Abstract
Background
Although intraocular pressure is an important risk factor in glaucoma, there is growing body evidence indicating an immunological component in the pathogenesis of normal-tension glaucoma (NTG). The aim of this study was to determine if NTG coexists with elevated levels of autoantibodies detected in rheumatic diseases.
Material/Methods
We enrolled 105 patients into the study: 35 with NTG, 34 with primary open-angle glaucoma (POAG), and 36 controls. All patients underwent ophthalmic examination and blood tests. Blood was examined for the level of: antibodies against antinuclear antibodies (ANA), antibodies to extractable nuclear antigens (ENA), immunoglobulins (IgG, IgA, IgM), rheumatoid factor, anti-citrullinated protein antibodies (ACPA), and antiphospholipid antibodies (anticardiolipin antibodies, beta2-glycoprotein I antibodies, antiprothrombin antibodies).
Results
The level of ANA was increased among 6 patients in the NTG group (17.1%), 8 in the POAG group (23.5%), and 6 in the control group (16.5%). The difference was not statistically significant (p=0.97). None of the patients in the NTG, POAG, or control group had positive antibodies to ENA. The level of immunoglobulins IgG, IgM, and IgA in the 3 groups was similar and within normal values. The median level of rheumatoid factor and ACPA was the highest in the NTG group, but it was within normal laboratory values. There was a statistically significant difference between antiprothrombin antibodies IgG between the NTG and POAG group (p=0.01), but not between the NTG and control group (p=0.24).
Conclusions
The results of our study do not confirm the hypothesis that NTG coexists with elevated blood levels of antibodies, which are a characteristic feature of rheumatic diseases.
MeSH Keywords: Autoantibodies - blood, Autoimmune Diseases, Low Tension Glaucoma - physiopathology
Background
Glaucoma is a group of diseases characterized by chronic progressive optic neuropathy that leads to degeneration of retinal ganglion cells and their axons, resulting in the typical appearance of the optic disc, which differentiates it from other optic neuropathies and visual-field defects [1–4]. There is thinning of the neuroretinal rim and the central depression of the optic disk expands. The causes of optic disk cupping include the loss of retinal ganglion cells, glial cells, and blood vessels [1]. Elevated intraocular pressure (IOP) is considered a major risk factor for the development of glaucomatous optic neuropathy. At present, IOP reduction has been the only effective treatment for glaucoma. In normal-tension glaucoma (NTG), however, IOP is not elevated and thus there must be other risk factors for glaucomatous optic neuropathy and subsequent damage to the optic nerve [5,6].
Glaucoma is a neurodegenerative disease; the numerous underlying pathogenetic mechanisms are responsible for the death of retinal ganglion cells and degeneration of their axons. There is increasing evidence of immune system involvement in neurodegenerative processes and numerous studies confirm the autoimmune component of glaucomatous optic neuropathy, with the immune system responsible for the degeneration of retinal ganglion cells and their axons [7].
Over 30 years ago, Cartwright et al. found that as many as 30% of patients with NTG had coexisting autoimmune disease compared to 8% of patients with ocular hypertension. Their study suggested that the immune system may be responsible for optic neuropathy in NTG [8] and prompted further studies in this area. Their findings implicate the humoral immune system in the pathogenesis and progression of glaucomatous optic neuropathy.
Wax et al. assessed levels of the monoclonal proteins IgM, IgG, and IgA in patients with NTG and patients with primary open-angle glaucoma (POAG) and found elevated levels in 18% of NTG patients, and no elevation in POAG patients [9]. Additionally, the NTG patients more frequently showed elevated serum levels of antibodies directed against extractable nuclear antigens (ENA) and of autoantibodies directed against Sjögren’s syndrome A antigens (SS-A/Ro) and Sjögren’s syndrome B antigens (SS-B/La). Antinuclear antibodies (ANA) are autoantibodies directed against non-extractable or extractable nuclear antigens. ANAs of importance in clinical practice include anti-Sm, anti-RNP (ribonucleoprotein), anti-SS-A/Ro, anti-SS-B-La, anti-Scl-70, and anti-Jo-1. The prevalence of ANAs in healthy individuals is 5% and their normal titer is 1:20, but it increases with age. ANAs may be also induced by pregnancy, drugs (e.g., gold salts, sulfasalazine, immunoglobulins, and TNF-α antagonists), infectious diseases, and malignancy [10].
A study by Hamman et al. assessed the serum levels of ANAs, anti-ENA, and anti-dsDNA autoantibodies, titers of IgA, IgM, and IgG immunoglobulins, and serum protein electrophoresis in patients with NTG and with POAG, and in control subjects. Compared with the other groups, the NTG patients had statistically significant increases in serum IgA levels and considerably higher ANA levels, although the latter difference was not statistically significant [11].
Antiphospholipid antibodies are a heterogeneous group of autoantibodies that react against plasma proteins. They have affinity to negatively-charged phospholipids such as cardiolipin, phosphatidylserine, phosphatidylinositol, and phosphatidic acid. The prevalence of antiphospholipid antibodies in healthy individuals is approximately 8% and their elevated titers are observed mostly in antiphospholipid syndrome and systemic lupus erythematosus [10]. Kremmer et al. found higher IgG and IgM antiphosphatidylserine antibodies in patients with NTG compared with POAG patients and controls, which might indicate both active (IgM) and chronic (IgG) autoimmune processes in NTG [12]. The multicenter population study titled the Canadian Glaucoma Study, from the Canadian Glaucoma Study Group, confirmed the relationship between anticardiolipin antibodies and the pathogenesis of glaucoma, with a 4-fold risk for the progression of glaucomatous optic neuropathy in patients with elevated anticardiolipin antibodies compared to individuals with normal levels. The study demonstrated that elevated levels of anticardiolipin antibodies in glaucoma patients are an independent factor of glaucoma progression with subsequent visual-field defects [13].
The aim of the study
The aim of the study was to determine if normal-tension glaucoma coexists with the elevated levels of autoantibodies detected in rheumatic diseases.
Material and Methods
This was a case-control study performed in 3 groups of patients: normal tension glaucoma (Group 1), primary open-angle glaucoma (Group 2), and healthy control patients without glaucoma (Group 3).
