Abstract
Rationale:
We hypothesized that untreated severe obstructive sleep apnea (OSA) is associated with elevated ambulatory blood pressure (BP) in subjects with high cardiovascular disease (CVD) risk despite medical management.
Methods:
Data from the baseline examination of the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT) study, a 4-site randomized controlled trial were analyzed. Individuals with moderate-severe OSA (apnea hypopnea index, AHI = 15-50) and cardiovascular risk were recruited from cardiology practices. Those with hypertension were included. Intensive antihypertensive regimen (IAR) was defined as ≥ 3 antihypertensives including a diuretic. Definitions were: controlled BP (BP < 130/80), uncontrolled elevated BP (BP ≥ 130/80 not on IAR) and resistant elevated BP (BP ≥ 130/80 mm Hg despite IAR). Associations of untreated severe OSA (AHI ≥ 30) and uncontrolled and resistant elevated BP were evaluated using logistic regression analyses adjusted for age, sex, race, body mass index, smoking status, diabetes, and CVD.
Results:
Among the 284 participants (mean age 63.1 ± 7.2 years, 23.6% with severe OSA), 61.6% had controlled BP, 28.5% had uncontrolled elevated BP, and 9.9% had resistant elevated BP. Among participants prescribed IAR, resistant elevated BP was more prevalent in those with severe compared to moderate OSA (58.3% vs. 28.6%, p = 0.01). Participants with severe OSA had a 4-fold higher adjusted odds of resistant elevated BP (OR 4.1, 95% CI: 1.7-10.2), a finding not reproduced in the absence of IAR use.
Conclusions:
Among patients with increased cardiovascular risk and moderate to severe OSA, untreated severe compared to moderate OSA was associated with elevated BP despite IAR suggesting untreated severe OSA contributes to poor BP control despite aggressive medication use.
Commentary:
A commentary on this article appears in this issue on page 845.
Citation:
Walia HK, Li H, Rueschman M, Bhatt DL, Patel SR, Quan SF, Gottlieb DJ, Punjabi NM, Redline S, Mehra R. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med 2014;10(8):835-843.
Keywords: sleep apnea, blood pressure, cardiovascular disease
Obstructive sleep apnea (OSA) is characterized by reduction in upper airway muscle tone resulting in repetitive complete (apnea) or partial (hypopnea) upper airway closure during sleep. OSA results in increased sympathetic nervous system activation, changes in baroreceptor function, increased oxidative stress, and endothelial dysfunction, which can increase blood pressure (BP).1–4 Respiratory events during sleep result in acute and transient BP perturbations,5,6 which persist during the daytime. These pathophysiological changes explain the association between OSA and hypertension (HTN) in several large-scale epidemiological studies.7
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are limited data regarding the severity of obstructive sleep apnea (OSA) and elevated blood pressure (BP) in patients with cardiovascular disease. Given the limited existing data, we examined the association between OSA severity and BP resistant to an intensive antihypertensive regimen in a group of individuals with high cardiovascular risk or established cardiovascular disease recruited from cardiology specialty clinics.
Study Impact: In this study of individuals at high cardiovascular risk or established cardiovascular disease, we observed a strong association of severe untreated OSA and resistant elevated BP despite treatment with an aggressive antihypertensive medication regimen even after consideration of well-recognized hypertension risk factors. Strategies to treat OSA in this subgroup should be strongly considered, as improved control in BP could lead to decreased cardiovascular morbidity and mortality.
Elevations in BP including high normal BP often progress to HTN.8 There are several large-scale epidemiological studies citing the importance of high normal BP and cardiovascular risk. In the Framingham Heart Study, high-normal BP (defined as systolic blood pressure of 130-139 or diastolic blood pressure of 85-89 mm Hg) was associated with an approximate 2-fold increased risk factor-adjusted cardiovascular disease (CVD) risk.9 These findings are consistent in cross-ethnic groups. For example, another large prospective study of approximately 9,000 middle-aged adults showed that the relative risk of CVD was increased by approximately two-fold for high normal BP compared to normal BP among African American patients.10 Furthermore, high normal BP has been shown to be associated with an increased risk of CVD mortality in Japanese men.11 Noteworthy to these studies is that these BP levels conferred increased CV risk even in the absence of demonstrating resistance to aggressive antihypertensive medication therapy.
There are several studies which have focused on the relationship of OSA and resistant HTN, which is intrinsically defined in part by lack of BP control despite an aggressive antihypertensive regimen, due to the exposures to surges in sympathetic neural tone, vasoconstriction, and increased aldosterone levels.12 However, there are very few studies which have examined elevation in BP including the high normal range BP, a recognized CVD risk, in the setting of OSA. In the limited data available, relationships of elevated BP despite the use of 3 or more antihypertensive drugs and OSA have been described, albeit in a relatively small sample and did not focus on a group at high cardiovascular risk.13
In particular, it is unclear whether OSA severity level confers increased risk for elevated BP, including the “high normal” range, in patients at risk for CVD or with known CVD. Given the limited existing data and its inherent limitations, we examined the association between OSA severity and BP resistant to an intensive antihypertensive regimen (IAR) in a group of individuals with high cardiovascular risk or established CVD recruited from cardiology specialty clinics. Given the association of BP ≥ 130/80 mm Hg with increased cardiovascular risk,9 a threshold BP cut off of 130/80 mm Hg was used in our analytic sample. We further stratified the groups into categories based on values of BP and medication usage. We hypothesized that severe OSA is associated with elevated ambulatory BP in patients with high cardiovascular risk or established CVD despite IAR usage even after consideration of potential confounding factors including age, sex, race, and obesity. In secondary analysis, we investigated whether severe OSA is associated with elevated ambulatory BP in patients using versus not using an intensive antihypertensive regimen (IAR), i.e., three or more antihypertensive medications including a diuretic.
