Miao D, Jiang M, Liu Z, Gu G, Hu Q, Kang T, Song Q, Yao L, Li W, Gao X, et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol Pharm. 2014;11:90–101. doi: 10.1021/mp400189j. [DOI] [PubMed] [Google Scholar]
- 46.Kuang Y, An S, Guo Y, Huang S, Shao K, Liu Y, Li J, Ma H, Jiang C. T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int J Pharm. 2013;454:11–20. doi: 10.1016/j.ijpharm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL, Han M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34:5628–39. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 48.Lv Q, Li LM, Han M, Tang XJ, Yao JN, Ying XY, Li FZ, Gao JQ. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int J Pharm. 2013;444:1–9. doi: 10.1016/j.ijpharm.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Gao X, Qian J, Zheng S, Xiong Y, Man J, Cao B, Wang L, Ju S, Li C. Up-regulating blood brain barrier permeability of nanoparticles via multivalent effect. Pharm Res. 2013;30:2538–48. doi: 10.1007/s11095-013-1004-9. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Sun X, Mei H, Wang Y, Liao Z, Chen J, Zhang Q, Hu Y, Pang Z, Jiang X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials. 2013;34:9171–82. doi: 10.1016/j.biomaterials.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 51.Ying X, Wen H, Lu WL, Du J, Guo J, Tian W, Men Y, Zhang Y, Li RJ, Yang TY, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010 Jan 25;141(2):183-92 [DOI] [PubMed]
- 52.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 53.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 54.Chen W, Tang T, Eastham-Anderson J, Dunlap D, Alicke B, Nannini M, Gould S, Yauch R, Modrusan Z, DuPree KJ, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci U S A. 2011;108:9589–94. doi: 10.1073/pnas.1017945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–18. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, ten Hagen TL, Bolkestein M, Gasselhuber A, Yatvin J, van Rhoon GC, Eggermont AM, Haemmerich D, Koning GA. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013 Apr 28;167(2):130-7 [DOI] [PubMed]
- 58.Liu P, Zhang A, Xu Y, Xu LX. Study of non-uniform nanoparticle liposome extravasation in tumour. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology. North American Hyperthermia Group. 2005;21:259–70. doi: 10.1080/02656730500068643. [DOI] [PubMed] [Google Scholar]
- 59.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–35. [PubMed] [Google Scholar]
- 60.Li Y-P, Pei Y-Y, Zhou Z-H, Zhang X-Y, Gu Z-H, Ding J, Zhou J-J, Gao X-J. PEGylated polycyanoacrylate nanoparticles as tumor necrosis factor-α carriers. J Control Release. 2001;71:287–96. doi: 10.1016/S0168-3659(01)00235-8. [DOI] [PubMed] [Google Scholar]
- 61.Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann N Y Acad Sci. 2004;1028:104–12. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- 62.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475–82. doi: 10.1172/JCI0215223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. 2006 Jan 1;12(1):175-82 [DOI] [PubMed]
- 64.Kuppen PJK, van der Eb MM, Jonges LE, Hagenaars M, Hokland ME, Nannmark U, Goldfarb RH, Basse PH, Fleuren GJ, Hoeben RC, et al. Tumor structure and extracellular matrix as a possible barrier for therapeutic approaches using immune cells or adenoviruses in colorectal cancer. Histochem Cell Biol. 2001;115:67–72. doi: 10.1007/s004180000224. [DOI] [PubMed] [Google Scholar]
- 65.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 66.Graff BA, Vangberg L, Rofstad EK. Quantitative assessment of uptake and distribution of iron oxide particles (NC100150) in human melanoma xenografts by contrast-enhanced MRI. Magn Reson Med. 2004 Apr;51(4):727-35 [DOI] [PubMed]
- 67.Pun SH, Tack F, Bellocq NC, Cheng J, Grubbs BH, Jensen GS, Davis ME, Brewster M, Janicot M, Janssens B, et al. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol Ther. 2004;3:641–50. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 68.Goodman TT, Olive PL, Pun SH. Increased nanoparticle penetration in collagenase-treated multicellular spheroids. Int J Nanomedicine. 2007;2:265–74. [PMC free article] [PubMed] [Google Scholar]
- 69.Kuhn SJ, Finch SK, Hallahan DE, Giorgio TD. Proteolytic surface functionalization enhances in vitro magnetic nanoparticle mobility through extracellular matrix. Nano Lett. 2006;6:306–12. doi: 10.1021/nl052241g. [DOI] [PubMed] [Google Scholar]
