Abstract
Study Objectives:
A majority of patients diagnosed with obstructive sleep apnea are position dependent whereby they are at least twice as severe when sleeping supine (POSA). This study evaluated the accuracy and efficacy of a neck-worn device designed to limit supine sleep. The study included nightly measurements of snoring, sleep/wake, time supine, and the frequency and duration of feedback to monitor compliance.
Methods:
Thirty patients between ages 18 and 75 years, BMI ≤ 35 with an overall apnea-hypopnea index (AHI) ≥ 5 and an overall AHI ≥ 1.5 times the non-supine AHI, and an Epworth score ≥ 5 were prospectively studied. Subjective reports and polysomnography were used to assess efficacy resulting from 4 weeks of in-home supine-avoidance therapy and to measure device accuracy. From 363 polysomnography reports, 209 provided sufficient positional data to estimate one site's prevalence of positional OSA.
Results:
In 83% of participants exhibiting > 50% reduction in overall AHI, the mean and median reductions were 69% and 79%. Significant reductions in the overall and supine AHI, apnea index, percent time SpO2 < 90%, and snoring contributed to significant improvements in stage N1 and N2 sleep, reductions in cortical arousals and awakenings, and improved depression scores. Supine position was under-detected by > 5% in 3% of cases. Sleep efficiency by neck actigraphy was within 10% of polysomnography in 87% of the studies when position feedback was delivered. The prevalence of POSA was consistently > 70% when the overall AHI was < 60.
Conclusions:
The neck position therapy device is accurate and effective in restricting supine sleep, improving AHI, sleep architecture and continuity, and monitoring treatment outcomes.
Citation:
Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med 2014;10(8):863-871.
Keywords: obstructive sleep apnea, positional therapy, supine sleep, snoring, prevalence
Obstructive sleep apnea (OSA) is a common chronic human affliction that is now quite easily diagnosed but remains difficult to treat. The vast majority of OSA patients have more obstructive events in the supine position, where gravity aids the collapsing forces on the upper airway.1 Also, compared to other recumbent positions, supine sleep reduces end-expiratory lung volume, which in turn decreases tracheal tug and lung oxygen stores, and contributes to greater hypoxemia during each obstructive breathing event. A formal diagnosis of positional OSA (POSA) is made when the difference between the apneahypopnea index (AHI) in the supine and non-supine positions exceeds one of the cutoff points defined in the literature.1–6 The prevalence of POSA among OSA patients is estimated at 56%3,4 if the standard definition of POSA by Cartwright (≥ twofold difference between the supine and non-spine AHI) is used.6
Clinical studies have demonstrated that position therapy can reduce the AHI to the “normal” range of less than 5 events per hour in some patients with POSA irrespective of the disease severity.7 This suggests that if someone has clinically important OSA when supine but has a relatively low AHI when non-supine, then keeping that person from sleeping supine could be the only treatment needed. The early versions of position therapy used objects of various sizes and shapes strapped to the patients back to make the supine position too uncomfortable to be maintained.7,8 Unfortunately, such an approach limits comfortable sleep, and similar to continuous positive airway pressure (CPAP), discomfort can result in poor long term compliance.9 More recently, vibro-tactile feedback from small devices have been introduced as a more sleep-friendly therapeutic intervention for POSA.10–12 The additional benefit of “electronic” position therapy approaches is that, like CPAP, utilization and effectiveness can potentially be monitored.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is now quite easy to diagnose but remains difficult to treat when both efficacy and compliance are considered. In this prospective study, we evaluated the impact of 4 weeks of neck-based vibro-tactile positional therapy on respiratory and sleep architecture measures, subjective symptoms, and compliance in patients with mild, moderate, and severe positional obstructive sleep apnea (POSA).
Study Impact: We found position therapy significantly reduced sleep disordered breathing and significantly improved sleep quality and moderately reduced symptoms across all OSA severity groups. The prevalence of POSA was relatively consistent across all patients who had an overall AHI < 60 events/hour.
This study provides an initial prospective evaluation of a neck-worn device that delivers vibro-tactile feedback as a deterrent to supine sleep. The primary endpoints include an evaluation of the effect of 4 weeks of position therapy on respiratory, sleep architecture, and subjective measures. Secondary endpoints include an assessment of the accuracy of neck-based actigraphy for the detection of sleep position and estimation of sleep and wake time. Supporting evidence includes a demonstration of the device's capability to monitor its utilization and effectiveness in the home. Finally, the prevalence of positional POSA in non-study patients evaluated by the study site with laboratory PSG while the study was ongoing is reported.
METHODS
Protocol Design
Volunteers were recruited under a protocol approved by the Chesapeake Institutional Review Board after review by the U.S. Food and Drug Administration (FDA). Enrollment required an age between 18 and 75 years, a screening overall apnea hypopnea index (AHI) ≥ 10 (based on a previously completed PSG or home sleep test [HST]), an Epworth Sleepiness Scale score (ESS) ≥ 5, and a body mass index (BMI) ≤ 35 kg/m2.
At the time of enrollment, subjects completed a battery of questionnaires to obtain pre-treatment measures of daytime sleepiness using the ESS, quality of life with the Functional Outcomes of Sleep Questionnaire (FOSQ) and Profile of Mood States (POMS), depression using the Patient Health Questionnaire (PHQ-9), insomnia using the Insomnia Severity Index (ISI), and anxiety by the Generalized Anxiety Disorder (GAD-7) questionnaire. In-home use of the Neck Position Therapy Device (NPTD) and generation of compliance reports were also explained.
To proceed onto therapy, volunteers needed to be diagnosed with POSA resulting from a baseline PSG with a minimum of 4 h sleep time, an overall AHI ≥ 5, and an overall AHI ≥ 1.5 times greater than the non-supine AHI. During the baseline PSG studies subjects wore the NPTD that was set to record-only mode (i.e., no positional feedback) and applied by the sleep technician. To provide a means to synchronize the PSG and the NPTD records, subjects were instructed to sit upright in bed for one-minute just prior to lights out and just after lights on. Technicians were instructed to attempt to acquire equivalent amounts of supine and non-supine sleep from each subject.