On 13 January 2010, the Bioethics Committee at the Medical Centre of Postgraduate Education gave their approval for the study. Before entering the study, each patient was verbally informed about their participation in a scientific study. The subject matter of the study was presented by a physician and the patient was given printed “Patient Information” about the study, which was written to be easy to understand for people without specialized medical knowledge. Next, each prospective participant gave informed consent to participate in the study by filling out and signing the Informed Consent for Clinical Studies form.
All patients had complete ophthalmologic examination and blood tests. The ophthalmologic examination included visual acuity, intraocular pressure measurement using applanation and pascal tonometry, detailed assessment of the anterior and posterior segments of the eyeball, pachymetry, gonioscopy, visual-field examination, and optical coherence tomography (OCT) of the optic nerve and the macula.
A panel of blood tests included determinations of the following: antinuclear antibodies (ANAs) by indirect immunofluorescence (IF) using as substrates monkey and guinea pig oesophagus, and HEp-2 cells, antibodies directed against extractable nuclear antigens (ENAs): Sm, RNP, SS-A/Ro, SS-B/La, PM-Scl, Scl-70, Jo-1, Ku, ACA, Mi-2, ribosomal, fibrillarin, RNA-polymerase I and cytoskeletal antigens by immunodiffusion using thymus extracts), IgG and IgM antiphospholipid antibodies (anticardiolipin, anti-beta2-glycoprotein I, anti-prothrombin), rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), and IgG, IgM, and IgA immunoglobulins.
The exclusion criteria were: lack of informed consent, ocular diseases that might lead to false results (e.g., corneal disorders with possible impact on visual acuity and visual field, including corneal ulceration, corneal injury, corneal dystrophy, corneal swelling; disorders of the ocular lens, including mature cataract, brown cataract and congenital cataract, with impact on visual acuity and visual field; retinal disorders with possible impact on visual acuity and visual field, including acquired macular disorders (e.g., age-related macular degeneration), retinal vascular disorders (e.g., diabetic retinopathy, retinal detachment; neurological disorders and optic nerve disorders, with possible impact on visual activity and visual field, including status after cerebrovascular accident, optic neuritis, optic neuropathy); severe systemic disease with possible impact on serum antibody levels, including chronic viral hepatitis, chronic inflammatory pulmonary disorders, systemic malignancies, viral infections (AIDS, infectious mononucleosis, influenza), bacterial infections (tuberculosis, syphilis, leprosy, brucellosis, salmonellosis, subacute endocarditis), parasitic diseases (malaria, filariasis, schistosomiasis), status after vaccination against viral disease, medical treatments affecting autoantibody levels, and intraocular pressure and pregnancy.
Statistical analysis
The statistical analyses were performed using STAT software. Prior to examination, a minimum number of patients required to be included in each group was established. Inclusion of 34 patients to each of the NTG, POAG, and controls group ensured (with 80% odds ratio) statistical significance at 5% (p=0.05). The normality of distribution of the study variables in particular groups was assessed with the Shapiro-Wilk test. Because of departures from normality assumptions, the non-parametric Kruskal-Wallis test was used for comparisons. The chi-square test was used to compare frequencies. The entire statistical analysis was performed at the significance level α=0.05. Results were considered statistically significant at p<0.05.
Results
We included a total of 105 patients aged 30–80 years in the study. Their demographic data are presented in Table 1.
Table 1.
Demographic particulars of patients in each group.
| Number of subjects | Age range (years) | Mean age (years) | |||
|---|---|---|---|---|---|
| % | From | To | |||
| Group 1 | |||||
| Females | 25 | 71% | 37 | 80 | 68.1 |
| Males | 10 | 29% | 54 | 80 | 66.9 |
| Total | 35 | 37 | 80 | 67.8 | |
| Group 2 | |||||
| Females | 22 | 65% | 51 | 80 | 66.6 |
| Males | 12 | 35% | 43 | 78 | 60.4 |
| Total | 34 | 43 | 80 | 64.4 | |
| Group 3 | |||||
| Females | 27 | 75% | 30 | 74 | 52.0 |
| Males | 9 | 25% | 40 | 75 | 59.0 |
| Total | 36 | 30 | 75 | 53.8 | |
In the NTG group, a coexisting autoimmune disease was identified in 3 patients (8.6%) by laboratory investigations within the study: Hashimoto’s thyroiditis in 2 patients and systemic lupus erythematosus in 1 patient. In the POAG group, 3 cases (8.8%) of a comorbid autoimmune disease were diagnosed: 1 case of Hashimoto’s thyroiditis and 2 cases of rheumatoid arthritis. In the control group, there were also 3 cases (8.3%) of a newly diagnosed coexisting autoimmune disease: 2 cases of Hashimoto’s thyroiditis and 1 case of psoriasis. The results are very similar and not statistically significant at p=0.97 for the comparison between Groups 1 and 2 and between Groups 1 and 3.