- 70.Neeves KB, Sawyer AJ, Foley CP, Saltzman WM, Olbricht WL. Dilation and degradation of the brain extracellular matrix enhances penetration of infused polymer nanoparticles. Brain Res. 2007;1180:121–32. doi: 10.1016/j.brainres.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–23. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–52. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 73.Jones PA, DeClerck YA. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980;40:3222–7. [PubMed] [Google Scholar]
- 74.Stetler-Stevenson WG, Liotta LA, Kleiner DE Jr. Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434-41 [DOI] [PubMed]
- 75.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 76.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008 May 1;122(9):2050-6 [DOI] [PubMed]
- 77.Brown PD, Giavazzi R. Matrix metalloproteinase inhibition: a review of anti-tumour activity. Ann Oncol. 1995 Dec;6(10):967-74 [DOI] [PubMed]
- 78.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–41. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei S, Kashiwagi M, Kota S, Xie Z, Nagase H, Brew K. Reactive site mutations in tissue inhibitor of metalloproteinase-3 disrupt inhibition of matrix metalloproteinases but not tumor necrosis factor-alpha-converting enzyme. J Biol Chem. 2005;280:32877–82. doi: 10.1074/jbc.C500220200. [DOI] [PubMed] [Google Scholar]
- 80.Chetty C, Lakka SS, Bhoopathi P, Kunigal S, Geiss R, Rao JS. Tissue inhibitor of metalloproteinase 3 suppresses tumor angiogenesis in matrix metalloproteinase 2-down-regulated lung cancer. Cancer Res. 2008;68:4736–45. doi: 10.1158/0008-5472.CAN-07-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361–8. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- 82.Song C, Zhu S, Wu C, Kang J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J Biol Chem. 2013;288:28021–33. doi: 10.1074/jbc.M113.498758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S, Cao J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biol Chem. 2011;286:33167–77. doi: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang T, Guan M, Xu C, Chen Y, Lu Y. Pigment epithelium-derived factor inhibits glioma cell growth in vitro and in vivo. Life Sci. 2007;81:1256–63. doi: 10.1016/j.lfs.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 85.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–72. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 86.Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur J Cancer. 1996;32A:2528–33. doi: 10.1016/S0959-8049(96)00398-X. [DOI] [PubMed] [Google Scholar]
- 87.Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941–6. doi: 10.1038/sj.bjc.6603043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci U S A. 2013;110:17047–52. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol (Camb). 2013 Jan;5(1):96-107 [DOI] [PMC free article] [PubMed]
- 90.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 91.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 92.Lunt SJ, Kalliomaki TM, Brown A, Yang VX, Milosevic M, Hill RP. Interstitial fluid pressure, vascularity and metastasis in ectopic, orthotopic and spontaneous tumours. BMC Cancer. 2008;8:2. doi: 10.1186/1471-2407-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simonsen TG, Gaustad JV, Leinaas MN, Rofstad EK. High interstitial fluid pressure is associated with tumor-line specific vascular abnormalities in human melanoma xenografts. PLoS One. 2012;7:e40006. doi: 10.1371/journal.pone.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 95.Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, Molokhia P, Attia K, Sullivan T, Kuter I, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol. 2005 Mar 20;23(9):1951-61 [DOI] [PubMed]
- 96.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 97.Vlahovic G, Rabbani ZN, Herndon JE, 2nd, Dewhirst MW, Vujaskovic Z. Treatment with Imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer. 2006;95:1013–9. doi: 10.1038/sj.bjc.6603366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, Hagedorn NL, McCarville MB, Stewart CF, Davidoff AM. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007 Jul 1;13(13):3942-50. [DOI] [PubMed]
- 99.Ley CD, Horsman MR, Kristjansen PEG. Early effects of combretastatin-A4 disodium phosphate on tumor perfusion and interstitial fluid pressure. Neoplasia. 2007;9:108–12. doi: 10.1593/neo.06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skliarenko JV, Lunt SJ, Gordon ML, Vitkin A, Milosevic M, Hill RP. Effects of the vascular disrupting agent ZD6126 on interstitial fluid pressure and cell survival in tumors. Cancer Res. 2006;66:2074–80. doi: 10.1158/0008-5472.CAN-05-2046. [DOI] [PubMed] [Google Scholar]