For the position therapy period, subjects were instructed to wear the NPTD for 30 nights while in bed and attempting to sleep. The first 2 nights provided an adaptation period in which no feedback was delivered. This allowed participants the opportunity to withdraw prior to initiating 28 consecutive nights of NPTD therapy and being categorized with an intention to treat. Subjects maintained daily logs so that potential interruption to their daytime or nighttime quality of life attributed to the position therapy could be evaluated, and comparisons between self-reported use and compliance measured with the NPTD could be compared. To remain in the study, subjects were required to generate a compliance report on the NPTD web-portal and mail the daily logs at the end of each week. Upon completion of 4 weeks of therapy, subjects completed the post-treatment battery of questionnaires and continued to receive positional therapy until completion of the follow-up PSG.
Polysomnography
All subjects were recruited from and studied at an American Academy of Sleep Medicine (AASM) accredited sleep center (Complete Sleep Solutions, Murrieta, CA). The baseline and follow-up PSG studies were conducted and scored according to the AASM criteria.13 Scoring of apneas required a 10-s cessation in breathing. Hypopneas required ≥ 30% reduction in airflow combined with 4% hypopnea desaturation. Subjects were studied in 1 of 4 rooms; 2 were equipped with Alice 3 and 2 with Alice 5 PSG systems (Philips Respironics, Monroeville, PA). Airflow was measured with Pro-Tech nasal pressure transducers. Respiratory effort was measured with Pro-Tech respiratory inductive plethysmography effort sensors. Chest position was measured with either Pro-Tech or SleepMate (Ambu, Inc. Glen Burke, MD) actigraphy-based position sensors. The Alice software synchronized and saved the video recording with the physiological signals. During scoring of the studies, technicians used video recordings to compare both torso and neck positions to the recorded chest position. Position obtained from the chest transducer and confirmed by video (i.e., video/chest) was used for the PSG report, and technician notes were used to identify periods when the chest was supine but the neck was non-supine left/right (i.e., head was turned and neck elevated > 30° from horizontal toward a lateral position).
Description of the NPTD
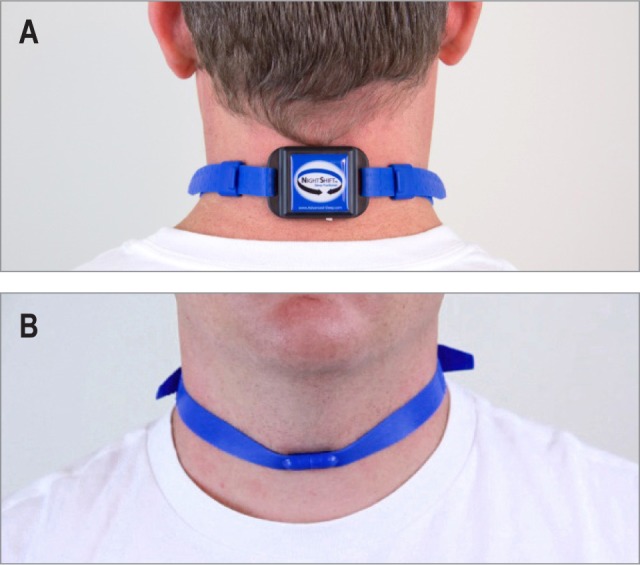
The battery-powered NPTD (Night Shift, Advanced Brain Monitoring, Carlsbad, CA) weighs 44 g and includes electronics housed in a 5.5 (l) × 3.8 (w) × 1.6 (h) cm enclosure affixed on the back of the neck with an adjustable non-latex silicone rubber strap secured by a magnetic clasp (see Figure 1).14
Figure 1. Photograph of neck device from (A) back and (B) front.
The NPTD measures snoring with a built-in, calibrated acoustic microphone. The raw audio input is sampled at 2 kHz, and root mean square (RMS) of the digitized signal is calculated using a 100-ms window. The resulting 10 Hz RMS signal is additionally filtered with a 0.5 Hz low-pass Butterworth fourth-order filter. A snore detection algorithm quantifies each snore based on the shape and the peak amplitude, prior to conversion to decibels (db). Loud snoring is defined as at least one snore with a magnitude ≥ 50 dB in a 30-s epoch. The percentage of time snoring > 50 dB is then determined for overall, supine, and non-supine epochs characterized as sleep by the NPTD.
A three-axis accelerometer is used to determine neck position and perform an actigraphy-based classification of sleep vs. wake. Neck positions are reported as upright, supine, lateral left, lateral right, and prone. Upright is assigned when the neck angle is ≥ 60°. Supine is assigned when the neck angle to the left/right is < 43°. Lateral left or right is assigned when the neck angle exceeded 47°. The NPTD assumes the patient has remained in the previously assigned position when the neck angle falls between 43° and 47°. Prone is the mirrored position of supine with the additional requirement that the Z-axis is < -15°. If the NPTD is worn upside down, the supine position will be accurately determined; however, reported time in the lateral left and right will be reversed. Thirty-second epochs are classified as sleep or wake by comparing the median filtered output derived from the three X, Y, and Z signals to a fixed threshold. If any of the 3 signals have an angle < 50° and exceed the actigraphy threshold, the epoch is classified as wake. In addition, long periods with gross movement extend the wake classification for up to 3 subsequent “silent” epochs. The initial 10-min period after the NPTD is turned on is automatically classified as wake.
Two x 1G haptic motors provide vibro-tactile feedback when the supine position is detected. Positional feedback is modulated by setting the number of motors to be excited (one or both) and varying the duration of the motor(s) excitation. Feedback is initiated at a very low frequency/duration, and gradually increased until the user exits the supine position. At any given intensity level, the feedback is repeated 6 times with an inter-feedback interval of 2 s. The feedback can be optionally paused for a predefined time interval or completely turned off. A total of 7 levels of feedback are delivered, with levels 5 through 7 utilizing both haptic motors. For this study vibro-tactile feedback was defaulted to initiate 15 min after the NPTD was turned on, to allow the user time to fall asleep. Upon conclusion of an upright period > 45 s, positional feedback was paused for 5 minutes.
