Abstract
Study Objectives:
The use of opioid medication for chronic pain has been increasing. The main aim of this study was to assess how many patients on opioids for chronic pain had sleep disordered breathing (SDB) and the type of SDB. The impact of these medications on daytime arterial blood gas (ABG) measurements and psychomotor vigilance was also studied.
Methods:
Twenty-four patients (aged 18-75 years) on long-term opioids were prospectively recruited. Patients underwent home polysomnogram (PSG), psychomotor vigilance testing (PVT), and awake daytime ABG. Overnight PSG findings were compared to those of patients matched for age, sex, and BMI referred to our sleep service for evaluation of SDB. PVT results in the patient cohort were compared to PVT in healthy controls.
Results:
Forty-six percent of opioid patients had severe SDB as defined by an apnea hypopnea index (AHI) > 30/h. The severity of SDB was similar in opioid-treated pain clinic patients and sleep clinic patients (mean ± SD AHI: Opioid-treated patients 32.7 ± 25.6; Sleep Study comparator group 28.9 ± 24.6, p = 0.6). Opioid patients had a higher frequency of central apneas and a lower arousal index (CAI: 3.9 ± 8.3 vs. 0.3 ± 0.5 events/h; p = 0.004, AI 8.0 ± 4.1 vs. 20.1 ± 13.8, p < 0.001). Pain clinic patients had impaired gas exchange during sleep and wakefulness. Nine of 20 (45%) had daytime hypercapnia, indicating a surprising number were in chronic respiratory failure. Morphine equivalent doses correlated with the severity of SDB. PVT was impaired when compared to a healthy PVT comparator group (RT: Opioid-treated patients 0.43 ± 0.27: Healthy PVT comparator group 0.28 ± 0.03 sec; p < 0.001).
Conclusions:
Patients on long-term opioids frequently have severe SDB, which in part is central in origin. PVT was markedly impaired. Half of the patients studied have evidence of chronic ventilatory failure.
Commentary:
A commentary on this article appears in this issue on page 853.
Citation:
Rose AR, Catcheside PG, McEvoy RD, Paul D, Kapur D, Peak E, Vakulin A, Antic NA. Sleep disordered breathing and chronic respiratory failure in patients with chronic pain on long term opioid therapy. J Clin Sleep Med 2014;10(8):847-852.
Keywords: opioids, sleep apnea, hypercapnia
Opioids are used widely in the treatment of malignant and non-malignant pain.1 There has been a significant increase in the use of these agents worldwide.2 In Australia over a period of 15 years (1992-2007), the number of opioid preparations available increased from 11 preparations of 4 medications to 70 preparations of 8 medications, and the total number of Pharmaceutical Benefit Scheme opioid prescriptions increased nationally by 290%.3
In recent years there has been an increasing focus on the control of pain and an emphasis on maintaining adequate anal-gesia with opioids. In the United States of America, chronic pain is estimated to affect around 68 million people each year, of whom 25% are elderly.1,2 There seems to be an increase in the prevalence of pain which parallels an increase in age, with over 50% of people over the age of 65 in the United Kingdom reporting pain or discomfort.4 Patients on long-term opioid therapy develop tolerance to the medication and as a consequence are often prescribed very high doses.3 Death from acute morphine overdose in humans is always due to a respiratory event. In excessive doses, opioids are known to suppress respiration via their effects on brainstem and peripheral chemoreceptors responsive to hypercapnia and hypoxia and to cause daytime somnolence and drowsiness.5–8 Despite the widespread use of opioid medication and its potential to suppress respiration, the effects on breathing during sleep have not been extensively studied. Improvement of SDB with bilevel ventilation has been observed in a small cohort of patients,9 and cessation of opiates has also been observed to abolish SDB in selected patients.10
BRIEF SUMMARY
Current Knowledge/Study Rationale: The use of opioid medication for chronic pain has been increasing. The main aim of this study was to assess how many patients on opiates had sleep disordered breathing and the type of SDB in patients on oral opioids for chronic pain.
Study Impact: Patients on long-term opioids frequently have severe SDB which in part is central in origin. PVT was markedly impaired. Half of the patients studied have evidence of chronic ventilatory failure.