- 101.Upreti M, Jyoti A, Sethi P. Tumor microenvironment and nanotherapeutics. Transl Cancer Res. 2013;2:309–19. doi: 10.3978/j.issn.2218-676X.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res. 2006;4:61–70. doi: 10.1158/1541-7786.MCR-06-0002. [DOI] [PubMed] [Google Scholar]
- 103.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 104.Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003;94:1021–8. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harada H, Kizaka-Kondoh S, Hiraoka M. Optical imaging of tumor hypoxia and evaluation of efficacy of a hypoxia-targeting drug in living animals. Mol Imaging. 2005;4:182–93. doi: 10.1162/15353500200505112. [DOI] [PubMed] [Google Scholar]
- 106.Cairns RA, Papandreou I, Sutphin PD, Denko NC. Metabolic targeting of hypoxia and HIF1 in solid tumors can enhance cytotoxic chemotherapy. Proc Natl Acad Sci U S A. 2007;104:9445–50. doi: 10.1073/pnas.0611662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A, Kyle A, Auf dem Keller U, Leung S, Huntsman D, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71:3364–76. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 108.Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed Engl. 2014;53:3362–6. doi: 10.1002/anie.201308368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, Agrawal N, Borzillary S, McCaffery JM, Watson EL, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci U S A. 2003;100:15083–8. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000 Feb;3(1):39-47 [DOI] [PubMed]
- 111.Gerweck LE, Kozin SV, Stocks SJ. The pH partition theory predicts the accumulation and toxicity of doxorubicin in normal and low-pH-adapted cells. Br J Cancer. 1999;79:838–42. doi: 10.1038/sj.bjc.6690134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vukovic V, Tannock IF. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. Br J Cancer. 1997;75:1167–72. doi: 10.1038/bjc.1997.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–18. doi: 10.1016/S0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 114.Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J Control Release. 2012 Jun 10;160(2):264-73 [DOI] [PMC free article] [PubMed]
- 115.Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007;15:538–45. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu H, Zhu L, Torchilin VP. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials. 2013;34:1213–22. doi: 10.1016/j.biomaterials.2012.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33:3942–51. doi: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sawant RR, Torchilin VP. Liposomes as ‘smart’pharmaceutical nanocarriers. Soft Matter. 2010;6:4026–44. doi: 10.1039/b923535n. [DOI] [Google Scholar]
- 119.Chen KL, Bothun GD. Nanoparticles meet cell membranes: probing nonspecific interactions using model membranes. Environ Sci Technol. 2014;48:873–80. doi: 10.1021/es403864v. [DOI] [PubMed] [Google Scholar]
- 120.Verma A, Stellacci F. Effect of surface properties on nanoparticle-cell interactions. Small. 2010;6:12–21. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]
- 121.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhattacharjee H, Balabathula P, Wood GC. Targeted nanoparticulate drug-delivery systems for treatment of solid tumors a review. Ther Deliv. 2010 Nov;1(5):713-34 [DOI] [PubMed]
- 123.Lu Y, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–93. doi: 10.1016/S0169-409X(02)00042-X. [DOI] [PubMed] [Google Scholar]
- 124.Daniels TR, Bernabeu E, Rodríguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012 Mar;1820(3):291-317 [DOI] [PMC free article] [PubMed]
- 125.Verdurmen WP, Wallbrecher R, Schmidt S, Eilander J, Bovee-Geurts P, Fanghanel S, Burck J, Wadhwani P, Ulrich AS, Brock R. Cell surface clustering of heparan sulfate proteoglycans by amphipathic cell-penetrating peptides does not contribute to uptake. J Control Release. 2013 Aug 28;170(1):83-91 [DOI] [PubMed]
- 126.Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983;258:9681–9. [PubMed] [Google Scholar]
- 127.Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–45. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 128.Paulos CM, Reddy JA, Leamon CP, Turk MJ, Low PS. Ligand binding and kinetics of folate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–14. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 129.Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–61. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 130.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010 Aug 3;145(3):182-95 [DOI] [PMC free article] [PubMed]