METHODS
Study Sample
The current study includes individuals participating in the baseline examination conducted for the Heart Biomarker Evaluation in Apnea (HeartBEAT), a randomized controlled trial aimed at comparing conservative medical therapy, supplemental nocturnal oxygen therapy, and positive airway pressure therapy on cardiovascular biomarkers in OSA (clinicaltrials.gov Trial Registration Number: NCT01086800). Participants were recruited from outpatient cardiology clinics at 4 sites— Brigham and Women's Hospital, Case Medical Center, Johns Hopkins Medical Center, and Veterans Affairs Boston Healthcare System. All of the sites follow standard American Heart Association/American College of Cardiology guideline-based approaches for primary and secondary CVD risk reduction.14,15 All recruited patients had cardiovascular risk factors or established CVD and moderate-to-severe OSA.
Study Protocol
Subjects were recruited using questionnaire screening and medical chart review. Inclusion criteria were age 45-75 years and high risk of cardiovascular disorders, defined as (1) established stable coronary artery disease (documented prior myocardial infarction or coronary revascularization > 3 months prior to entry or angiographically documented ≥ 50% stenosis in a major coronary artery); or (2) ≥ 3 cardiovascular risk factors characterized by: (a) physician treated hypertension (HTN; systolic blood pressure > 140 mm Hg or diastolic blood pressure > 90 mm Hg or antihypertensive medication use including ACE inhibitor, angiotensin receptor blocker, β-adrenergic blocker, α-adrenergic blocker, diuretic, and calcium channel blocker usage); (b) diabetes mellitus treated by a physician; (c) body mass index (BMI) ≥ 30 kg/m2; or (d) physician treated dyslipidemia.
Exclusion criteria were: heart failure with an ejection fraction < 30% or NYHA classification > 2, poorly controlled HTN (≥ 170/100 mm Hg), poorly controlled diabetes (HbA1c > 9.0%), prior stroke with functional impairment, severe uncontrolled medical problems, or medications that might influence measurements or impair ability to participate in study. For this analysis, patients without HTN as defined above were excluded. Subjects who met the inclusion criteria underwent overnight type III sleep testing16 (Embletta-Gold, Embla, Broomfield, CO USA); those with an apnea hypopnea index of 15-50 events/h were considered eligible and were included in the study. Those with nocturnal oxygen saturation < 85% for > 10% of the screening sleep monitoring record or central apnea index > 5 events/h were excluded from the study. Institutional review board approval was obtained from all sites, and full written informed consent was obtained.
Data Collection
Sleep Apnea Assessment
Sleep apnea severity was derived from the results of an in-home sleep study that was scored by a single registered polysomnologist following the 2007 American Academy of Sleep Medicine guidelines17 and was modified for scoring of portable sleep monitoring. Severe OSA was defined by AHI ≥ 30 events/h and moderate OSA by AHI 15-29 events/h. An Embletta portable unit with nasal cannula, thermistor, finger oximeter, abdomen, thoracic belt, and 3-lead EKG with positional sensor was used. An apnea was defined as a complete cessation of airflow, measured using nasal pressure for ≥ 10 sec. An apnea was scored as obstructive if there was continued or increased inspiratory effort, and was scored as central if there was absence of inspiratory effort. Hypopnea was defined as 50% reduction in breathing amplitude lasting ≥ 10 seconds associated with ≥ 3% oxygen desaturation. The apnea hypopnea index (AHI; the number of apneas and hypopneas per hour of estimated sleep time) was measured from the sleep study.
24-Hour Ambulatory Blood Pressure Monitoring
Participants were instructed in the use of the Spacelabs 90217 Ambulatory Blood Pressure Monitor (Issaquah, Washington). The device was programmed to measure systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) every 20 minutes from 06:00 to 22:00 and every 30 min between 22:00 and 06:00 for a 24-h period. Participants were instructed to engage in usual activities and continue usual medication regimens including antihypertensive therapy. Participants completed a diary indicating bed and wake times; these time periods were used to identify periods of wake and sleep for BP analysis. The mean number of days between the screening sleep study and baseline ambulatory BP collection was 23 ± 12 days. The mean number of baseline sleep ambulatory blood pressure readings was 15 ± 4, and mean number of awake readings was 35 ± 9.
Resting Blood Pressure Monitoring
Resting BP was measured after the participant had been sitting quietly ≥ 5 min, following a standard protocol using a calibrated appropriate bladder size that was identified from a standard chart. Measurements were repeated 3 times and their average was taken in consideration.
Statistical Plan
In the primary analysis, we examined the relationship of severe versus moderate OSA (predictor) with 3 blood pressure groups including controlled BP, uncontrolled elevated blood pressure, and resistant elevated BP (outcomes). We characterized the BP outcomes groups based on (1) threshold of BP and (2) the medication usage. As data demonstrate increased cardiovascular risk associated with systolic BP > 130, a BP cutoff of ≥ 130/80 mm Hg was selected.9 The definition of an IAR was adopted from the JNC7 guidelines, which is defined as ≥ 3 antihypertensive medications including a diuretic.18 The antihypertensive medication classes included ACE inhibitors or angiotensin receptor blockers, β-adrenergic blockers, α-adrenergic blockers, calcium channel blockers, nitrates, aldosterone antagonists, and diuretics. The BP outcome groups were based upon the threshold of the 24-h ambulatory blood pressure and defined as:
Elevated BP: SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg (includes number 2 and 3)
Uncontrolled elevated BP: SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg without use of an IAR (≥ 3 antihypertensives with 1 being a diuretic)
Resistant elevated BP: SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg with the use of an IAR, i.e. presence of ≥ 3 antihypertensive including a diuretic
Controlled BP: SBP < 130 mm Hg or DBP < 80 mm Hg irrespective of antihypertensive medication usage.