SDB has been identified in patients on long-term methadone maintenance programs.6,7,11 Teichthal et al. described a 30% prevalence of predominantly central sleep apnea in this group.7 However, patients who receive opioids for chronic pain differ from patients who are on a methadone maintenance program. Chronic pain patients have a higher prevalence of obesity and have various other comorbidities (physical and psychiatric) and often are on multiple medications. Despite the known propensity for opioids to suppress respiration, contribute to hypoxemia during wakefulness12 and sleep,13 and possibly induce SDB, there have been no systematic studies of awake arterial blood gas composition in opioid-treated chronic pain patients. Finally, SDB and high dose opioids have the potential, separately or together, to adversely affect daytime vigilance and increase the risk of accidents. The paucity of data on sleep and breathing and daytime vigilance among patients on long-term opioids for chronic pain prompted us to undertake this observational study.
METHODS
This was a collaborative project between the Adelaide Institute for Sleep Health at the Repatriation General Hospital, and the Pain Management Unit (PMU) at Flinders Medical Centre (FMC). The FMC Pain Management Unit is responsible for the acute and the chronic pain service of this tertiary teaching institution. The study was approved by the the Repatriation General Hospital Ethics Committee (ID 50/07). All subjects gave written informed consent prior to participation.
Subject Selection
Chronic pain patients
The first author attended the outpatient facility of the PMU on a weekly basis and prospectively screened case notes of patients to identify those potentially eligible for the study.
Inclusion criteria: Patients aged between 18 and 75 years on prescribed oral long-acting opioids within prespecified dose ranges (morphine 40-500 mg/day, oxycodone 30-350 mg/day, and methadone 20-100 mg/day) were invited to participate in this study. The dose ranges were representative of those generally used in this pain clinic. Patients needed to be on these agents for ≥ 6 months and at stable doses for ≥ 4 weeks prior to enrolment and resident within an 80-km radius of the Flinders Medical Centre. Consecutive patients who agreed to participate were included in the study.
Exclusion criteria: Patients with clinically significant congestive cardiac failure, previous cerebrovascular accidents, severe chronic obstructive pulmonary disease (FEV1 < 50% predicted), history of substance abuse, and major psychiatric illness were excluded from the study.
Control Subjects
Two separate control groups were selected retrospectively from available data for relevant comparisons with the opioid-treated patient group.
Sleep Clinic comparator group was used to examine differences in type and/or severity of sleep disordered breathing between opioid-treated chronic pain patients and a group with similar characteristics and likely to have a similar range of medical comorbidities except for opioid use. We compared polysomnography results in our chronic pain patients with an age, gender, and BMI-matched group of patients who had been referred to our tertiary sleep medicine service for evaluation of SDB and had undergone a sleep study between 2006 and 2008. For each opioid treated patient, a sleep study control matched for gender, age (± 2 years), and BMI (± 2 kg/m2) was drawn with removal from all available matches. Of the 24 patients, 20 sleep study controls could be identified with this method. Of these sleep study control patients who were referred for evaluation for possible obstructive sleep apnea (OSA), two were on opiate medication.
The Healthy PVT Comparator group was a different control group of 20 healthy subjects recruited from the general community as control subjects for OSA patients for another study,14 in which Palm psychomotor vigilance test (PVT) results were also obtained. Clinically significant sleep disorders were excluded in this control group by sleep questionnaires, and polysomnography.14 Given the limited number of healthy controls it was not possible to specifically match gender, age, or BMI with the opioid-treated patient group. However, the PVT control group did not differ in mean age or gender distribution from the opioid group, but had a lower BMI (24.5 ± 2.6 vs 34.8 ± 9.4 kg/m2, p < 0.001).