- 131.Kim HS, Kim JS, Lee YK, Koo KH, Park YS. An efficient liposomal gene delivery vehicle using Sendai F/HN proteins and protamine. Cancer Gene Ther. 2008;15:214–24. doi: 10.1038/sj.cgt.7701121. [DOI] [PubMed] [Google Scholar]
- 132.Ting CL, Wang ZG. Interactions of a charged nanoparticle with a lipid membrane: implications for gene delivery. Biophys J. 2011;100:1288–97. doi: 10.1016/j.bpj.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maitani Y, Igarashi S, Sato M, Hattori Y. Cationic liposome (DC-Chol/DOPE=1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int J Pharm. 2007;342:33–9. doi: 10.1016/j.ijpharm.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 134.Koshkaryev A, Piroyan A, Torchilin VP. Bleomycin in octaarginine-modified fusogenic liposomes results in improved tumor growth inhibition. Cancer Lett. 2013;334:293–301. doi: 10.1016/j.canlet.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33:3942–51. doi: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Navarro G, Sawant RR, Essex S. Tros de Ilarduya C, Torchilin VP Phospholipid–polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Deliv Transl Res. 2011 Feb 1;1(1):25-33 [DOI] [PMC free article] [PubMed]
- 137.Zuhorn IS, Engberts JB, Hoekstra D. Gene delivery by cationic lipid vectors: overcoming cellular barriers. Eur Biophys J. 2007;36:349–62. doi: 10.1007/s00249-006-0092-4. [DOI] [PubMed] [Google Scholar]
- 138.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koshkaryev A, Piroyan A, Torchilin VP. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol Ther. 2012;13:50–60. doi: 10.4161/cbt.13.1.18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6:1949–57. [PubMed] [Google Scholar]
- 141.Kuwazuru Y, Yoshimura A, Hanada S, Utsunomiya A, Makino T, Ishibashi K, Kodama M, Iwahashi M, Arima T, Akiyama S. Expression of the multidrug transporter, P-glycoprotein, in acute leukemia cells and correlation to clinical drug resistance. Cancer. 1990;66:868–73. doi: 10.1002/1097-0142(19900901)66:5<868::AID-CNCR2820660510>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 142.Bai F, Wang C, Lu Q, Zhao M, Ban FQ, Yu DH, Guan YY, Luan X, Liu YR, Chen HZ, et al. Nanoparticle-mediated drug delivery to tumor neovasculature to combat P-gp expressing multidrug resistant cancer. Biomaterials. 2013;34:6163–74. doi: 10.1016/j.biomaterials.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 143.Sun YL, Chen JJ, Kumar P, Chen K, Sodani K, Patel A, Chen YL, Chen SD, Jiang WQ, Chen ZS. Reversal of MRP7 (ABCC10)-mediated multidrug resistance by tariquidar. PLoS One. 2013;8:e55576. doi: 10.1371/journal.pone.0055576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patel NR, Rathi A, Mongayt D, Torchilin VP. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int J Pharm. 2011;416:296–9. doi: 10.1016/j.ijpharm.2011.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baumert C, Günthel M, Krawczyk S, Hemmer M, Wersig T, Langner A, Molnár J, Lage H, Hilgeroth A. Development of small-molecule P-gp inhibitors of the N-benzyl 1,4-dihydropyridine type: novel aspects in SAR and bioanalytical evaluation of multidrug resistance (MDR) reversal properties. Bioorg Med Chem. 2013;21:166–77. doi: 10.1016/j.bmc.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 146.Zhu H, Liu Z, Tang L, Liu J, Zhou M, Xie F, Wang Z, Wang Y, Shen S, Hu L, et al. Reversal of P-gp and MRP1-mediated multidrug resistance by H6, a gypenoside aglycon from Gynostemma pentaphyllum, in vincristine-resistant human oral cancer (KB/VCR) cells. Eur J Pharmacol. 2012;696:43–53. doi: 10.1016/j.ejphar.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 147.Patil Y, Sadhukha T, Ma L, Panyam J. Nanoparticle-mediated simultaneous and targeted delivery of paclitaxel and tariquidar overcomes tumor drug resistance. J Control Release. 2009 May 21;136(1):21-9 [DOI] [PubMed]
- 148.Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–7. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 149.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–9. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 150.Navarro G, Sawant RR, Biswas S, Essex S, Tros de Ilarduya C, Torchilin VP. P-glycoprotein silencing with siRNA delivered by DOPE-modified PEI overcomes doxorubicin resistance in breast cancer cells. Nanomedicine (Lond) 2012;7:65–78. doi: 10.2217/nnm.11.93. [DOI] [PMC free article] [PubMed] [Google Scholar]