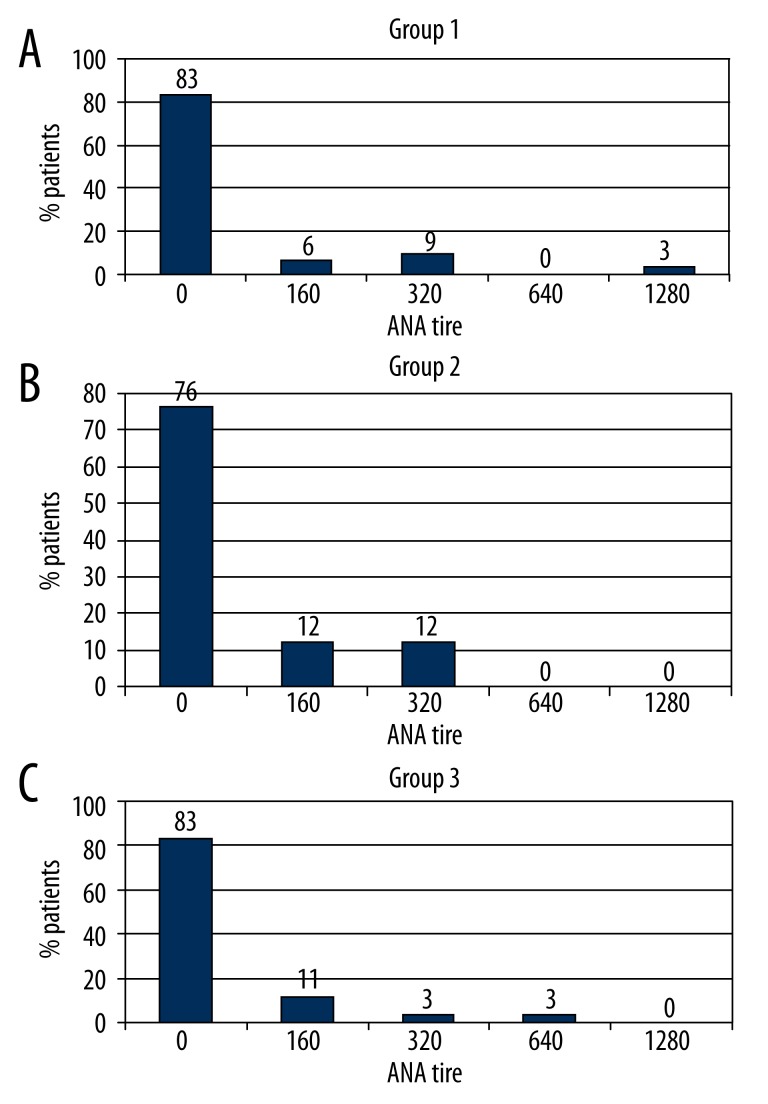
ANAs were found in 6 of 35 NTG patients (17.1%), 8 of 34 POAG patients (23.5%), and 6 of 36 controls (16.5%). It should be emphasized that the percentage of patients with ANAs in each of the study groups was much higher than reported for the general population. Since fewer than 25% of patients in each study group had positive ANA test results, both medians and quartiles are equal at 0. A comparison of ANA blood levels between the 3 groups did not demonstrate statistical significance (p=0.73). These results are shown in Figure 1.
Figure 1.
Distribution of incidence of positive antinuclear antibodies (ANA) by titer in the 3 groups.
There were no anti-ENA antibodies identified in the serum of any patients in neither group 1, 2 nor 4.
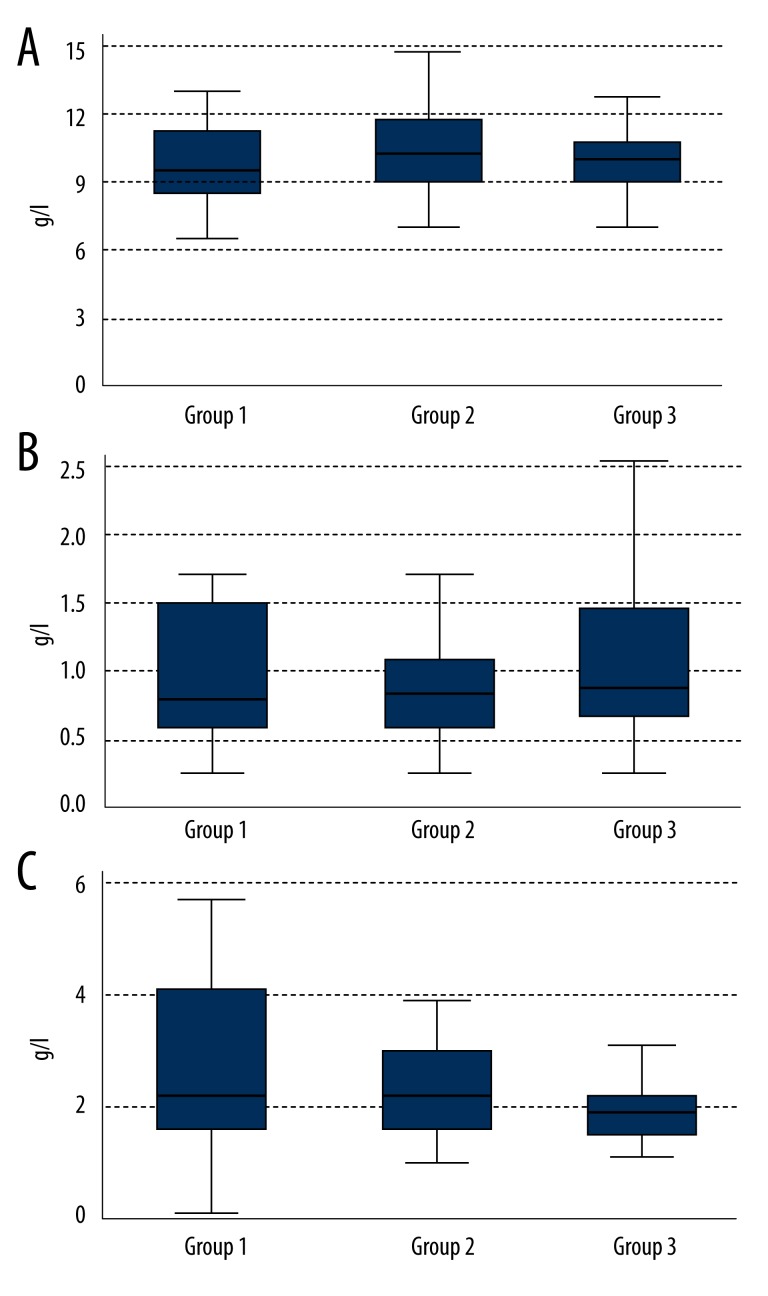
IgG, IgM, and IgA median levels (Figure 2) were similar in the 3 groups and remained within normal limits:
Figure 2.
Levels of immunoglobulins IgG (A), IgM (B), and IgA (C) in the 3 groups.
-
IgG immunoglobulin [normal range: 7–16 g/L]:
Group 1: 9.31 g/L (Q1–8.35; Q3–10.79);
Group 2: 10.19 g/L (Q1–8.65; Q3–11.68);
Group 3: 9.79 g/L (Q1–8.86; Q3–10.86).
The difference is not statistically significant (p=0.35).