Data are acquired and analyzed in real time with derived measures for sleep/wake, snoring magnitude, and position for each 30-s epoch saved to the microcontroller flash memory. The NPTD memory can store up to 6 nights' of detailed snoring, sleep, and position measures (one set of values for each 30-s epoch), as well as a summary of key daily parameters by month for 4 months, and the average values across the days in the month for 12 months. All the information is accessed via assessment/compliance reports generated in PDF format from a web-enabled portal. The NPTD can record and provide vibro-tactile feedback for 3 nights before charging is required.
Data Reduction and Analysis
In addition to the standard sleep study reports, Alice software was used to export epoch level resolution of chest position and sleep stage. To compare PSG and neck-device epoch classifications of sleep or wake, and supine or non-supine, the clock times from the files were aligned, with accurate synchronization confirmed by the change to the upright position at the start of the recording. The percentage of time supine, as determined by PSG (chest sensor and video recording) and NPTD (neck accelerometer) during the baseline and follow-up PSG studies were compared from lights out to lights on. Video recordings were reviewed to provide the gold standard for classification of the supine position based on the neck at < 30° angle from horizontal. Technician notes from the video review during scoring were used to identify when technician edits were applied to the body position and when the neck position conflicted with body position. Bland-Altman plots were used to compare the supine sleep detection from the neck and chest. From the PSG hypnograms, the number of awakenings (return to an awake state from any REM or NREM stage were tallied and divided by the hours of total sleep time.
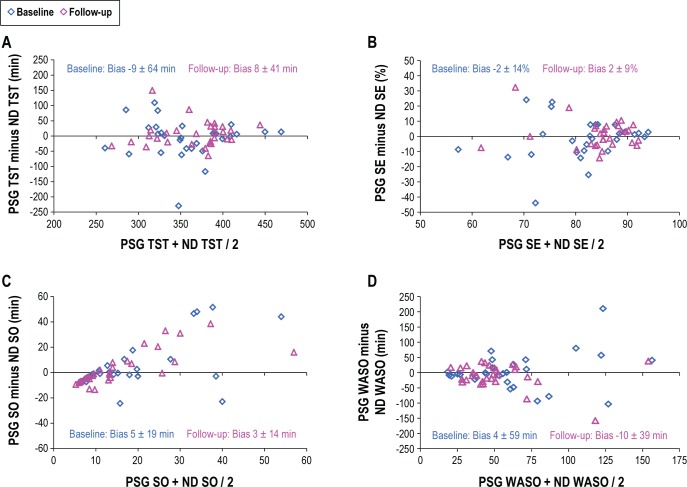
Two-tailed t-tests, which assumed equal variances, were used to measure significant differences between baseline and follow-up respiratory, sleep architecture, sleep/wake, and subjective measures. A minimum of 12 min of sleep time was required for supine AHI values to be reported as > 0. The overall percent time snoring ≥ 50 dB from the neck (loud snoring) was computed from lights out to lights on during periods detected as sleep by neck (NPTD) actigraphy. Bland-Altman plots were used to compare differences in the total sleep time (TST), sleep efficiency (SE), sleep onset (SO), and wake after sleep onset (WASO) derived from the respective PSG and NPTD.
To assess position therapy compliance, the utilization rate was defined as the number of nights that the device was worn ≥ 4 h divided by the days during the intention to treat period, similar to that used for CPAP.15 Repeated measures analysis of variance (ANOVA) was used to evaluate changes in utilization, efficacy, and response to feedback measured with the NPTD across the 4 weeks of therapy. Repeated measures ANOVA was also used to evaluate changes across the daily sleep diary reports with respect to weekly changes in: (a) average number of previous night's recalled avoidance feedback, (b) total number of times the device fell off, (c) average shoulder or neck discomfort or pain score resulting from sleeping on back, and (d) average effect of position therapy on sleep quality.
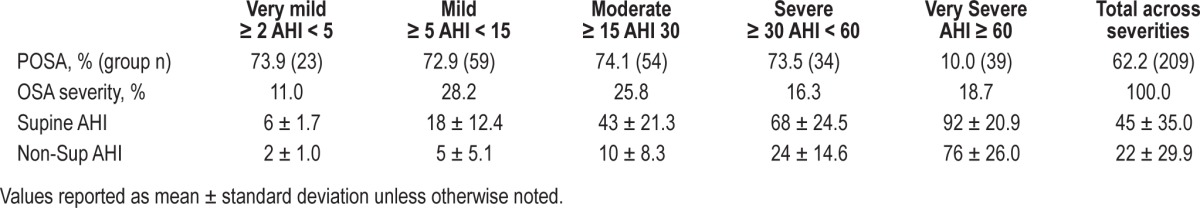
PSG reports from 363 consecutive patients evaluated by PSG for various reasons at the study site during the 150-day study recruitment period (but not enrolled to the study) were retrospectively analyzed to assess the prevalence of positional sleep apnea in the patient sample from which this study cohort was selected. Supine and non-supine AHI were calculated for all patients with ≥ 20 min of sleep in both positions; the percentage of patients with the supine to non-supine AHI ratio of ≥ 2 was determined for each of the standard OSA severity categories based on the overall AHI (very mild: 2-4/h; mild: 5-14/h; moderate: 15-29/h; severe: 30-59/h, and very severe: > 60/h).
RESULTS
Thirty-six subjects were initiated into the study. All but one subject wore the NPTD during their baseline study. Five subjects were dropped as a result of their baseline PSG results. Two had very severe sleep disordered breathing with an overall to non-supine ratio < 1.5; 2 had an overall AHI < 5; and one had < 4 h of sleep time. One subject was dropped for protocol non-compliance due to failure to maintain and submit daily sleep diaries. Of the 22 males and 8 females who completed the protocol, 37% had mild overall OSA severity (AHI ≥ 5 and < 15), 33% had moderate overall severity (AHI ≥ 15 and < 30), and 30% had overall severe OSA (AHI ≥ 30).