All 284 patients were included in the analysis. Univariate and multivariable logistic regression analyses were used to determine the odds of uncontrolled BP and resistant elevated BP for severe versus moderate OSA. We used a hierarchical approach to the logistic regression modeling considering an unadjusted model (Model 1), Model 2 adjusted for age, sex, race, and body mass index (BMI, kg/m2), and Model 3 which included Model 2 covariates and also smoking status (which was defined as ever smoked vs. never smoked), diabetes and CVD. CVD was defined as history of prior myocardial infarction, coronary artery revascularization or angiographically documented stenosis > 50% or a history of stroke. Associations for groups classified by BP and OSA status and were compared using the χ2 test.
Secondary analysis investigated whether severe OSA is associated with resistant elevated BP (SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg) in the group of patients using the IAR. Adjusted odd ratios were estimated based on maximal likelihood method. In addition, we determined the relationship of severe OSA and systolic BP or diastolic BP by comparing mean systolic BP or diastolic BP differences using analysis of variance and compared the proportion of high normal systolic BP (≥ 130 mm Hg) or high diastolic BP (≥ 80 mm Hg) using the χ2 test. Secondary analyses were also performed considering resting BP measures in the definitions of the respective BP outcomes.
Analyses were performed using SAS version 9.2 (SAS Inc., NC). Two-sided p values are reported, and p < 0.05 is considered statistically significant.
RESULTS
Subject Characteristics
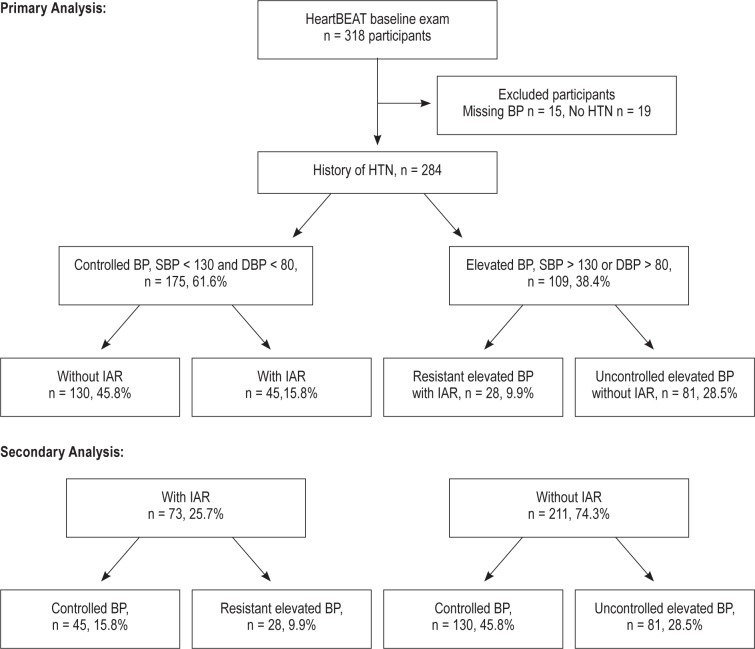
Of a total 318 participants in the baseline study, 284 were included in the final analytic sample based on self-reported HTN history (n = 281) or baseline blood pressure ≥ 140/90 mm Hg (n = 3); 19 participants without HTN and 15 without valid ambulatory BP were excluded. Of the 109 (38.4%) subjects with elevated BP on ambulatory blood pressure monitoring (BP ≥ 130/80 mm Hg), 28 (9.9%) were classified as having resistant elevated BP and the remaining 81 (28.5%) as uncontrolled elevated BP. A total of 73 (25.7%) subjects were identified to be on an IAR, of which 45 (15.8%) had controlled BP and 28 (9.8%) had resistant elevated BP. A total of 211 (74.3%) subjects were not on IAR; of these participants, 130 (45.8%) had controlled BP and 81 (28.5%) had uncontrolled elevated BP. Among 175 (61.6%) participants with controlled BP, 130 (45.8%) patients were not on IAR, whereas 45 (15.8%) participants were on IAR (Figure 1).
Figure 1.
IAR, intensive antihypertensive regimen.