-
IgM immunoglobulin [normal range: 0.4–2.3 g/L]:
Group 1: 0.80 g/L (Q1–0.60; Q3–1.50);
Group 2: 0.90 g/L (Q1–0.60; Q3–1.10);
Group 3: 1.00 g/L (Q1–0.65; Q3–1.50).
The difference is not statistically significant (p=0.37).
-
IgA immunoglobulin [normal range: 0.7–4.0 g/L]:
Group 1: 2.15 g/L (Q1–1.35, Q3–4.15);
Group 2: 2.17 g/L (Q1–1. 69; Q3–2.93);
Group 3: 1.86 g/L (Q1–1.40; Q3–2.17).
The difference is not statistically significant (p=0.14).
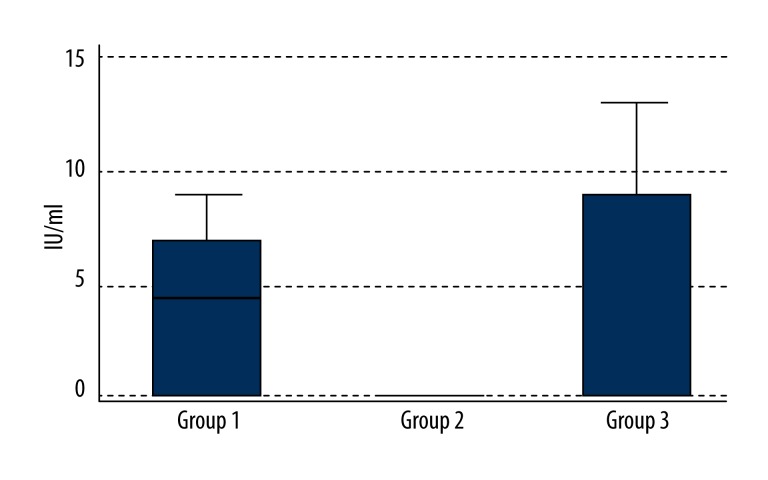
Rheumatoid factor median levels [normal range: 0–14 IU/mL] were as follows (Figure 3):
Figure 3.
Levels of rheumatoid factor (RF) in the blood in the 3 groups.
Group 1: 4.40 IU/mL (Q1–0.00; Q3–7.20);
Group 2: 0.00 IU/mL (Q1–0.00; Q3–0.00);
Group 3: 0.00 IU/mL (Q1–0.00; Q3–8.55).
The only statistically significant difference was between Groups 1 and 2 (p=0.01). However, it should be emphasized that in spite of this statistically significant difference between the NTG patients and the POAG patients, none of the NTG patients had an elevated blood rheumatoid factor level (outside the laboratory’s normal value range).
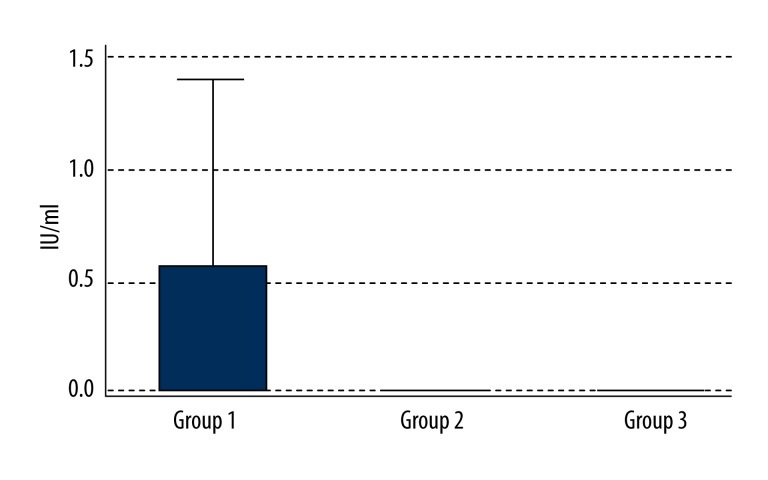
The median levels of anti-citrullinated protein antibodies (normal range: 0–5 U/mL) were not elevated in any of the study groups and were as follows (Figure 4):
Figure 4.
Levels of ACPA in the 3 groups.
Group 1: 0.00 U/mL (Q1–0.00; Q3–0.60);
Group 2: 0.00 U/mL (Q1–0.00; Q3–0.00);
Group 3: 0.00 U/mL (Q1–0.00; Q3–0.00).
The differences between Groups 1 and 3 (p=0.0009) and between Groups 2 and 3 (p=0.041) were statistically significant. However, in this case too, in spite of the statistical significance, none of the NTG or POAG patients had an elevated ACPA level in the blood.
Tests for IgG and IgM antiphospholipid antibodies (anticardiolipin, anti-beta2-glycoprotein I, and anti-prothrombin) did not demonstrate their elevated titers in any of the study groups (median levels presented below):
-
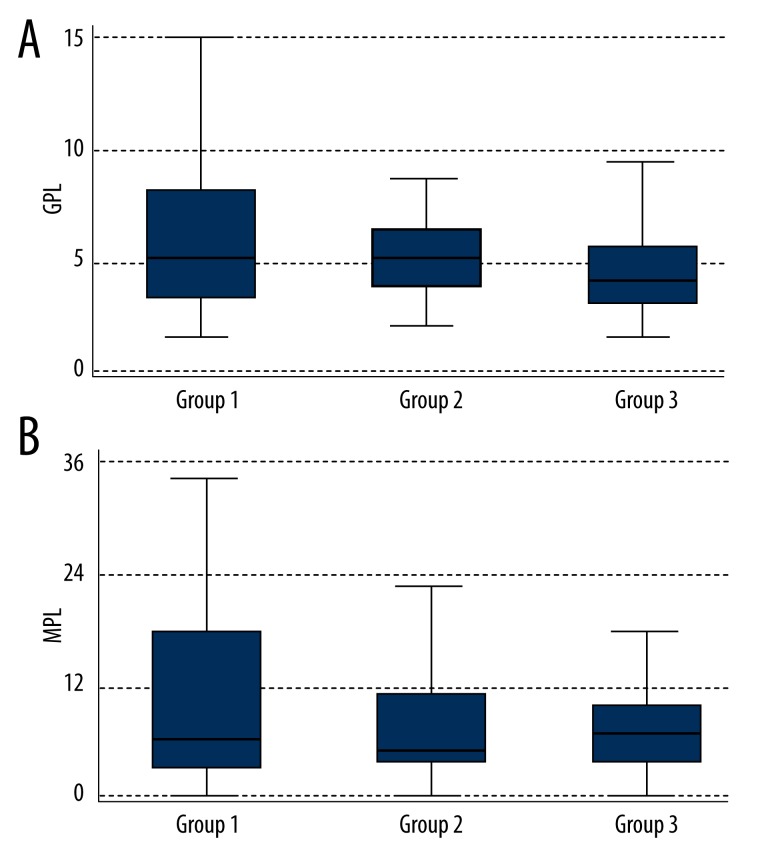
IgG anticardiolipin antibodies (normal range: 0–10 GPL [IgG phospholipid units]) (Figure 5A):
Group 1: 5.29 GPL (Q1–3.27; Q3–8.27);
Group 2: 5.29 GPL (Q1–3.59; Q3–6.71);
Group 3: 4.11 GPL (Q1–2.85; Q3–6.05).