Primary Endpoints
Respiratory Measures
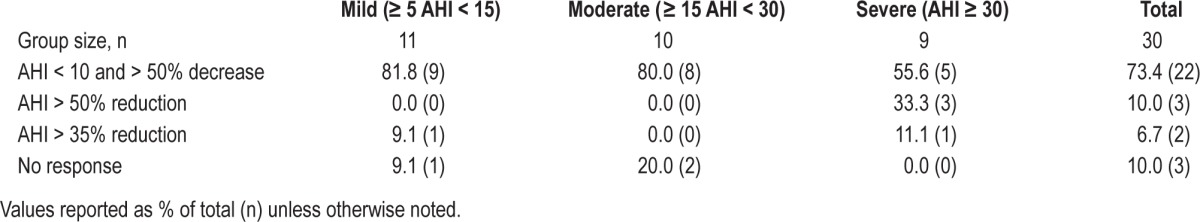
Based on effectiveness of the supine sleep restriction, the overall and supine AHI, apnea index, and percent time SpO2 < 90% were significantly reduced (Table 1). Across all participants, the mean percent reduction in AHI was 69% and the median reduction was 79%. At follow-up, 73% percent of participants achieved an AHI < 10 with a ≥ 50% reduction in overall AHI, and an additional 17% showed potentially important reductions in overall AHI of 50% or 35% (Table 2). Three non-responders exhibited > 60% increase in non-supine AHI at follow-up.
Table 1.
Sleep and respiratory measures obtained by PSG without and with position avoidance feedback.
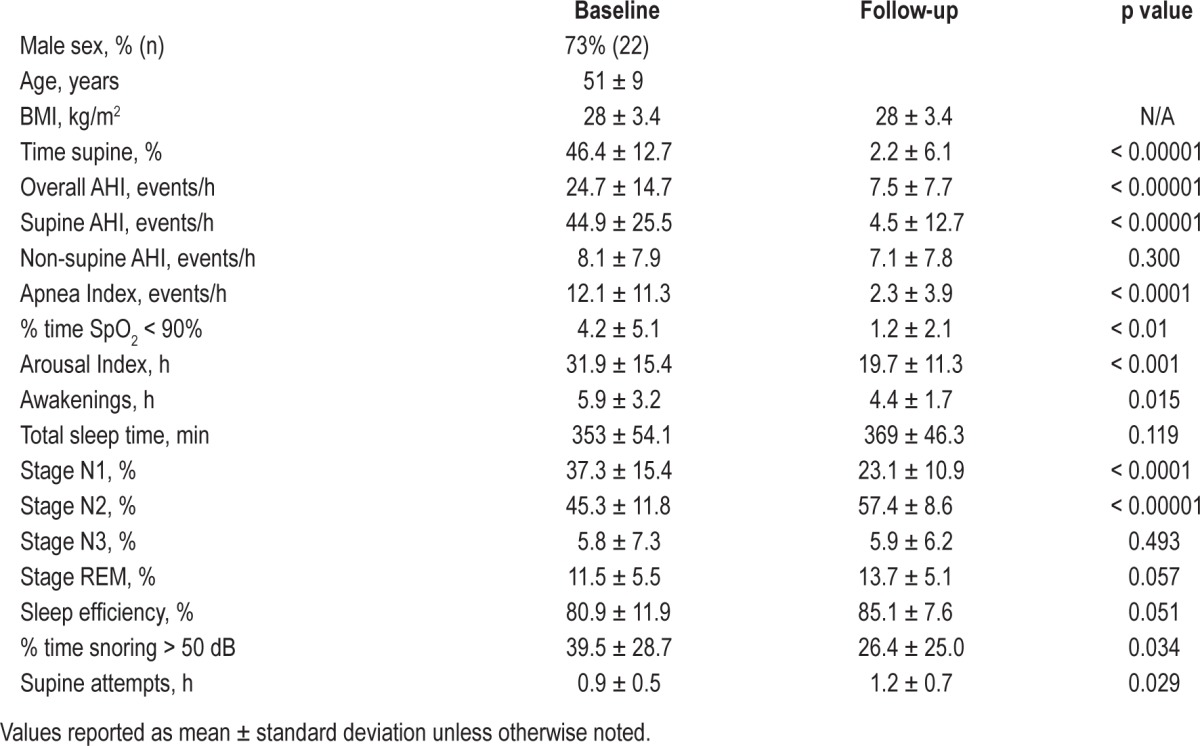
Table 2.
Distribution of response to neck position therapy stratified by OSA severity.
Notable reductions in loud snoring were, on average, achieved with the NPTD as a result of supine position avoidance. Fifty-nine percent of participants exhibited reductions in the percent of time with loud snoring of ≥ 5% or more, while only 10% of subjects had an increase in the percent of time with loud snoring. The reduction in loud snoring was statistically significant in those with a baseline apnea index < 10 (n = 17, p = 0.04), but not for the 12 participants with an apnea index ≥ 10.
Sleep Architecture Measures
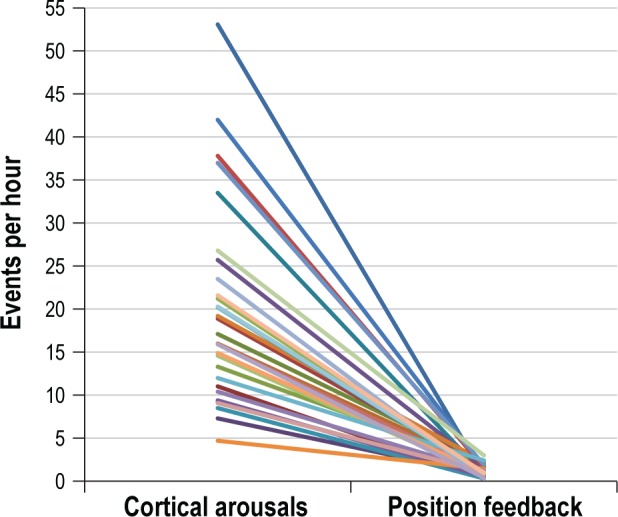
Compared to the baseline PSG, a significant reduction in the percent time stage N1, decrease in the number of cortical arousals and awakenings per hour of sleep, and a significant increase in stage N2 sleep were observed. Positive trending improvements in the percent of sleep time in stage REM and sleep efficiency were also noted. A significant increase in the number of supine attempts/h was observed during the follow-up PSG when positional feedback was delivered. The relationship between cortical arousals and positional feedback attempts per hour is presented in Figure 2.