Subject Assessment
Consecutive chronic pain patients who were eligible and willing to participate underwent clinical review with detailed clinical assessment including spirometry, sleep, and mood questionnaires, and PVT. An arterial blood gas sample was drawn between 14:00 and 16:00 with the patient breathing room air. At the end of the clinical review, patients were prepared in the laboratory for home-based polysomnography (PSG, Somte, and Compumedics Australia) before returning home to sleep. PSG recordings included the standard sleep montages of EEG, EMG, EOG, and respiratory signals of thoracic and abdominal effort and their sum (inductance coils), nasal airflow (pressure transducer), and oximetry. The device was activated to record from 21:00. The patients turned the recording device off in the morning and returned it to the laboratory. The polysomnograms15 were scored by a single Board of Registered Polysomnographic Technologists certified technician using the American Academy of Sleep Medicine 1999 criteria.16 Sleep stages and arousals were scored according to conventional criteria.12 Hypopneas were scored as per the so called “Chicago” criteria: (i) Those with > 50% decrease in a valid measure of airflow without a requirement for associated oxygen desaturation or arousal, and (ii) Those with a lesser airflow reduction in association with oxygen desaturation > 3% or an arousal.16
Psychomotor Vigilance Testing
The Walter Reed palm-held psychomotor vigilance test (Palm PVT) runs on a Palm operating system based personal data assistant (PDA). The Palm PVT shows close agreement with conventional desktop computer-based PVT normally considered the gold standard test for assessing psychomotor vigilance and sleepiness.17,18 The Palm PVT uses a liquid crystal display (LCD) screen to display a black and white circular annuli stimulus in the form of a bull's eye with 33 mm diameter. The subject was instructed, on seeing the visual stimulus, to respond by pressing a button from which reaction time and minor lapses (responses ≥ 500 msec. and < 3 sec) were measured. The test was programmed to run for a period of 10 minutes, with consecutive stimuli appearing at random intervals ranging between 2 and 10 seconds.
Questionnaire Data
All opioid-treated chronic pain patients completed the following questionnaires during the clinical review: the Epworth Sleep Score (ESS)19 and the Depression, Anxiety and Stress Scale (DASS 21)20 questionnaires were administered before the PSG was performed.
Morphine Equivalence (MEQ)
As the number of available opioid medications is increasing, it is useful when comparing patients on different agents to be able to compare equivalent doses. For this purpose morphine equivalence was computed. A multiplication factor of 1.5 was used to calculate the MEQ for oxycodone (i.e., 1 mg of oxycodone is equivalent to 1.5 mg of morphine). A factor of 3.5 was used for computing the MEQ for methadone.21,22
Statistical Analysis
Gender comparisons between groups were assessed via χ2 tests. Anthropometric and sleep study variables were assessed for normality using Kolmogorov-Smirnov tests and compared between opioid treated and each control group using 1-way ANOVA or Kruskal-Wallis 1-way ANOVA for normally and non-normally distributed data, respectively, followed by Bonferroni-adjusted pairwise tests to examine significant between group effects (IBM SPSS Statistics, Release 20). PVT variables were compared between opioid and healthy control groups using Mann-Whitney U-tests. Pearson correlation and backwards-stepwise linear regression analyses were used to explore relationships between MEQ and AHI and central apnea index, and MEQ and PaCO2 and PaO2, and PVT variables. Age, gender, and BMI were entered into regression models of MEQ vs AHI and CAI; and age, gender, FEV1 (% predicted), and FER (% predicted) into models of MEQ vs PaCO2 and PaO2. All data are reported as mean ± SD; p < 0.05 was considered statistically significant.
RESULTS
Of 56 potentially eligible opioid treated chronic pain patients, a total of 24 (50% male) agreed to participate in the study. Anthropomorphic and polysomnography results from the opioid treated versus healthy and sleep clinic control groups are presented in Table 1. Chronic pain patients were on average middle-aged and obese. Six (25%) were morbidly obese (BMI > 40 kg/m2). By design, the anthropomorphic characteristics of chronic pain patients and sleep clinic controls were not different, but both these groups had significantly higher BMI and ESS scores than the healthy age- and gender-matched control group. In opioid treated patients, the MEQ dose was 141 ± 97, median 120 [interquartile range 68-170] mg/24 h, indicating high opioid dose usage.
Table 1.