Figure 5.
Levels of anticardiolipin antibodies IgG (A) and IgM (B) in the 3 groups.
The difference was not statistically significant (p=0.39).
-
IgM anticardiolipin antibodies (normal range: 0–20 MPL [IgM phospholipid units]) (Figure 5B):
Group 1: 6.77 MPL (Q1–2.66; Q3–17.04);
Group 2: 5.01 MPL (Q1–3.01; Q3–10.65);
Group 3: 6.59 MPL (Q1–3.38; Q3–9.40).
The difference is not statistically significant (p=0.68).
-
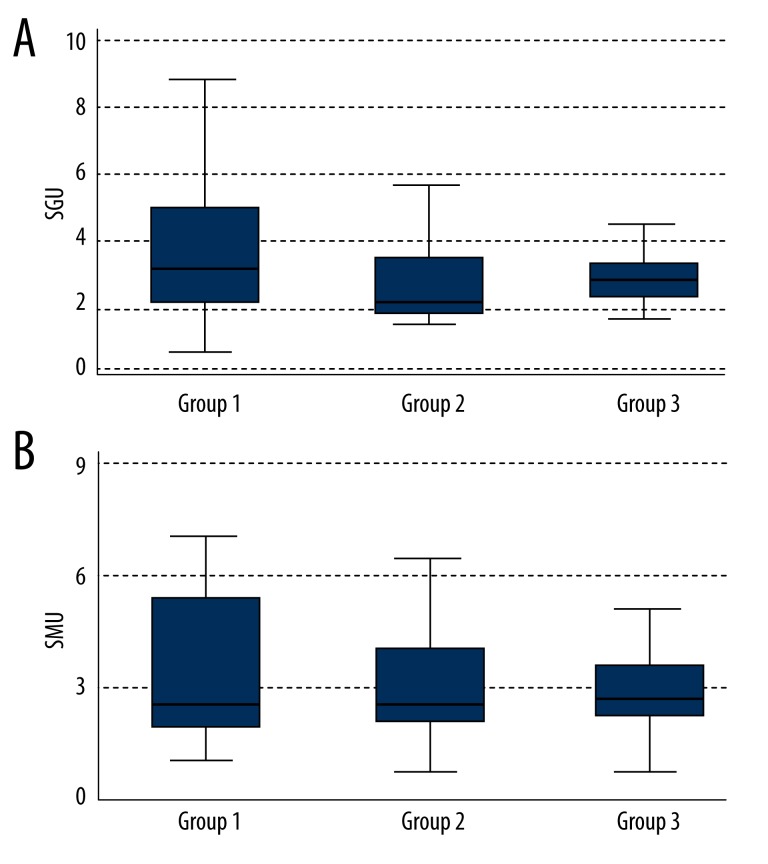
IgG anti-beta2-glycoprotein I antibodies (normal range: 0–20 SGU [standard IgG unit]) (Figure 6A):
Group 1: 3.31 SGU (Q1–2.10; Q3–4.93);
Group 2: 2.28 SGU (Q1–1.83; Q3–3.34);
Group 3: 2.79 SGU (Q1–2.38; Q3–3.34).
Figure 6.
Levels of anti-beta-2-glycoprotein I antibodies IgG (A) and IgM (B) in the 3 groups.
The difference was not statistically significant (p=0.13).
-
IgG anti-beta2-glycoprotein I antibodies (normal range: 0–20 SMU [standard IgM unit]) (Figure 6B):
Group 1: 2.55 SMU (Q1–1.76; Q3–5.11);
Group 2: 2.58 SMU (Q1–1.95; Q3–3.98);
Group 3: 2.69 SMU (Q1–2.00; Q3–3.48).
The difference was not statistically significant (p=0.92).