Figure 2. Comparison of cortical arousals and supine attempts per hour during the follow-up PSG.
Subjective Measures
The impact of 4 weeks of position therapy on subjectively measured impairment and quality of life are presented in Table 3. Subjects showed a significant improvement in depression scores, and marginally significant improvements in ESS, POMS, and ISI.
Table 3.
Subjective measures obtained by questionnaire.

Secondary Endpoints
Supine Detection
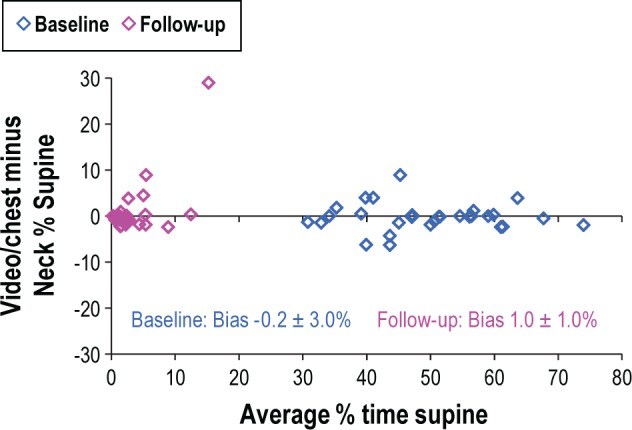
The NPTD measures of the percent of recording time spent in the supine position were in close agreement with the percent of time supine determined by video inspection of the 29 baseline and 30 follow-up studies (Figure 3). For the baseline PSG records, 3 cases had an absolute difference between neck supine and chest plus video supine > 5%. For the follow-up studies, NPTD estimates were within 5% of values based on the chest sensor plus video in 28 of 30 studies. Larger disagreements between the PSG and NPTD estimates occurred in patients who spent a portion of the night with the head turned far to the side while the torso was supine. Incorrect detection of supine sleep which would result in non-delivery of therapy for > 5% of sleep time was limited to 2 of 59 cases (3%).
Figure 3. Bland Altman Plot comparing the percent of time supine as scored by as scored by video editing of the chest sensor to the percent of time supine determined with the NPTD.
Sleep/Wake Detection
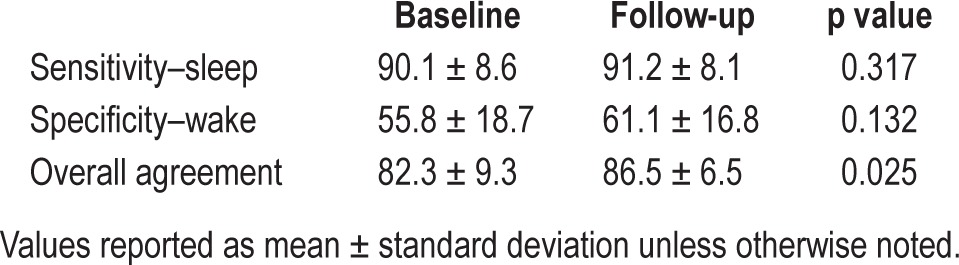
Table 4 presents the mean sensitivity (sleep) and specificity (wake) of neck actigraphy as compared to PSG. Significant improvements in overall accuracy were observed during the follow-up PSG when positional feedback was delivered.
Table 4.
Measures of behavioral sleep/wake by neck actigraphy vs. PSG.
Differences in TST, SE, SO, and WASO are presented in Bland-Altman plots (Figure 4). No statistically significant differences between PSG and NPTD were observed during either the baseline or the follow-up for TST, SE, SO, and WASO. Variability about the mean was reduced by over one-third for TST, SE, and WASO when therapy was delivered during the follow-up PSG.
Figure 4. Bland-Altman plots comparing PSG to NPTD for: (A) total sleep time (TST), (B) sleep efficiency (SE), (C) sleep onset (SO), and (D) wake after sleep onset (WASO).
Supporting Evidence
NPTD Home Use
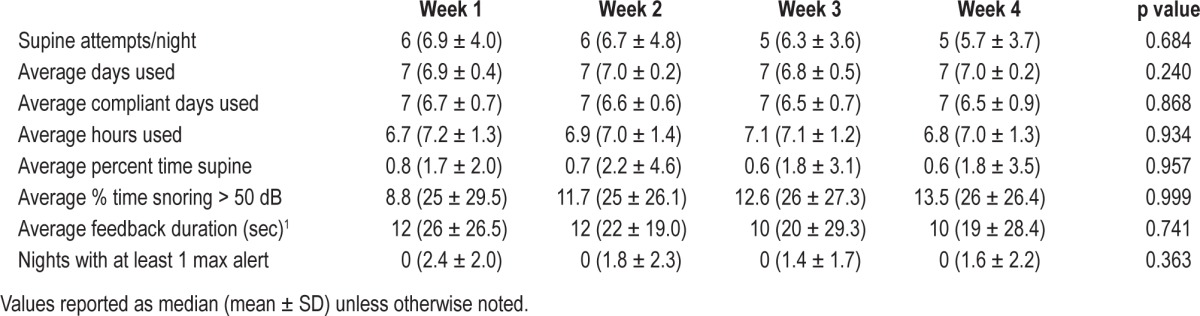
Across subjects, the device was worn in 99% of the possible treatment nights, with a median compliance rate of 96% (range 71% to 100%). Twenty-seven of 30 subjects maintained the non-supine position over > 98% of the time across nights of use; the remaining 3 spent 96%, 93%, and 84% of the time in bed in the non-supine position. The mean number of supine attempts per night trended lower across the 4 weeks of treatment, but no consistent pattern suggesting a training effect was apparent. Most did not require delivery of the maximum feedback intensity (i.e., max alert) to change positions. The participants were in general not awakened by the feedback, as they recalled only one-third of all stimuli delivered (median: 2; range 1-4). Repeated measures ANOVA showed that the compliance measures did not significantly vary throughout the 4 weeks of treatment (Table 5). The average number of perceived stimuli per night also did not change over time.