Anthropometric, polysomnography, and psychomotor vigilance test data
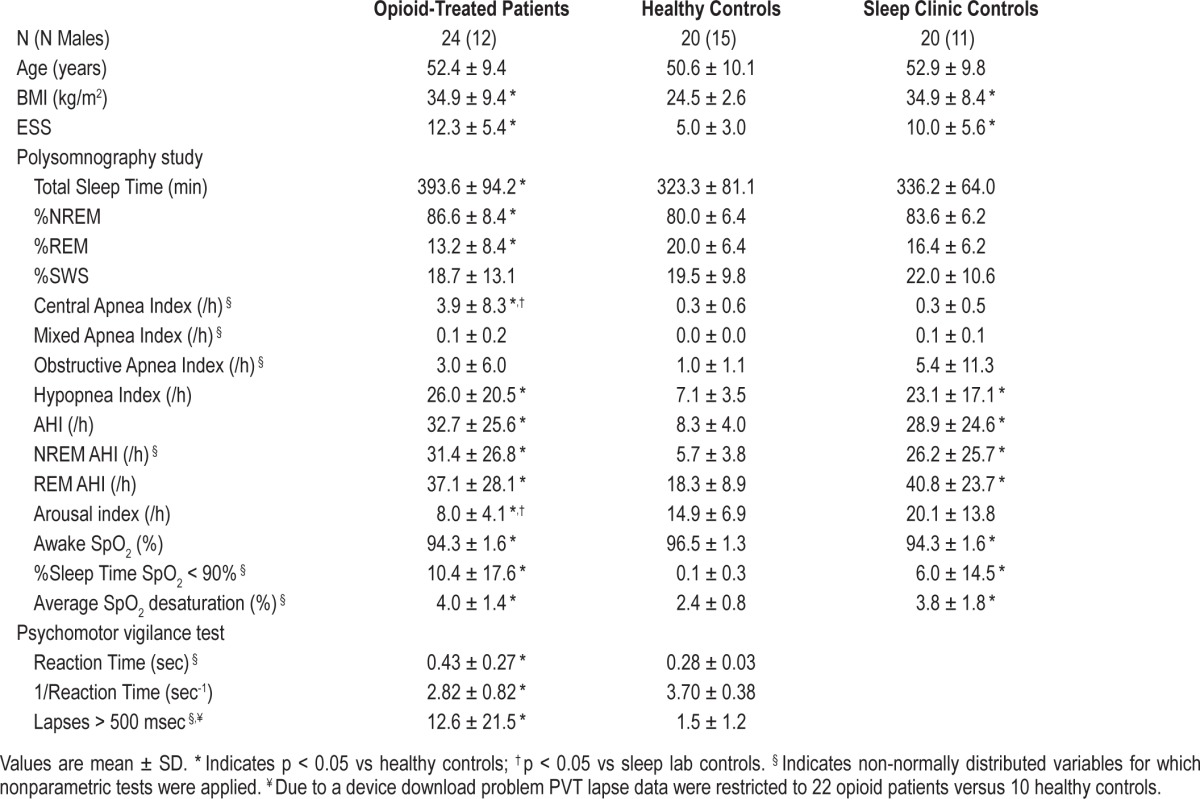
Most (22/24, 92%) of the opioid-treated patients were prescribed at least one other agent with a central nervous system action: selective serotonin reuptake inhibitors (8 patients), pregabalin (8), tricyclic antidepressants (5), baclofen (4), benzodiazepines (10), tramadol (1), mirtazapine (1), and serotonin noradrenaline reuptake inhibitor (1). Twelve patients were on ≥ 2 of these agents in addition to their opioid therapy.
Severe SDB was very common in chronic pain patients with comparable AHI, hypopnea, and oxygen saturation indices compared to the sleep clinic referred patient group: 17 (71%) of opioid-treated patients had what might be regarded as clinically significant sleep disordered breathing (defined as AHI ≥ 15); 11 (46%) had severe sleep disordered breathing (AHI ≥ 30). Central apneas events were more frequent; 4/24 (17%) patients had a CAI > 5/h. Despite frequent respiratory events, arousal frequency was significantly reduced in opioid-treated patients compared to healthy controls and the sleep lab control group.
PVT data from opioid-treated patients versus the healthy PVT comparator group are also presented in Table 1. Full reaction time data were available from both groups, but a device download problem and missing data restricted PVT lapse measurements to 22 opioid patients and 10 healthy controls. Opioid patients showed significantly slower reaction times and more frequent lapses than healthy controls. PVT reaction times positively correlated with average SpO2 < 90% (R2 = 0.549, p = 0.005) and percentage of sleep time spent with SpO2 < 90% (R2 = 0.446, p = 0.029), but not MEQ, PaCO2, or PaO2. In multiple linear regression models, the percentage of time spent with SpO2 < 90% was the only significant predictor of impaired reaction time. Five of the 20 healthy comparator group had an AHI < 5/h.