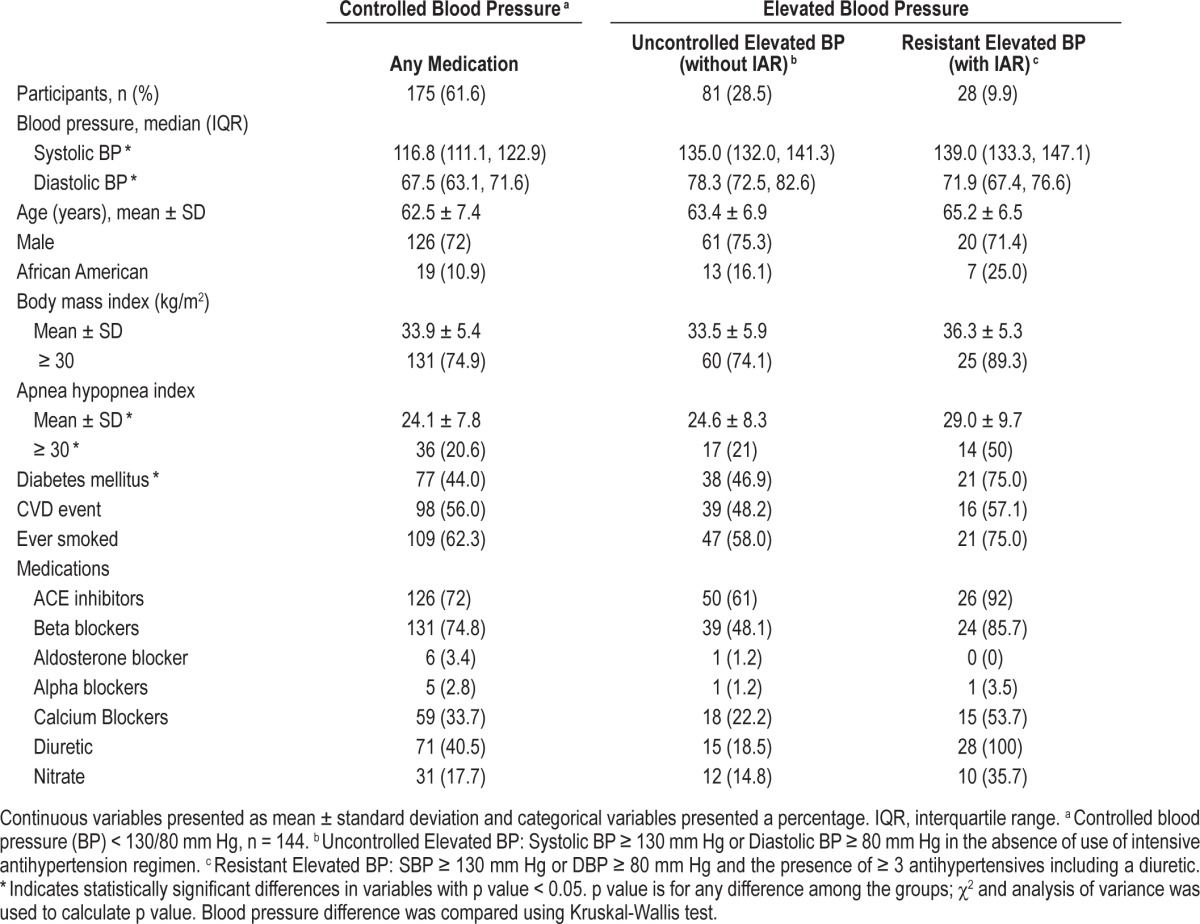
There were 175 (61.6 %), 81 (28.5%), and 28 (9.9%) participants in the controlled BP, uncontrolled elevated BP, and resistant elevated BP categories, respectively. Patient characteristics among the 3 BP groups are listed in Table 1. The mean AHI was higher in the resistant elevated BP group (29.0 in resistant elevated BP vs. 24.6 in the uncontrolled elevated BP group, and 24.1 in the controlled BP group), and the proportion of severe OSA was more common in subjects with resistant elevated BP (50% in resistant elevated BP, 21% in uncontrolled elevated BP, and 20.6% in the controlled BP group). Participants in the resistant elevated BP group had a higher BMI and were more likely to be diabetic than other groups.
Table 1.
Baseline subject characteristics (n = 284).
Association of Severe Obstructive Sleep Apnea with Resistant Elevated and Uncontrolled Elevated Blood Pressure
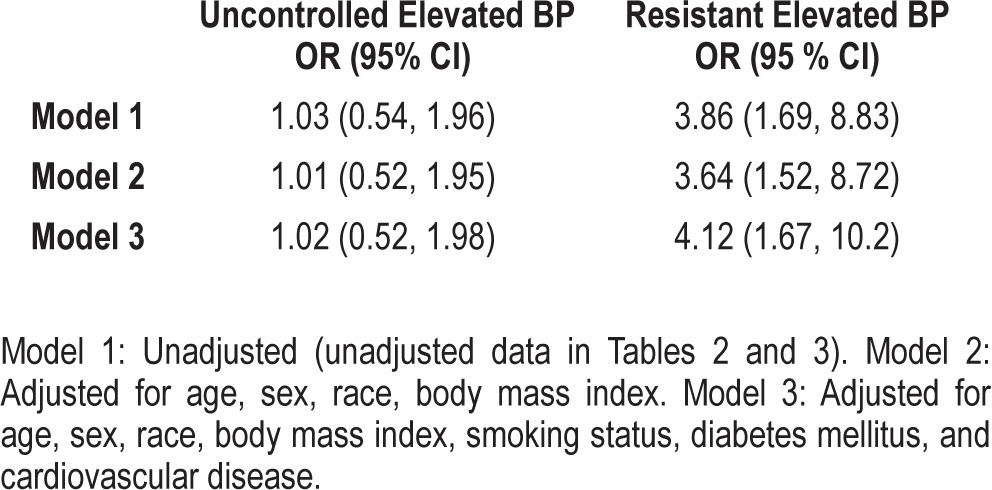
In the unadjusted model (Table 2 and Model 1), there was a greater than 3-fold increased odds of resistant elevated BP (OR = 3.86, 95% CI 1.69, 8.83) with the presence of severe OSA, which was not substantively attenuated after adjusting for BMI (OR = 3.64, 95% CI, 1.52-8.72). This relationship persisted after statistical adjustment for smoking, diabetes mellitus, and CVD: OR = 4.12, 95% CI, 1.67-10.2. Unlike resistant elevated BP, severe OSA was not associated with increased odds of uncontrolled elevated BP (Table 3).
Table 2.
Factors associated with uncontrolled and resistant elevated blood pressure (n = 284).