-
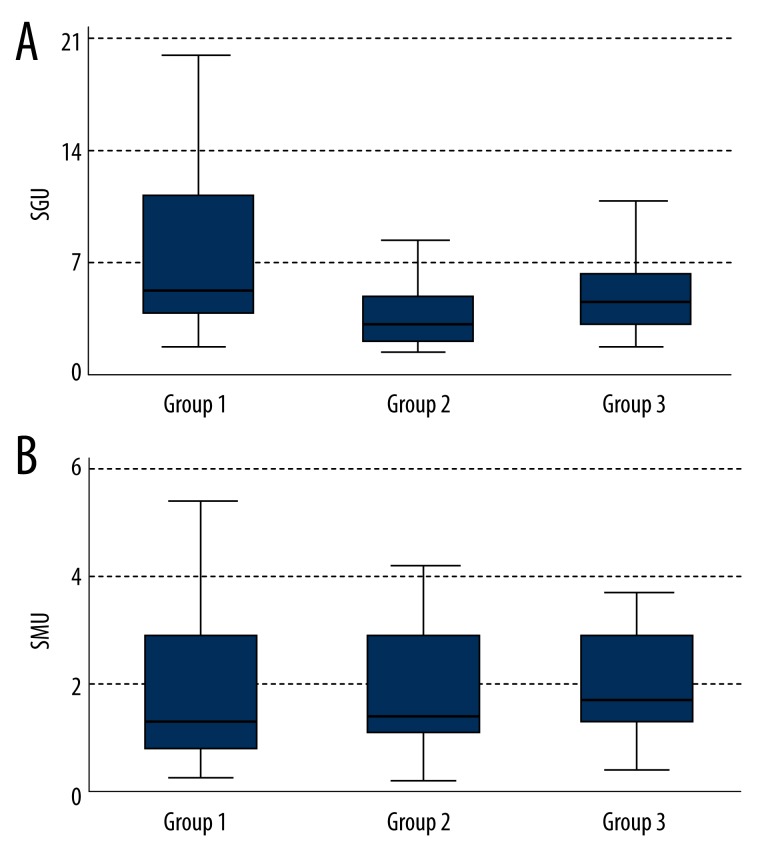
IgG anti-prothrombin antibodies (normal range: 0–7 SGU) (Figure 7A):
Group 1: 5.43 SGU (Q1–3.35; Q3–10.72);
Group 2: 2.86 SGU (Q1–1.91; Q3–5.14);
Group 3: 4.79 SGU (Q1–2.56; Q3–6.21).
Figure 7.
Levels of antiprothrombin antibodies IgG (A) and IgM (B) in the 3 groups.
The difference was statistically significant (p=0.03). Comparison between groups found a statistically significantly higher titer of IgG anti-prothrombin antibodies in the NTG patients than in the POAG patients (p=0.01) but not between NTG and control group (p=0.24).
-
IgM anti-prothrombin antibodies (normal range: 0–6 SMU) (Figure 7B):
Group 1: 1.21 SMU (Q1–0.67; Q3–2.69);
Group 2: 1.28 SMU (Q1–1.01; Q3–2.71);
Group 3: 1.61 SMU (Q1–1.19; Q3–2.47).
The difference was not statistically significant (p=0.32).
Discussion
A pathogenetic association between autoimmune disorders and the development of glaucomatous optic neuropathy has considered for approximately 30 years. However, in spite of numerous published studies suggesting the autoimmune etiology of glaucomatous optic neuropathy, so far none of them has offered convincing evidence for the role of the immune system in the pathogenesis of NTG.
The first study that put forward the hypothesis of the autoimmune component of glaucoma was published in the United States in 1992 by Cartwright et al., who reported autoimmune comorbidities up to 30% of patients with NTG, compared to just 8% of patients with ocular hypertension. Our study, carried out more than 30 years later, produced different results. Only 8.6% of our NTG patients had autoimmune disease and in this respect did not significantly differ from either the POAG patients or controls. It should be emphasized, however, that there have been no other published studies reporting the percentage of NTG patients with autoimmune comorbidities, apart from the study by Cartwright et al. and the present study. The difference in the results may be accounted for by relatively small groups of subjects in the 2 studies (67 patients in Cartwright’s study and 35 patients in the present study). Additionally, Cartwright et al. recruited subjects with ocular hypertension as controls. Thus, they did not assess the percentage of POAG patients with coexisting autoimmune disease and whether the autoimmune component was found exclusively in NTG or also in POAG [8,14]. In the present study, 3 groups of subjects were compared – NTG patients, POAG patients, and controls – and the results do not indicate that autoimmune diseases are more frequent in NTG and POAG patients.
Wax et al. found elevated serum immunoglobulin levels in patients with NTG [9]. In a later study, Hammam et al. confirmed higher serum concentrations of just IgA in patients with NTG, compared to POAG patients and controls [11]. Our results do not confirm those findings. IgG, IgA, and IgM concentrations were not significantly increased in any of the study groups. Many factors may be responsible for these differences in the results. Firstly, Wax et al. only reported the proportion of NTG patients with elevated immunoglobulin concentrations (8/44) without differentiating immunoglobulins into IgG, IgM, and IgA. Thus, an association between NTG and any particular class of immunoglobulins cannot be considered as proven. In our opinion, including all classes of immunoglobulins in a single statistical analysis is not a correct approach, since different pathophysiological mechanisms are responsible for increased concentrations of different classes of immunoglobulins. Importantly, the mean age of patients with NTG in the study by Wax et al. was 68.8 years and the prevalence of paraprotein in the blood serum increases with age; they are found in 1–1.7% of people aged over 50 years, in 3% of those aged over 70 years, and in nearly 6.0% of people in the ninth decade of life [15]. Thus it may be assumed that the elevated immunoglobulin levels reported by Wax et al. were partly accounted for by patient age. It was only later that Hammam et al. assessed particular classes of immunoglobulins in the sera of patients with NTG, POAG, and controls. Only the mean IgA concentration was statistically significantly higher in the NTG patients. The authors, however, did not consider an important fact – although that concentration was higher than the IgA levels in the control group and in the POAG patients, it still remained within the laboratory’s normal value range [11]. In our opinion, the study does not provide evidence for elevated IgA concentrations in the sera of patients with NTG, which would be the cause of glaucomatous optic neuropathy. Unlike the 2 studies presented above, we did not find any differences in the serum IgGs, IgMs, and IgAs between the 3 groups of subjects. In view of some limitations of those 2 studies, we believe that the results of our study are reliable and we conclude that immunoglobulin levels are not elevated in NTG serum.
A later study by Wax et al. also deals with the question of the autoimmune component of glaucoma. They found more frequent occurrence of ANAs and antibodies directed against ENAs (e.g., RNP, SS-A/Ro, and SS-B/La) in patients with NTG. In their study, 41% of patients with NTG were ANA-positive compared to 29% of patients with POAG, but the difference was not statistically significant. Additionally, the incidence of ENAs was higher in the NTG patients than in the POAG patients and the difference was statistically significant [9]. In another study, Hammam et al. confirmed the results of the study by Wax et al. reporting a higher incidence of serum ANAs in NTG, but the difference was not statistically significant. A large proportion of the study patients tested positive for ANAs: 32.3% of the NTG patients, 12.5% of the POAG patients and 15.6% of the controls. Unlike Wax et al., Hammam et al. did not observe a statistically significant increase in the incidence of antibodies directed against ENAs in the patients with NTG (12.9%) vs. 9.4% of the patients with POAG and 3.1% of the controls [11].