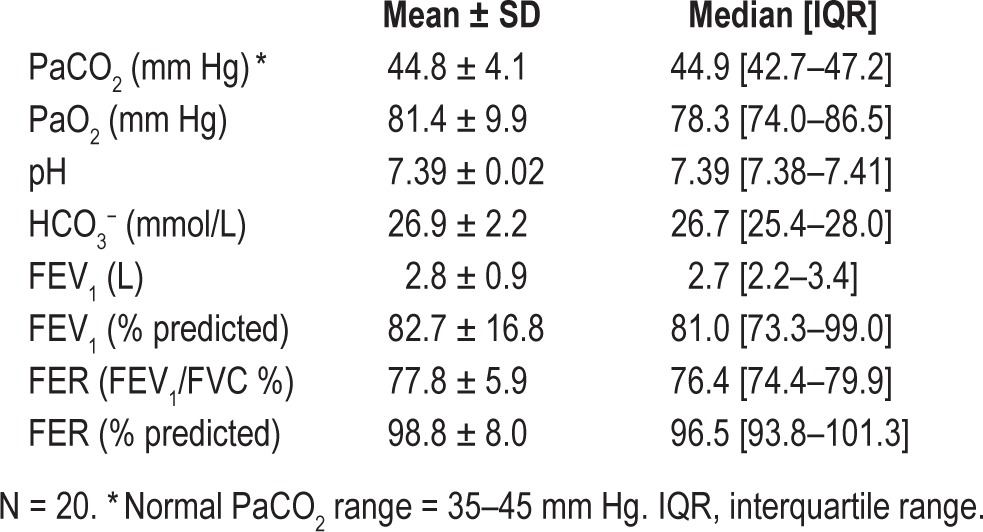
Twenty chronic pain patients agreed to arterial blood sampling for arterial blood gas (ABG) analysis during their clinical review (Table 2). Mean PaCO2 for the group was at the upper limit of normal, but a high proportion of patients (9/20, 45%) had an elevated arterial PaCO2 (> 45 mm Hg). Lung function was relatively normal (Table 2), and none of the spirometry results examined differed between hypercapnic versus normocapnic (all p-values > 0.8).
Table 2.
Arterial blood gas and pulmonary function results in opioid-treated chronic pain patients
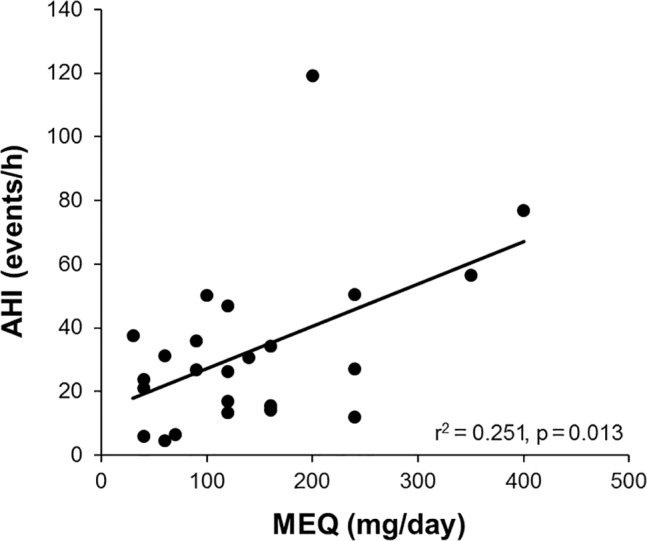
MEQ correlated positively with total AHI (Figure 1), NREM AHI (R2 = 0.250, p = 0.013), hypopnea index (R2 = 0.168, p = 0.046), and central apnea index (R2 = 0.167, p = 0.047), and there was a trend for a negative correlation with awake arterial oxygen saturation (R2 = 0.149, p = 0.062) but not with awake PaCO2. There was a significant positive correlation between wake PaCO2 and sleep time spent with SpO2 < 90% (R2 = 0.214, p = 0.040), but not lung function. In stepwise linear regression, MEQ and BMI were the only significant independent predictors of AHI (standardized β = 0.501, p = 0.007 and 0.397, p = 0.028, respectively; adjusted model R2 = 0.353, p = 0.004), and MEQ the only significant independent predictor of central apnea index. None of the variables examined were significant independent predictors of PaCO2 or PaO2.
Figure 1. Relationship between morphine equivalent dose (MEQ) and apnea hypopnea index (AHI), N = 24.
There was no significant difference in the ESS between patients who had severe sleep apnea and those who did not (AHI > 30, ESS - 12.1 ± 5.6 vs AHI ≤ 30, ESS - 12.6 ± 5.5, p = 0.807).