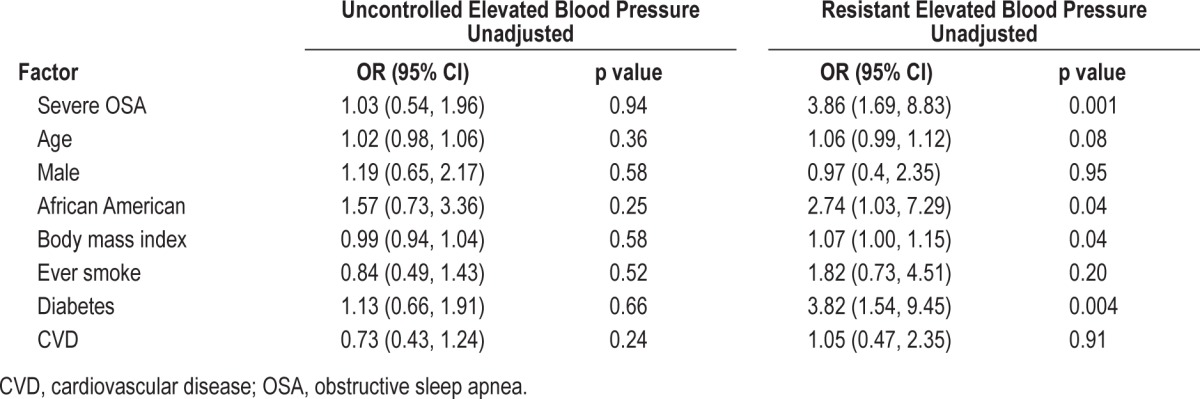
Table 3.
Logistic regression analyses of severe obstructive sleep apnea with uncontrolled and resistant elevated blood pressure.
Proportions of Suboptimally Controlled Blood Pressure in Moderate and Severe Obstructive Sleep Apnea by Intensive Antihypertensive Medication Group
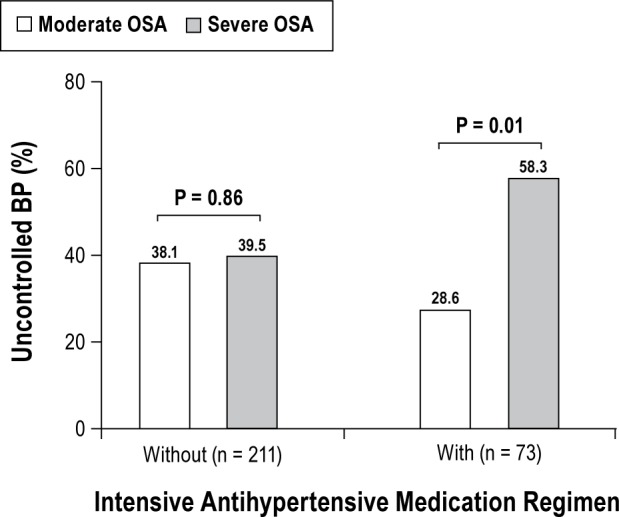
In the group on IAR, the percentage of resistant elevated BP almost doubled in the severe OSA group (58.3%) compared to the moderate OSA group (28.6%), p = 0.01. Conversely, there was no difference in percentage of suboptimally controlled BP between moderate OSA (38.1%) and severe OSA (39.5%) in subjects who were not on IAR (Figure 2).
Figure 2. Proportions of elevated blood pressure between moderate and severe obstructive sleep apnea by intensive antihypertensive medication use (n = 284).
Secondary Analysis
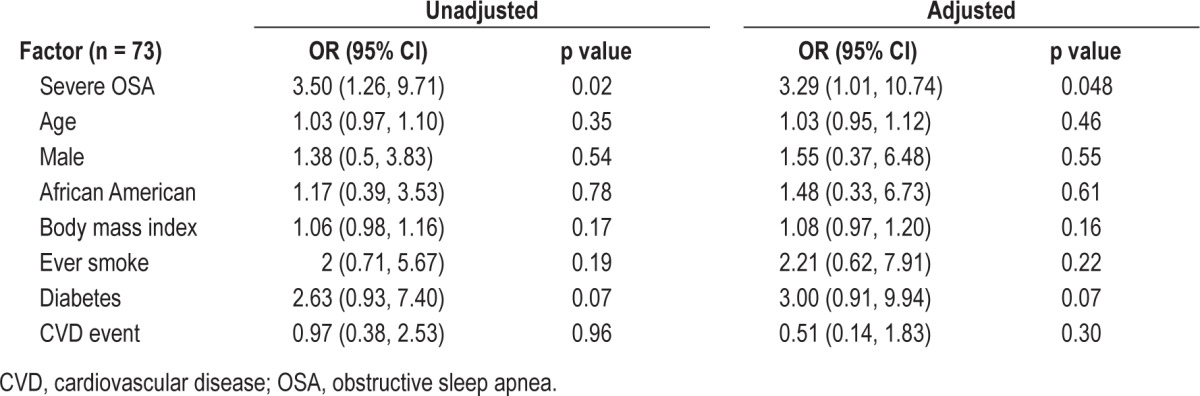
Our secondary analysis was then focused on the 73 participants who were on the IAR. Among those who were on this regimen, the odds of having resistant elevated BP with severe OSA was more than 3-fold higher in the unadjusted model and reduced slightly in the fully adjusted model (OR 3.29 95 % CI 1.01, 10.74, p = 0.048; Table 4).
Table 4.
Severe obstructive sleep apnea associated with resistant elevated BP among participants using an intensive antihypertensive regimen.