The results of our study are not in agreement with those in the studies discussed above. None of the study patients with NTG tested positive for ANAs. Of key importance is the method used to determine serum ANAs. The laboratory that carried out the tests in the present study detected ANAs at titers ≥1:160 (i.e., the minimum titer relevant for the diagnosis of rheumatic disease) [11]. On the other hand, the 2 studies cited earlier reported ANA titers as low as 1:40. In the study by Hammam et al., all ANA-positive patients except 1 had ANA titers of ≥1:40 [11]. In the study by Wax et al., half of all NTG patients tested positive for ANAs but the titers were 1:40 or 1:80 [9]. This difference in the ANA testing method was the most likely cause of the difference in results between our study and the earlier studies. Still, the elevations of serum ANAs reported by Wax et al. and Hammam et al. were not statistically significant and it may be assumed that ANAs are not involved in the pathogenesis of glaucomatous optic neuropathy. Interestingly, in both our study and the studies by the 2 other groups of authors, relatively large proportions of patients with and without glaucoma tested positive for ANAs. According to epidemiological reports, ANAs are increased in only 5% of the general population. Their titers increase with age and during pregnancy, some treatments (e.g., therapy with gold salts, sulfasalazine, immunoglobulins, or TNF-α antagonists), infectious diseases, and malignancies [10]. In our study, elevated ANA titers were found in as many as 17.1%, 23.5%, and 16.6% of patients with NTG, POAG and controls, respectively, while the corresponding values in the study by Hammam et al. were 32.3%, 12.5%, and 15.6%. Neither of the 2 groups of authors indicated whether their exclusion criteria included pregnancy, certain medications, infectious disease, or malignancy, which may account for the increased ANA titers. In our study, no subjects were pregnant, had infectious disease or malignancy, or took medicinal products that could have affected their ANA levels. Thus, it may be assumed that it was the older age that accounted for such a large number of ANA-positive subjects in our study. Also, nowadays more and more apparently healthy individuals tend to test positive for ANAs, associated with both connective tissue disorders and other diseases. For example, thyroid antibodies such as anti-TPO or anti-TG are found in as many as 9% to 27% of the general population [16–18].
Of all NTG and POAG patients and controls in the present study, none tested positive for antibodies directed against ENAs such as RNP, SS-A/Ro, and SS-B/La, Sm, PM-Scl, Scl-70, Jo-1, Ku, ACA, Mi-2 or ribosomal, fibrillarin, RNA-polymerase I, or cytoskeletal antigens. ENAs are detected in systemic connective tissue disorders, mostly in systemic lupus erythematosus and Sjögren’s syndrome [10]. Thus, a question arose of why Wax et al. and Hammam et al. found increased ENAs in subjects without diagnosed rheumatic disease. The answer was provided by another study by Wax et al. [19]. Considering that in their study group of NTG patients, so many had increased antibodies directed against SS-A/Ro and SS-B/La antigens without any symptoms of Sjögren’s syndrome, the authors decided to reassess the patients’ sera. A new test, this time using the immunoblot method, not ELISA, did not find increased ENAs, but elevated levels of antibodies directed against the human protein HSP60. The earlier erroneous results were due to the cross-reactivity of anti-SS-A/Ro and SS-B/La antibodies with anti-HSP60 antibodies, which produced false-positive test results for anti-SS-A/Ro and anti-SS-B/La antibodies [19]. Like Wax et al. in their first study, Hammam et al. also tested their patients for the anti-ENA antibodies using ELISA, which might have resulted in false-positive results [11]. The laboratory we used to test for the anti-ENA antibodies used the immunodiffusion method, not ELISA.
Kremmer et al. studied the IgG and IgM antiphospholipid antibody profiles in patients with NTG, patients with POAG, and controls. The NTG patients demonstrated elevated IgG antiphospholipid antibodies, including the subspecies of antiphosphatidylserine antibodies, compared with the POAG patients and controls. IgM antiphospholipid antibodies and their 2 subspecies – antiphosphatidylserine and anti-beta-2-glycoprotein I antibodies – were elevated in both POAG and NTG patients compared to controls. According to the authors, these findings suggested the autoimmune and prothrombotic component of the pathogenesis of glaucoma, including NTG [12]. Another study investigating an association between anticardiolipin antibodies and the progression of glaucomatous optic neuropathy (a multicenter population study, the Canadian Glaucoma Study [CGS]) confirmed that the risk for the progression of glaucoma is as much as 4 times higher in patients with elevated serum anticardiolipin antibodies compared to individuals without increased anticardiolipin antibodies. Although the difference was statistically significant, the percentage of subjects with increased anticardiolipin antibodies (5.5% of the 258 study subjects) did not differ from that of the general population, which is 2% to 7% [10,13]. The above results are in agreement with the findings of Tsakiris et al. who did not detect any significant differences in antiphospholipid antibody levels (anticardiolipin antibodies and lupus anticoagulants) between NTG and POAG patients and controls [20]. Our study included determinations of antiphospholipid antibodies in the blood. Because of their diagnostic value, tests for the following subspecies of antiphospholipid antibodies were performed: anticardiolipin, anti-beta-2-glycoprotein I, and antiprothrombin. We did not find any significant differences in the concentrations of IgG and IgM anticardiolipin antibodies and IgG and IgM antibodies directed against beta-2-glycoptotein I between the NTG, POAG, and control groups. Similarly, there were no differences in the concentrations of IgM antiprothrombin antibodies among the 2 groups. Differences in the increases of IgG antiprothrombin antibodies were found only between the NTG and the POAG patients. Elevated antiprothrombin antibody levels in the blood correspond to elevated lupus anticoagulant levels and are an independent risk factor for thrombotic events in systemic lupus erythematosus and venous and arterial thrombosis [10,21]. Similarly, anti-phosphatidylserine antibodies, found by Kremmer et al. to be higher in NTG patients, are another risk factor for thrombosis. In this respect our findings and those by Kremmer et al. seem to be in agreement and suggest that patients with NTG are at greater risk for thrombotic events than patients with POAG. Interestingly, both studies found increased IgG immunoglobulins, which suggests a chronic autoimmune process in the pathogenesis of NTG. In our study, we did not find increased blood concentrations of anticardiolipin antibodies in the NTG patients. This confirms the results of studies by Tsakiris et al. and the CGS [13,20]. Additionally, in the present study we did not find increased anti-beta-2-glycoprotein I antibodies in the patients with NTG. That might be possibly accounted for by small sizes of the 2 studies and differences between the study populations.