DISCUSSION
The impact of opioids on sleep and breathing has been best studied by Teichtahl et al. in methadone maintenance patients.7 They found a 30% prevalence of significant SDB, defined as an AHI > 5/h. Ten of the 15 patients had an elevated central sleep apnea index (CAI) > 5/h coexisting with obstructive sleep apnea. The remaining five had only central sleep disordered breathing (CAI > 5/h).11 The opioid-treated patients in our study differed from those of Teichtahl et al. The patients in our study were older (52.4 ± 9.4 years vs 35 ± 9 years) and obese (BMI 34.9 ± 9.4 kg/m2 vs 27 ± 6 kg/m2). The mean AHI in our patient group was 32.7 ± 25.6/h which was higher than the methadone maintenance patients (mean AHI 17.5 ± 17.3/h).11,23–25
In our study, opioid-treated patients had similar mean AHI values compared with the age, gender, and BMI matched control patients who were referred to our sleep laboratory for investigation of possible OSA. These data do not allow us to determine the prevalence and severity of SDB in opioid-treated pain patients compared with non-opioid-treated pain patients or with healthy matched control subjects. However, the comparison with age, gender, and BMI-matched sleep clinic patients referred with a presumptive diagnosis of obstructive sleep apnea, shows that opioid-treated patients have SDB as commonly as a group from the general community group who were considered at high risk for sleep apnea, and shows that at equivalent levels of sleep disordered breathing opioid patients have a tendency towards longer sleep, and significantly fewer arousals and more central apneas. Given they had a similar number of respiratory events as the sleep study comparator group, it is possible that their arousal threshold was blunted by the opiates.
Arterial carbon dioxide elevation has been demonstrated in animal studies following intravenous administration of opioid agonists.27 A novel and very important observation in our study was the increase in wake arterial carbon dioxide tension values in 45% of participants who had arterial blood gas measurements. These patients had no acute symptoms and did not have significant respiratory disease but showed a significant positive correlation between wake PaCO2 and sleep time spent with SpO2 < 90%. We postulate there is a general state-independent suppression of respiratory drive due to opioid administration. This hypoventilation manifests as hypercapnia during wakefulness and produces central apneas and intermittent hypoxemia while asleep. Resting hypoventilation could also contribute to greater desaturation during SDB events by virtue of mild hypoxemia awake placing patients closer to the steep part of the oxyhemoglobin dissociation curve. The association could, however, be bi-directional, with opioid-induced sleep apnea also contributing to awake central hypoventilation.
The finding of reduced psychomotor vigilance in the opioid-treated patients is an important new observation. Much of the available literature suggests there is no impairment of psycho-motor abilities in opioid maintained patients28; however, most patients undergoing psychomotor vigilance testing have been methadone maintenance patients.29 Our patients, recruited from a chronic pain clinic, consistently performed poorly on the 10-minute PVT test, with significantly higher mean reaction times and more frequent minor lapses than healthy age-matched control subjects. Though the sample size is relatively small, the present findings raise questions regarding the ability of such patients to be vigilant in more complex tasks such as driving. It may be that other centrally acting medications used by our patient group in addition to opioids and the presence of SDB contribute to the impairment of psychomotor vigilance. Further research in this area is warranted.
Subjective daytime sleepiness was a common problem for most of our opioid-treated patients, as over 70% had an elevated ESS. However, there was no difference in the ESS between patients who had severe sleep apnea and those who did not. These data raise the possibility that SDB may not greatly contribute to excessive daytime sleepiness in this patient group in whom there are potentially multiple causes of subjective sleepiness. While there was no correlation with the prevalence or severity of SDB, it is possible that SDB was a contributor to daytime sleepiness. However, there was strikingly little sleep fragmentation, as demonstrated by the arousal index in opioid-treated patients. An alternative explanation is that opioid medication had a direct sedative effect on the central nervous system. Also, as expected, depression scores were found to be high among the opioid-treated patients.30,31 Depression itself can contribute to daytime sleepiness.32 Most opioid-treated patients were also taking one or two other centrally acting medications such as benzodiazepines, tricyclic antidepressants, or GABA analogues. These medications may also have contributed to the finding of increased daytime sleepiness and SDB among the patients. It may be that chronic pain causes sleep disruption and contributes to daytime sleepiness.