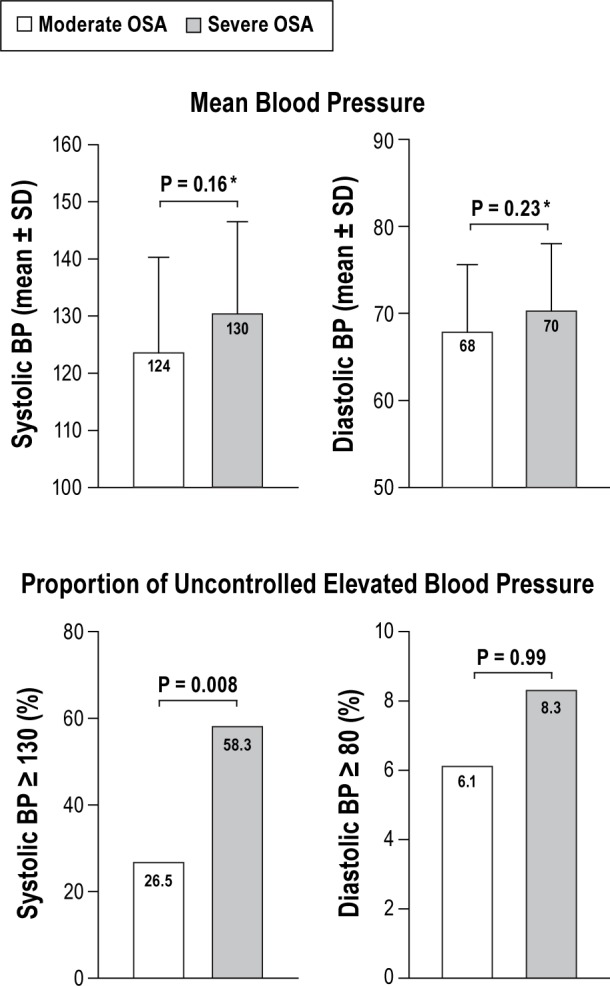
The mean difference in SBP and DBP values and percentage difference in those with high SBP (≥ 130 mm Hg) or high DBP (≥ 80 mm Hg) between moderate and severe OSA were examined. Mean systolic BP in severe OSA was on average 6 mm Hg higher than in moderate OSA; however, this did not achieve statistical significance. The proportion of participants with high systolic BP (≥ 130 mm Hg) was significantly higher in severe OSA (58.3%) than moderate OSA (26.5%), p = 0.008. There was no significant difference between moderate and severe OSA and mean DBP and proportion of high DBP (Figure 3).
Figure 3. Comparison of blood pressure between moderate and severe obstructive sleep apnea among participants using an intensive antihypertensive regimen (n = 73).
*Adjusted for age, gender, race and BMI.
Association of Severe OSA Using Different BP Groups Using Resting BP
Ambulatory BP and resting blood pressure values were moderately correlated (Pearson correlation coefficient value of 0.55, p < 0.0001). Similar analyses were performed considering resting BP values which demonstrated consistent findings. Similar BP cutoff values were ascertained as ambulatory blood pressure > 130/80 mm Hg. There was an approximately 3-fold higher odds of resistant elevated BP (OR 2.75, 95 % CI 1.23-6.14, p = 0.01) in the severe OSA group in the model adjusted for age, sex, race, BMI, smoking, diabetes mellitus, and CVD. Although this is significant, the strength of association was slightly lower than the 24-h ambulatory blood pressure findings; uncontrolled elevated BP was not associated with severe OSA.
Among patients on IAR, the odds of having resistant elevated BP for severe OSA was more than 3-fold higher in the unadjusted model and increased in the fully adjusted model (OR = 5.47, 95 % CI 1.34-22.4, p = 0.02), the latter consistent with a higher magnitude of association compared to the 24-h ambulatory blood pressure findings.
DISCUSSION
In this examination of individuals at high cardiovascular risk or established CVD, we observed a strong association of severe untreated OSA and resistant elevated BP (almost 4-fold higher odds) despite treatment with an aggressive antihypertensive medication regimen even after consideration of well-recognized hypertension risk factors. Moreover, of those participants on an IAR, the percentage of those with severe OSA was almost double that of those with a moderate degree of OSA. Finally, in secondary analyses conducted to investigate whether those with difficult to control blood pressure were more likely to have severe OSA, we discovered a 3-fold increase in odds of resistant elevated blood pressure associated with severe OSA among those on an intensive antihypertensive medication regimen. Also noted was a higher proportion of individuals with increased systolic blood pressure, but not diastolic blood pressure in severe compared to moderate levels of OSA. Interestingly, although the correlation of resting and 24-h ambulatory blood pressure was moderate, we observed consistent results when resting as opposed to ambulatory BP was considered in analyses.
We chose to examine the relationships of elevated BP using the 130/80 threshold, given that this level has been shown to be associated with adverse cardiovascular consequences.9 Specifically, a recent large meta-analysis involving about 470,000 participants from 18 prospective cohort studies with SBP of > 120-139 or DBP > 80-89 mm Hg showed patients with these BP levels were at elevated risk of CVD after adjustment of cardiovascular risk factors.19 The risk was consistent even in the low range of hypertension (SBP of 120-129/80-84 mm Hg). Even mildly elevated BP levels have been also associated with increased risk of carotid atherosclerosis and increased atheroma.20,21
There are limited data exploring the relationship of OSA and resistant elevated BP levels. Martinez-Garcia et al. reported that the prevalence of sleep apnea syndrome was very high in patients with difficult to control HTN (SBP > 125 mm Hg or mean DBP > 80 mm Hg despite the use of ≥ 3 antihypertensive medications). Similar to our work, the group found higher uncontrolled ambulatory SBP in severe OSA and noted that patients with severe OSA reported greater use of medications than those without severe OSA.13 However, there were some differences from the current study design and findings. For example, we chose to take into consideration confounding by cardiovascular comorbidity and smoking history, and perhaps had increased study power to examine the subgroup-specific relationships in the IAR group. Unlike our current study, increased DBP was associated with severe OSA in the prior study, which is potentially attributable to the differences in the baseline characteristics of the two study samples. For example, our study focused on patients with increased cardiovascular risk factors, which include systolic hypertension,22 or established coronary heart disease, and were of an older age range than the prior study.