To the best of our knowledge, until now there have been no studies investigating a possible association between blood levels of rheumatoid factor and anti-citrullinated protein antibodies and the development of NTG. In the present study we performed tests for these antibodies to find out whether they have a role in the pathogenesis of glaucomatous optic neuropathy. We considered this to be important because these antibodies play an important role in the initiation of rheumatoid arthritis (RA) and in its diagnosis. RA, which may lead to permanent and incurable disability, affects 1% of the general population [22]. We wanted to determine if the rheumatoid factor and ACPA additionally influences the development of glaucomatous optic neuropathy. We found that the RF levels were higher in the NTG patients than in the POAG patients and the levels of ACPA were higher in the NTG patients than in controls, which were both statistically significant differences. In spite of this statistical significance of the differences, suggesting that the antibodies found in RA are increased in patients with NTG, we do not think there is any association between these antibodies and the pathogenesis of glaucomatous optic neuropathy, because the blood levels of ACPA and RF did not exceed the laboratory normal value range in any of the study patients with NTG.
Conclusions
The results of our study do not confirm the hypothesis that NTG coexists with elevated blood levels of antibodies, which are a characteristic feature of rheumatic diseases. The study patients with NTG were only found to have a higher mean level of IgG anti-prothrombin antibodies compared to the patients with POAG, but it was not different from the level in controls. That is why no association between NTG and increased blood levels of antibodies can be demonstrated based on the results of our study.
Footnotes
Source of support: The study was funded from the science budget in the years 2010–2014 as own research project under the title “Is there any association between elevated levels of antibodies occurring in autoimmune diseases and normal-tension glaucoma?” (No. N N402 283139)
References
- 1.Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Primary Open-Angle Glaucoma. N Eng J Med. 2009;360(11):1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 4.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative Normal-Tension Glaucoma Study Group. Comparison of Glaucomatous Progression Between Untreated Patients with Normal-Tension Glaucoma and Patients with Therapeutically Reduced Intraocular Pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 6.Collaborative Normal-Tension Glaucoma Study Group. The Effectiveness of Intraocular Pressure Reduction in the Treatment of Normal-Tension Glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 7.Tezel G, Wax MB. Glaucoma. Chem Immunol Allergy. 2007;92:221–27. doi: 10.1159/000099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartwright MJ, Grajewski AL, Friedberg ML, et al. Immune-related disease and normal-tension glaucoma. A case-control study. Arch Ophthalmol. 1992;110:500–2. doi: 10.1001/archopht.1992.01080160078035. [DOI] [PubMed] [Google Scholar]
- 9.Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1994;117:561–68. doi: 10.1016/s0002-9394(14)70059-5. [DOI] [PubMed] [Google Scholar]
- 10.Puszczewicz M. Reumatologia. Wydawnictwo Medical Tribune; Polska, Warszawa: 2010. pp. 32–39. [in Polish] [Google Scholar]
- 11.Hammam T, Montgomery D, Morris D, Imrie F. Prevalence of serum autoantibodies and paraproteins in patients with glaucoma. Eye (Lond) 2008;22:349–53. doi: 10.1038/sj.eye.6702613. [DOI] [PubMed] [Google Scholar]
- 12.Kremmer S, Kreuzfelder E, Klein R, et al. Antiphosphatidylserine antibodies are elevated in normal tension glaucoma. Clin Exp Immunol. 2001;125:211–15. doi: 10.1046/j.1365-2249.2001.01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chauhan BC, Mikelberg FS, Balaszi AG, et al. Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126:1030–36. doi: 10.1001/archopht.126.8.1030. [DOI] [PubMed] [Google Scholar]
- 14.Dana MR, Wilensky JT. Immune-Related Disease and Normal-Tension Glaucoma. Arch Ophthalmol. 1993;111:22–23. doi: 10.1001/archopht.1993.01090010024012. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA. Diagnostic criteria of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:347–69. [PubMed] [Google Scholar]
- 16.Sinclair D. Analytical aspects of thyroid antibodies estimation. Autoimmunity. 2008;41:46–54. doi: 10.1080/08916930701619466. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem. 2006;43:173–83. doi: 10.1258/000456306776865043. [DOI] [PubMed] [Google Scholar]
- 18.McConnell RJ, Brenner AV, Oliynyk VA, et al. Factors associated with elevated serum concentrations of anti-TPO antibodies in subjects with and without diffuse goitre. Results from the Ukrainian-American Cohort Study of thyroid cancer and other thyroid diseases following the Chornobyl accident. Clin Endocrinol (Oxf) 2007;67:879–90. doi: 10.1111/j.1365-2265.2007.02979.x. [DOI] [PubMed] [Google Scholar]
- 19.Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–87. [PubMed] [Google Scholar]
- 20.Tsakiris DA, Osusky R, Kaiser HJ, et al. Lupus anticoagulants/anticardiolipin antibodies in patients with normal tension glaucoma. Blood Coagul Fibrinolysis. 1992;3:541–45. doi: 10.1097/00001721-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bizzaro N, Ghirardello A, Zampieri S, et al. Anti-prothrombin antibodies predict thrombosis in patients with systemic lupus erythematosus: a 15-year longitudinal study. J Thromb Haemost. 2007;5:1158–64. doi: 10.1111/j.1538-7836.2007.02532.x. [DOI] [PubMed] [Google Scholar]
- 22.Puszczewicz M. Reumatologia. Wydawnictwo Medical Tribune; Polska, Warszawa: 2010. pp. 75–82. [in Polish] [Google Scholar]