Limitations of the Study
Our study has several limitations. First, the total numbers of subjects studied is relatively small. Second, most patients were on other non-opioid centrally acting medications that might have independently contributed to sleep disordered breathing, daytime sleepiness, and vigilance. Thus it is difficult to ascribe the abnormalities to opioids alone. However regardless of the relative contribution of opioids versus other medications, chronic pain patients are frequently prescribed several centrally acting medications, and our study has shown for the first time a range of clinically relevant and important associated respiratory and central nervous system abnormalities in this patient group. Comparisons were made with retrospective control groups. While members of the Sleep Comparator Group were matched for gender, age, and BMI, the healthy PVT comparator group had a lower BMI. Polysomnographic data from both control groups were not rescored by the sleep technologist involved in this prospective investigation of opioid patients. We also did not have an esophageal manometer in place to allow unequivocal scoring of the central/mixed or obstructive nature of respiratory events. However, given that hypopneas dominated the elevated AHI, it appears unlikely that such measures would have substantially altered the main findings.
In summary, this study confirms the presence of severe SDB in 46% of chronic pain patients on long-term opioid therapy. While central apnea activity was increased compared with patients referred to a sleep clinic for investigation of OSA (Sleep Clinic Comparator Group), our opioid-treated chronic pain patients clearly had a mixture of obstructive and central respiratory events. Higher levels of obesity, older age, and the frequent use of multiple centrally acting medications may explain these differences. Interestingly, while similar levels of SDB were observed in opioid-treated chronic patients and sleep clinic patients, chronic pain patients appeared by comparison to have minimal sleep fragmentation, raising questions about the contribution of sleep apnea to their daytime sleepiness and functional impairment. An important observation was the presence of impaired gas exchange, both during sleep and wakefulness, with hypercapnia (i.e., chronic ventilatory failure) in almost half the opioid patient group. Another new and potentially very important observation in this study was the impaired psychomotor vigilance observed in this group of chronic pain patients. In addition to opioid therapy, other centrally acting agents and coexisting sleep disordered breathing could contribute to this observation.
In future studies of opioid-treated chronic pain patients, it would be interesting to assess the prevalence of SDB, hypercapnia, and impaired psychomotor vigilance associated with separate classes of opioid medications. It will also be important to assess the impact of CPAP or other therapies for SDB on daytime sleepiness and measure of mood and vigilance. The impaired psychomotor vigilance described here is novel and warrants further investigation. Given the large numbers of people on opioid medications for chronic pain, this impaired vigilance may have a very significant public health impact when tasks that require vigilance such as driving are considered.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Antic has received research funding from the National Health and Medical Research Council of Australia, Philips Respironics and Fisher and Paykel, equipment donations from ResMed, Philips Respironics and SomnoMed, and lecture fees and payment for development of educational presentations from ResMed, Astra Zeneca and GSK. Dr. McEvoy has received research funding from the National Health and Medical Research Council of Australia, Philips Respironics, and Fisher and Paykel, equipment donations from ResMed, Philips Respironics and SomnoMed, and lecture fees from Philips Respironics. Dr. Catcheside has received research funding from the Australian Research Council and National Health and Medical Research Council of Australia, and equipment support from Philips Respironics, Air Liquide Healthcare and Gorman Promed Pty Ltd. Dr. Kapur has received research funding and educational support from Janssen Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Antic had full access to all of the data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. Study concept and design: Antic, Rose, McEvoy, Catcheside, Kapur. Acquisition of data: Antic, Rose, McEvoy, Catcheside, Kapur, Paul, Vakulin. Analysis and interpretation of data: Antic, Rose, McEvoy, Catcheside, Kapur, Peak, Vakulin. Drafting of the manuscript: Antic, Rose, McEvoy, Catcheside. Critical revision of the manuscript for important intellectual content: Antic, Rose, McEvoy, Catcheside, Kapur, Paul, Vakulin. Statistical analysis: Antic, Catcheside. Obtained funding: Antic, Rose, McEvoy, Catcheside. Administrative, technical or material support: Antic, Rose, McEvoy, Catcheside. Study supervision: Antic, Rose, McEvoy, Catcheside.