A large randomized controlled trial designed to investigate the effect of sleep apnea treatment on resistant hypertension (BP > 130/80 mm Hg from 24-h ambulatory blood pressure monitoring) in a group of participants who were of comparable age, sex, and obesity in reference to the current study demonstrated improvements in the mean arterial blood pressure, but not systolic blood pressure, with continuous positive airway pressure treatment compared to no sleep apnea treatment.23 In contrast to this trial, our study sample involved participants with a higher prevalence of cardiovascular disease (> 50% versus 21% in the aforementioned trial) and also had less severe apnea. It is possible that the increased cardiovascular risk of our study participants resulted in observation of a relationship of severe OSA and SBP, a finding not observed in terms of OSA treatment effect in the clinical trial.
Elevated SBP has implications as well. There has been a continuous positive association of SBP and heart failure risk shown in elderly patients with a SBP as low as < 115 mm Hg.24 Control of SBP has translated into reduction in mortality, stroke, and heart failure events.25,26 It has also been shown that in patients with CVD or target organ damage, 9 patients would require SBP reduction by 12 mm Hg over 10 years to prevent one death.27
There are several potential mechanisms that may explain the association between resistant elevated BP and severe OSA. Severe OSA may cause endothelial dysfunction, which is primarily driven by OSA-associated intermittent hypoxia.28 Observational data indicate that aldosterone excess could play a role in the relation between the severity of OSA and in resistant HTN.29–31 Increased sympathetic activity in patients with OSA linked to increased chemoreflex drive is also thought to be one of the contributory causes for HTN.32 In particular, untreated OSA might reduce the effectiveness of antihypertensive medications through pharmacokinetic or chronotherapeutic effects, which may be activating a hypertensive pathway that is more resistant to drugs. Alternatively, fatigue observed in OSA patients could result in suboptimal medication adherence. Of note, while use of CPAP therapy has been shown to be effective in reducing blood pressure in untreated non-resistant HTN and OSA, certain antihypertensive medication classes have been observed to reduce blood pressure to an even more pronounced degree.33 Although these data suggest that suboptimal medication adherence may play a role in the interpretation of our findings, the extent of blood pressure response to antihypertensive medication in OSA may be physiologically blunted in the resistant HTN phenotype.
It is now accepted by many that ambulatory blood pressure monitoring, as utilized in the current study, has greater prognostic value than office BP measurements.34–36 In recently published guidelines for the management of HTN, more strict BP control during a 24-h period is identified for management of high-risk hypertensive patients. Given the high prevalence of resistant elevated BP in OSA, the use of ambulatory blood pressure monitoring may be useful to this patient population as well. Nevertheless, it is worth noting that results from the analyses deriving BP indices from baseline resting values versus 24-h ambulatory measures yielded consistent findings.
A key point to note is that this study considered a lower blood pressure cutoff and also excluded those patients with poorly controlled BP (≥ 170/110 mm Hg); therefore, our results could underestimate the true association of severe OSA and resistant blood pressure. Furthermore, patients enrolled in the trial were under the care of cardiology subspecialists adhering to national treatment guidelines. It is noteworthy that despite the fact that many of these patients were on intensive antihypertensive medication treatment, there appeared to be a significant contribution of untreated severe OSA to resistance of BP control using the BP cutoff of 130/80. These data suggest that severe OSA contributes to poor BP control despite aggressive medication usage.
Strengths of the study include the collection of data from multiple clinical settings, thus enhancing generalizability. The use of 24-h ambulatory blood pressure monitoring provided information which has a prognostic value greater than spot office BP measurements and is a better predictor of cardiovascular risk. Standardized methods were also used for the collection of sleep and BP measures, which were scored by certified technicians using centralized reading and subject to quality control procedures. We considered various confounding factors, such as age, gender, race, BMI, cardiovascular risk factors, and cardiovascular disease status.
The study also had a few limitations: in particular, subjects in the study included those with moderate to severe OSA without the ability to compare to those without sleep apnea or with lesser degrees of sleep apnea. We could not assess whether individuals with mild to moderate sleep apnea were at increased risk for elevated BP than individuals without OSA. Dosages of the medications were not available, and the actual compliance with the medications could not be determined. However, there is no evidence to support that patients with severe OSA were less compliant with medications.
Future investigations should focus on better understanding the mechanisms such as baroreflex, autonomic, and aldosterone levels on BP control in OSA, the effect of BP variability, and the responses to specific pharmacological and other interventions. Strategies to treat OSA in this subgroup should be strongly considered, as improved control in BP could lead to decreased cardiovascular morbidity and mortality. Rigorous trials are needed to assess the effect of OSA treatment on control of BP in individuals on IAR treatment.