Table 5.
Compliance information obtained across four weeks of in-home use.
How the NPTD was adjusted for larger necks may have influenced how often it fell off during the night. Twenty subjects reported the NPTD remained in place during sleep without incident across the 4 weeks of therapy (neck size 39 ± 3.8 cm). Two reported the device fell off once, and 6 subjects reported the NPTD fell off between 2 and 4 times (neck sizes ranged from 39 to 45.7 cm). One subject with a neck circumference of 44 cm reported the device fell off 9 times in the first 2 weeks and 11 times overall. Another subject with a neck circumference of 44.5 cm reported the device fell off 12 times in weeks 3 and 4, and a total of 16 times.
In total, 6 patients reported symptoms of minor back, shoulder, or neck discomfort in the morning across ≥ 2 weeks of therapy. Of these, one subject identified noticeable/extreme back, shoulder, or neck pain across the first 2 weeks of therapy. A second participant experienced noticeable/extreme pain during week 3. All remained in the study until completion.
Across all subjects and nights, the percentage of occurrences that perceived sleep quality was worse/substantially worse as a result of Night Shift trended downward from 13.4% in week 1, 10.2 in week 2, 9.5% in week 3, and 7.4% in week 4.
Prevalence of POSA
Of the 363 PSG reports available for the retrospective analysis, 26 (7%) had an AHI < 2, and 128 (35%) had < 20 min of sleep time in either supine or non-supine position; thus, a total of 209 reports were ultimately used for the assessment of POSA prevalence. Table 6 presents the prevalence distributions stratified by and across OSA severities, with the accompanying group AHI distributions. The prevalence of POSA was consistently > 70% across all severity categories, other than those with very severe OSA (the AHI was < 60 in over 80% of the studies). Additional statistical analyses showed no evidence of a significant relationship in this sample between the presence/absence of POSA and variables such as age, BMI, TST or total time spent in the supine position.
Table 6.
Prevalence of POSA stratified by AHI severity.
DISCUSSION
Several recent studies demonstrated success with supine avoidance therapy when enrollment was limited to mild and moderate OSA.7,10–12 To our knowledge, this is the first study that has demonstrated consistent improvement in sleep disordered breathing and sleep architecture across patients with mild, moderate, and severe POSA. In this cohort, the mean reduction of overall AHI was 69% and the median reduction was 79%. Eighty-three percent of participants with POSA achieved at least a 50% reduction in overall AHI; 80% met Sher's criteria for surgical success (i.e., 50% reduction in AHI and post-treatment AHI < 20/h)16; and 73% demonstrated a 50% reduction in AHI with a post-treatment AHI < 10/h. One should note that the percentage of patients with > 50% reduction in AHI was similar in all OSA severity categories. Of interest, 78% of those with severe OSA exhibited a notable decrease in non-supine AHI at follow-up, a pattern that was not consistently observed in those with mild to moderate OSA.
Sleep quality during the course of positional therapy is an important outcome measure, since positional therapy has the potential to disrupt sleep and reduce total sleep time (especially among the elderly who, once awakened, might have problems falling back to sleep). The quality of sleep (as judged by TST, SE, number of arousals, and percentage of shallow N1 sleep) clearly improved when the NPTD was used. The reduction in sleep disordered breathing contributed to deeper and more efficient sleep. These findings suggest that vibro-tactile positional feedback does not significantly disrupt sleep architecture, or more precisely, that the number of eventual disruptions triggered by the delivery of feedback is vastly eclipsed by disruptions attributed to obstructive supine sleep. As expected, improved sleep quality contributed to clinically relevant downward trend in daytime somnolence, symptoms associated with depression and insomnia, and improved quality of life.
It is difficult to explain why significant improvements in sleep architecture and sleep continuity only contributed to modest improvements in subjective scores. Applying an ESS change ≥ 2 as a clinically relevant threshold, subjective sleepiness did not increase in any of the study participants; 83% of those with an ESS ≤ 7 and 73% of those with an ESS > 13 reported an improvement. However, only 15% with an ESS between 8 and 12 reported ESS improvements of 2 or more. Of the six subjective measures we evaluated, the FOSQ showed the least statistical improvement. A moderate association was observed between the ESS and depression change scores (r = 0.63, p < 0.001).
In a comparable study, Van Mannan found no significant differences in sleep architecture or sleep continuity but noted significant improvements in ESS and FOSQ responses after 30 days of position therapy.10 In both studies, 50% of participants exhibited an ESS improvement of 2 or more. In the Van Mannan cohort, the ESS improvements were more equally distributed across ESS ranges. Differences in the two studies with respect to improvement of the FOSQ are difficult to explain, other than patients living in Southern California reported improved quality of life scores before and after the study, with less group variability than those living in the Netherlands.
Of the three subjects who were totally non-responsive to the NPTD, night-to-night variability in post-treatment AHI17,18 was likely a contributing factor in two cases. Both subjects exhibited > 60% increase non-supine AHI compared to baseline, yet reported substantial improvements in FOSQ and other subjective scores after four weeks of position avoidance therapy. Given these findings, it would be beneficial to cross-validate the NPTD with a sham controlled study in a larger, more diverse population.