REFERENCES
- 1.Erstad BL, Puntillo K, Gilbert HC, et al. Pain management principles in the critically ill. Chest. 2009;135:1075–86. doi: 10.1378/chest.08-2264. [DOI] [PubMed] [Google Scholar]
- 2.Bell JR. Australian trends in opioid prescribing for chronic non-cancer pain, 1986-1996. Med J Aust. 1997;167:26–9. doi: 10.5694/j.1326-5377.1997.tb138759.x. [DOI] [PubMed] [Google Scholar]
- 3.Leong M, Murnion B, Haber PS. Examination of opioid prescribing in Australia from 1992 to 2007. Intern Med J. 2009;39:676–81. doi: 10.1111/j.1445-5994.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–57. doi: 10.1093/ageing/afs200. [DOI] [PubMed] [Google Scholar]
- 5.Gutstein HB, Akil H. Opioid analgesics. In: Brunton L, editor. Goodman and Gilman's Pharmacological Basis of Therapeutics. 11th ed. McGraw-Hill; 2006. pp. 547–90. [Google Scholar]
- 6.Santiago TV, Pugliese AC, Edelman NH. Control of breathing during methadone addiction. Am J Med. 1977;62:347–54. doi: 10.1016/0002-9343(77)90831-2. [DOI] [PubMed] [Google Scholar]
- 7.Teichtahl H, Wang D, Cunnington D, et al. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest. 2005;128:1339–47. doi: 10.1378/chest.128.3.1339. [DOI] [PubMed] [Google Scholar]
- 8.Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: systematic review and meta-analysis of randomized controlled trials. JAMA. 2005;293:3043–52. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- 9.Alattar MA, Scharf SM. Opioid-associated central sleep apnea: a case series. Sleep Breath. 2009;13:201–6. doi: 10.1007/s11325-008-0221-7. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Livingston M, Scharf SM. Reversal of central sleep apnea following discontinuation of opioids. J Clin Sleep Med. 2012;8:579–80. doi: 10.5664/jcsm.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–56. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- 12.Mogri M, Desai H, Webster L, et al. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13:49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 13.Farney RJ, Walker JM, Cloward TV, et al. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–9. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- 14.Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447–55. doi: 10.7326/0003-4819-151-7-200910060-00005. [DOI] [PubMed] [Google Scholar]
- 15.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Thorne DR, Johnson DE, Redmond DP, et al. The Walter Reed palm-held psychomotor vigilance test. Behav Res Methods. 2005;37:111–8. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- 18.Lamond N, Dawson D, Roach GD. Fatigue assessment in the field: validation of a hand-held electronic psychomotor vigilance task. Aviat Space Environ Med. 2005;76:486–89. [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–43. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- 21.Pergolizzi J, Boger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 22.Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3:455–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Webster LR, Choi Y, Desai H, et al. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9:425–32. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Teichtahl H, Goodman C, et al. Subjective daytime sleepiness and daytime function in patients on stable methadone maintenance treatment: possible mechanisms. J Clin Sleep Med. 2008;4:557–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Heinzer R. [Opioids and sleep disordered breathing] Rev Med Suisse. 2009;5:2322–4. 2326–8. [PubMed] [Google Scholar]
- 26.Pavlova MK, Duffy JF, Shea SA. Polysomnographic respiratory abnormalities in asymptomatic individuals. Sleep. 2008;31:241–8. doi: 10.1093/sleep/31.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang PK, Weinger MB, Negus SS. Elucidation of dose-effect relationships for different opiate effects using alfentanil in the spontaneously ventilating rat. Anesthesiology. 1992;77:153–61. doi: 10.1097/00000542-199207000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Fishbain DA, Cutler RB, Rosomoff HL, et al. Are opioid-dependent/tolerant patients impaired in driving-related skills? A structured evidence-based review. J Pain Symptom Manage. 2003;25:559–77. doi: 10.1016/s0885-3924(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 29.Specka M, Finkbeiner T, Lodemann E, et al. Cognitive-motor performance of methadone-maintained patients. Eur Addict Res. 2000;6:8–19. doi: 10.1159/000019004. [DOI] [PubMed] [Google Scholar]
- 30.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–27. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Christo PJ, Grabow TS, Raja SN. Opioid effectiveness, addiction, and depression in chronic pain. Adv Psychosom Med. 2004;25:123–37. doi: 10.1159/000079062. [DOI] [PubMed] [Google Scholar]
- 32.Bixler EO, Vgontzas AN, Lin HM, et al. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90:4510–5. doi: 10.1210/jc.2005-0035. [DOI] [PubMed] [Google Scholar]