DISCLOSURE STATEMENT
This study was supported by NIH National Heart Lung Blood Institute RC2 HL101417, NIH M01 RR00080, NIH UL1 RR024989 from the National Center for Research Resources (NCRR). Dr. Mehra was supported by NIH NHLBI 1R01HL109493 and R21HL108226. Dr. Bhatt is on the Advisory Board of Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; is on the Board of Directors of Boston VA Research Institute, and Society of Cardiovascular Patient Care; is chair of American Heart Association Get With The Guidelines Steering Committee; is on the Data Monitoring Committees of Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, and Population Health Research Institute; has received honoraria from the American College of Cardiology (Editor, Clinical Trials, Cardiosource), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), and WebMD (CME steering committees); has received research grants from Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Roche, SanofiAventis, and The Medicines Company; has participated in unfunded research for FlowCo, PLx Pharma, and Takeda. Additional disclosures for Dr. Bhatt are Clinical Cardiology (Associate Editor) and Journal of the American College of Cardiology (Section Editor, Pharmacology). Dr. Mehra serves on the Medical Advisory Board for Care Core National, has received funding from the National Institutes of Health for research and her institution has received positive airway devices from Philips Respironics for research for which she is the Principal Investigator. Dr. Quan is Editor-in-Chief of the Journal of Clinical Sleep Medicine and has served as a consultant for Saatchi and Saatchi. Dr. Patel has served as a consultant to Apnex Medical, Apnicure, and Vertex Pharmaceuticals. His institution, Brigham and Women's Hospital has received grant support and/or equipment for research studies from ResMed Inc, ResMed Foundation, and Philips Respironics. Dr.Gottlieb is a consultant for ResMed Corporation and PI or co-investigator on multiple VA-funded sleep apnea research studies. Dr. Redline is PI for NIH funded research of sleep apnea and cardiac disease, PI of a grant from ResMed, and has received equipment from Philips Respironics and ResMed for research. Dr. Punjabi has received research support from ResMed. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138:434–43. doi: 10.1378/chest.09-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey JA. Sleep apnea causes daytime hypertension. J Clin Invest. 1997;99:1–2. doi: 10.1172/JCI119119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher EC. Hypertension in patients with sleep apnoea, a combined effect? Thorax. 2000;55:726–8. doi: 10.1136/thorax.55.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7:677–85. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 5.Morgan BJ, Dempsey JA, Pegelow DF, et al. Blood pressure perturbations caused by subclinical sleep-disordered breathing. Sleep. 1998;21:737–46. doi: 10.1093/sleep/21.7.737. [DOI] [PubMed] [Google Scholar]
- 6.Okabe S, Hida W, Kikuchi Y, et al. Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax. 1995;50:28–34. doi: 10.1136/thx.50.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–6. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 10.Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119:133–41. doi: 10.1016/j.amjmed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Sairenchi T, Iso H, Irie F, et al. Age-specific relationship between blood pressure and the risk of total and cardiovascular mortality in Japanese men and women. Hypertens Res. 2005;28:901–9. doi: 10.1291/hypres.28.901. [DOI] [PubMed] [Google Scholar]
- 12.Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26:281–7. doi: 10.1038/jhh.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Garcia MA, Gomez-Aldaravi R, Gil-Martinez T, Soler-Cataluna JJ, Bernacer-Alpera B, Roman-Sanchez P. [Sleep-disordered breathing in patients with difficult-to-control hypertension] Arch Bronconeumol. 2006;42:14–20. doi: 10.1016/s1579-2129(06)60108-0. [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–86. doi: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R. Guidelines for the management of hypertension and target organ damage: reply. J Hypertens. 2013;31:2464–5. doi: 10.1097/HJH.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 16.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Wang S, Cai X, et al. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC Med. 2013;11:177. doi: 10.1186/1741-7015-11-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonati L, Cuspidi C, Sampieri L, et al. Ultrasonographic evaluation of cardiac and vascular changes in young borderline hypertensives. Cardiology. 1993;83:298–303. doi: 10.1159/000175985. [DOI] [PubMed] [Google Scholar]
- 21.Sipahi I, Tuzcu EM, Schoenhagen P, et al. Effects of normal, pre-hypertensive, and hypertensive blood pressure levels on progression of coronary atherosclerosis. J Am Coll Cardiol. 2006;48:833–8. doi: 10.1016/j.jacc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 22.Izzo JL, Jr., Levy D, Black HR. Clinical Advisory Statement. Importance of systolic blood pressure in older Americans. Hypertension. 2000;35:1021–4. doi: 10.1161/01.hyp.35.5.1021. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Kalogeropoulos AP, Georgiopoulou VV, et al. Systolic blood pressure and incident heart failure in the elderly. The Cardiovascular Health Study and the Health, Ageing and Body Composition Study. Heart. 2011;97:1304–11. doi: 10.1136/hrt.2011.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA. 1999;282:539–46. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 26.Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278:212–6. [PubMed] [Google Scholar]
- 27.Ogden LG, He J, Lydick E, Whelton PK. Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension. 2000;35:539–43. doi: 10.1161/01.hyp.35.2.539. [DOI] [PubMed] [Google Scholar]
- 28.Lavie L. Obstructive sleep apnoea syndrome–an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 29.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–9. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 30.Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Pimenta E, Stowasser M, Gordon RD, et al. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest. 2013;143:978–83. doi: 10.1378/chest.12-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182:954–60. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 34.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 35.Verdecchia P, Angeli F, Cavallini C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation. 2007;115:2091–3. doi: 10.1161/CIRCULATIONAHA.107.697086. [DOI] [PubMed] [Google Scholar]
- 36.McManus RJ, Caulfield M, Williams B. NICE hypertension guideline 2011: evidence based evolution. BMJ. 2012;344:e181. doi: 10.1136/bmj.e181. [DOI] [PubMed] [Google Scholar]