Ten percent of the subjects in this study slept more than 5% supine when supine-avoidance feedback was delivered as compared to 29% for the Van Maanan cohort.10 This discrepancy is likely attributed to differences in the two approaches used for delivery of supine avoidance feedback. The NPTD will not initiate feedback for 15 minutes after the device is turned on and for 5 minutes after any sustained upright period greater than one minute. The chest-device delays feedback for 30 minutes after being turned on, and again for 30 minutes after any sustained upright time. The occurrences of false-negative delivery of vibro-tactile therapy (i.e., percent supine errors > 5%) were similar for the NPTD (2 of 59 cases) and torso supine sleep reported by Bignold et al. (1 of 15 cases).11 In all three studies, subjects were accepting of vibro-tactile feedback and compliant in its use.
While patient selection clearly contributes to position therapy efficacy, where the supine position is detected, and how feedback is delivered may also affect outcomes. Van Kersteren el al. demonstrated that subtle changes in head position which are not reported by torso position have clinically important implications for OSA severity.19 The NPTD ensures that positions most vulnerable to airway collapse due to the effects of gravity are avoided. The NPTD avoids delivery of feedback when the chest is supine and the neck is upright, so users can read or watch television in bed with the device turned on to avoid falling asleep without therapy.
The definition of POSA used in this study was selected as a result of the FDA requirement that the NPTD demonstrate a 50% reduction in overall AHI. The classic definition of POSA (i.e., supine AHI at least two times greater than the non-supine AHI) does not consider the impact of supine sleep time and/or supine AHI severity in its contribution to the overall AHI. Rather than create a sophisticated algorithm to screen for various combinations of supine AHI severities and durations, we utilized alternative criteria whereby the overall AHI had to be at last 1.5 times greater than the non-supine AHI for our enrollment. To demonstrate these alternative criteria did not bias the results, we compared the two POSA definitions using the data provided in Table 6. The net difference was to reclassify 6% of patients with an AHI < 15 (8 of 132) and 2% of patients with an AHI ≥ 15 as non-positional vs. the classic definition of POSA. This does, however, suggest the NPTD would likely be much less useful for OSA patients who already have the habit of sleeping in the non-supine position. In retrospect, the classic definition of POSA2,6,10 is preferable so long as the contribution of the supine sleep disordered breathing on the overall AHI is considered in combination with the selected SpO2 desaturation criteria. As compared to a 4% desaturation, a 3% reduction in oxyhemoglobin desaturation will result in a greater number of hypopnea events being identified. If the increase in non-supine events occurs at a greater rate than supine events, then alternative definitions of POSA may be necessary.
Using the classic definition, POSA prevalence at our study site was approximately 5% greater than previous reports.1,3 The percentage of patients with POSA and mild OSA was identical to Benoist5 (72.9 vs. 73.3%, respectively), and very close for moderate OSA (74.1% and 78.1%, respectively). We chose to evaluate the POSA prevalence with severe and very severe OSA and found that POSA was distributed quite similarly across all AHI severities < 60. When our severe and very severe OSA groups were combined, the POSA prevalence for those with an AHI ≥ 30 was greater than for Benoist (41.1% vs. 30.0, respectively). The percentage of PSG studies which could not be classified due to insufficient supine or non-supine sleep time was equivalent to another POSA prevalence report.3 Unfortunately, due to the overwhelming directive toward CPAP therapy, it is common for OSA diagnostic studies to be conducted with an insufficient assessment of positional severity. In some cases, home sleep testing devices are used that do not measure position,20 and in other cases split-night PSG studies are performed with insufficiently recorded amounts of supine or non-supine sleep time.3 Given the influence positional severity has on outcomes across all OSA therapies,2,21 it raises the question as to why the adequate characterization of positional OSA severity is an option rather than a requirement for the delivery of an acceptable OSA diagnostic study.
BMI ≤ 35 was used an inclusion criteria as a precaution for potential neck and shoulder problems that might have resulted from increased non-supine sleep in those morbidly obese. Several of the studied subjects complained of minor shoulder or neck soreness as a result of increased non-supine sleep; however, the discomfort was insufficient to withdraw from the study. A post hoc analysis confirmed there was no relationship between increased BMI and complaints of discomfort. Although the BMI criteria resulted in exclusion of a quarter of potential candidates from the study site, an analysis of the POSA prevalence data stratified for BMI > 35 suggested greater bias toward non-positional OSA, although the sample size of high BMI individuals (n = 53) was small. Because supine avoidance feedback is delivered to the neck rather than the torso, there is no obvious reason why an obese POSA patient would not be effectively treated with the NPTD. Additional investigations are needed to evaluate efficacy and potential side effects with the NPTD used on larger patients. Additionally, it may be useful to develop a questionnaire to help identify patients with histories of neck or back issues that might be exclusion criteria for position therapy.
In this study, 45% of those with mild OSA, 11% with moderate OSA, and 56% with severe OSA had at least a 10% reduction in the frequency of loud snoring. The significant reduction in loud snoring (defined as at least one snore in a 30-s epoch > 50 dB) reported in this study conflicts with Bignold et al. report that supine avoidance does not reduce snoring.11 Differences in the characterization of loud snoring (50 dB vs. 70 dB) were likely attributed to differences in the measurement with an acoustic microphone at the neck vs. nasal prongs. The NPTD automatically excluded loud sounds acquired when actigraphy-based wake or upright times which were likely equivalent to the hand scoring of intensity employed by Bignold. The finding that over half of the patients with an apnea index < 10 exhibited an important reduction in snoring was consistent with Ravesloot's conclusion.2 Given that changes in loud snoring may be most useful in assessing the benefit of supine avoidance therapy in benign snorers, additional studies should be conducted to evaluate the benefit of position therapy in those who exhibit insufficient sleep disordered breathing during their diagnostic study to qualify for healthcare system provided OSA therapy.
The NPTD sensitivity to the detection of sleep improved slightly and its specificity with respect to detection of wake improved considerably during the follow-up PSG when subjects were delivered supine avoidance therapy. It is common for Bland-Altman plots comparing actigraphy to PSG to distribute most of the results above the zero line, indicating the bias toward over-reporting of sleep. Differences between NPTD and PSG were equally distributed above and below the zero line for TST, SE, and WASO. The initial under-reporting of SO was a result of automatic assignment of wake to the first 10 minutes after the device is turned on.
One of the important advantages of the NPTD, as compared to pillow-based position therapy devices, is the capability to monitor compliance and efficacy. Based on the percent time supine, number of supine attempts/night, number of times maximum feedback was required, and the average response to feedback, these data suggest subjects do not acclimate and become non-responsive to vibro-tactile feedback. These findings suggest, but do not definitively prove, the treatment outcomes were a result of the therapeutic impact of the device and not a training/behavioral effect. We found that those who responded slowly to feedback remained slow responders across the treatment period.
To overcome one of the limitations of this study, an evaluation is underway to assess long-term compliance with the NPTD. This study will allow us to assess changes in subjective measures and compliance across a six-month observation window. This study will also allow us to evaluate whether one can become behaviorally trained to avoid the supine position without feedback,22 or if position therapy will need to be delivered nightly to be effective, as with other OSA treatments. Another study is underway to evaluate the benefit of positional therapy in combination with suboptimal outcomes resulting from oral appliance therapy.
It is often presumed that that CPAP should be offered as the initial treatment option across all OSA severity ranges, and only patients with mild to moderate sleep disordered breathing who are non-compliant with CPAP are candidates for a position therapy (or other non-CPAP treatment). To challenge this conventional thinking, a randomized trial is required with control groups having either NPTD or CPAP. Endpoints based on utilization and subjective outcomes would be justified based on the assumption that a treatment that is 60% effective and used 100% of the time by a majority of patients may be more useful than a treatment that is 100% effective but used only 40% of the time by only half of the patients. The results from such a study, in combination with better profiling of patients during the diagnostic study, might contribute to adoption of a wider range of initial treatment options or combinations of therapy, and improved long-term outcomes.
DISCLOSURE STATEMENT
This study was funded by Advanced Brain Monitoring, Inc. All authors are salaried employees of Advanced Brain Monitoring, Inc., the sponsor of this research. As co-inventors of the neck-device, Levendowski, Popovic, and Westbrook would only benefit financially if the Night Shift intellectual property was acquired by a third party. None of the authors with this conflict were involved with subject recruitment, data acquisition, or data reduction. The results summarized and reported by these authors were submitted to the U.S. Food and Drug Administration, whereby civil or criminal penalties apply for untruthful and inaccurate statements. Complete Sleep Solutions was paid the standard fee for each PSG study and an hourly rate for performance of study-related recruitment tasks. The work was performed at Advanced Brain Monitoring, Inc. and Complete Sleep Solutions, Murrieta, CA.
ACKNOWEDGMENTS
The authors thank the staff at Complete Sleep Solutions for their assistance with subject recruitment, Kara Henninger for her assistance with recruitment, and Delmer Henninger, M.D., A.B.S.M., James Smith, R.P.S.G.T., and Glen Edwards, R.P.S.G.T., for their scoring and video editing. Additionally, the contributions of Brastislav Veljkovic, Zoran Matic, and Milenko Cventinovic were essential to the development of the NPTD.
REFERENCES
- 1.Oksenberg A, Khamaysi I, Silverberg DS, Tarasiuk A. Association of body position with the severity of apneic events in patients with severe nonpositional obstructive sleep apnea. Chest. 2000;118:1018–24. doi: 10.1378/chest.118.4.1018. [DOI] [PubMed] [Google Scholar]
- 2.Ravesloot MJ, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea – a review of the literature. Sleep Breath. 2013;1:39–49. doi: 10.1007/s11325-012-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mador MJ, Kufel TJ, Magalang UJ, Rejesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–37. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 4.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleeping position in obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2006;263:946–50. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 5.Benoist LB, Morong S, van Maanen JP, Hilgevoord AAJ, de Vries N. Evaluation of position dependency in non-apneic snorers. Eur Arch Otorhinolaryngol. 2014;271:189–94. doi: 10.1007/s00405-013-2570-5. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–14. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 7.Permut I, Diaz-Abad M, Chitila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs. continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–81. doi: 10.1378/chest.115.3.771. [DOI] [PubMed] [Google Scholar]
- 9.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17:771–9. doi: 10.1007/s11325-012-0764-5. [DOI] [PubMed] [Google Scholar]
- 11.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–83. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Maanen JP, Richard W, van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21:322–9. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 14.Levendowski DL, Veljkovic B, Seagraves S, Westbrook PR. Capability of a neck worn device to measure sleep/wake, airway position, and differentiate benign snoring from obstructive sleep apnea. J Clin Monit Comput. 2014 Mar 6; doi: 10.1007/s10877-014-9569-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kribbs NB, Pack AI, Kline RL, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 16.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 17.Levendowski DJ, Zack N, Rao S, et al. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath. 2009;13:163–7. doi: 10.1007/s11325-008-0214-6. [DOI] [PubMed] [Google Scholar]
- 18.Levendowski DJ, Steward D., Woodson BT, Olmstead R, Popovic D, Westbrook PR. The impact of obstructive sleep apnea variability measured in-lab versus in-home on sample size calculations. Int Arch Med. 2009;2:2. doi: 10.1186/1755-7682-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Kesteren ER, van Maanen JP, Hilgevoord AA, Laman DM, de Vries N. Quantitative effects of trunk and head position on the apnea hypopnea index in obstructive sleep apnea. Sleep. 2011;34:1075–81. doi: 10.5665/SLEEP.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collop NA, Tracy SL, Kapur V, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7:531–48. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JW, Enciso R, Levendowski DJ, Morgan TD, Westbrook PR, Clark TG. Treatment outcomes of mandibular advancement devices in positional and nonpositional OSA patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:724–31. doi: 10.1016/j.tripleo.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cartwright RD, Lloyd S, Lilie J, Kravtiz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1984;8:87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]