Abstract
Study Objectives:
The cardiovascular complications caused by obstructive sleep apnea (OSA) decrease after continuous positive airway pressure treatment (CPAP). Mean platelet volume (MPV), platelet distribution width (PDW), and red cell distribution width (RDW) are newly recognized tools for assessing cardiovascular risk.
Methods:
From a selection of patients with symptoms of nocturnal snoring and/or excessive daytime sleepiness, 36 males with an apnea-hypopnea index (AHI) > 30/h and 22 age-matched normal male controls with AHI < 5/h were included. Patients with OSA underwent another night of CPAP titration, and 11 patients were excluded at the 6-month evaluation due to poor compliance with the home CPAP therapy. Complete blood count parameters of compliant patients and the control group were evaluated.
Results:
Compared to controls, MPV values were significantly higher (p = 0.025) in OSA patients, but no significant differences in PDW or RDW were found (p > 0.05). Six months of CPAP therapy resulted in significantly lower MPV values but increased values of PDW and RDW in patients with severe OSA (p = 0.001, p = 0.007, p = 0.001, respectively).
Conclusion:
Our data suggest that complete blood count parameters in OSA patients such as MPV, PDW and RDW change significantly after CPAP therapy.
Citation:
Sökücü SN, Özdemir C, Dalar L, Karasulu L, Aydın Ş, Altın S. Complete blood count alterations after six months of continuous positive airway pressure treatment in patients with severe obstructive sleep apnea. J Clin Sleep Med 2014;10(8):873-878.
Keywords: continuous positive airway pressure, obstructive sleep apnea, mean platelet volume, platelet distribution width, red cell distribution width
Obstructive sleep apnea (OSA) is characterized by collapse of the upper airway during sleep, recurring apnea, intermittent hypoxemia, and daytime sleepiness. The severity of OSA is evaluated in terms of the number of apnea/hypopnea episodes per hour of sleep and is expressed as the apnea-hypopnea index (AHI).1
Red blood cell distribution width (RDW) is a numerical measure of the size variability of circulating erythrocytes and is routinely reported as a component of the complete blood count in the differential diagnosis of anemia. Disorders related to ineffective erythropoiesis or increased red blood cell destruction cause greater size heterogeneity and thus a higher RDW.2,3 Several studies have reported increased platelet activation and aggregation in patients with OSA.4,5 It has been shown that platelet size, as measured by mean platelet volume (MPV), correlates with platelet reactivity, and is regarded as an easy and useful tool for indirect monitoring of platelet activity. As larger platelets have greater thrombotic potential, the RDW, MPV, and platelet distribution width (PDW) have been shown to be related to cardiovascular morbidity and mortality in patients with cardiac diseases.6–8
Although the underlying mechanisms and etiologies are not completely understood, OSA can lead to important cardiovascular complications that decrease after CPAP therapy.9,10 In OSA patients, RDW, MPV, and PDW are newly recognized indicators of the severity of the condition that are associated with no additional cost compared to a routinely performed complete blood count.11–14
BRIEF SUMMARY
Current Knowledge/Study Rationale: Mean platelet volume (MPV), platelet distribution width (PDW), and red cell distribution width (RDW) are newly recognized tools for assessing cardiovascular risk. These markers increase in patients with OSA. The effect of CPAP treatment on these parameters was evaluated.
Study Impact: Our data suggest that complete blood count parameters such as MPV, PDW, and RDW changed significantly after CPAP therapy in patients with OSA. A complete blood count is simple to perform and can be useful in the follow up of CPAP therapy.
Continuous positive airway pressure (CPAP) therapy is the gold standard in patients with severe OSA. MPV is reduced after CPAP therapy in severe OSA patients, indicating that CPAP therapy has cardioprotective effects.15 To our knowledge, the effect of CPAP therapy on RDW and PDW in OSA patients has not been reported; thus, we decided to investigate this possibility.
METHODS
Patients
The patients and control subjects were selected consecutively from patients admitted to our hospital sleep clinic with symptoms of nocturnal snoring and/or excessive daytime sleepiness. All patients and control subjects underwent polysomnographic evaluations in our clinic between January 2012 and July 2012.
Demographic and health-related data, including age, sex, body mass index (BMI), and medical conditions, as well as medical histories regarding drug usage and cardiovascular disease were obtained from medical records. A physical examination, pulmonology function test, chest X-ray, and electrocardiography were performed before PSG, and chemistry values including glucose, creatinine, triglycerides, cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), as well as complete blood counts, were assessed.
Inclusion criteria included symptoms of nocturnal snoring and/or excessive day time sleepiness and an AHI > 30/h on the polysomnographic evaluation. The control group was composed of patients with the same complaints but with an AHI < 5/h. Exclusion criteria were any known cardiac disease, lung disease, diabetes (defined as fasting plasma glucose > 126 mg/dL and/ or antidiabetic treatment), chronic renal or hepatic diseases, use of acetylsalicylic acid or other anticoagulant therapy during the previous week, hemoglobin < 13 mg/dL (to exclude anemia), central sleep apnea syndrome diagnosed in the polysomnographic evaluation, and previous treatment for OSA.
Of 55 consecutive severe OSA patients, 36 male patients met the study criteria. The study group underwent another night of CPAP titration, and 11 patients were excluded at the 6-month evaluation due to poor compliance with the home CPAP therapy. Therefore, the study was completed with 25 male patients (mean age 47.44 ± 11.68 years; AHI 59.25 ± 18.99 events/h). The control group was composed of 21 age-matched consecutive male subjects (mean age 40.76 ± 11.62 years) without OSA (AHI < 5 events/h).
Informed written consent was obtained from all subjects. This study protocol was approved by the institutional ethics committee.
Measurement of Laboratory Parameters
Complete blood count values were measured in the study group 1 day after the polysomnographic evaluation and after 6 months of CPAP therapy at home. No intervention other than CPAP treatment was introduced at follow-up. Morning venous blood samples were drawn from participants after an overnight fast > 8 h, between 07:00 and 08:00 and analyzed within 1 hour. The RDW, MPV, PDW, and platelet count were determined using an Abbott Cell-Dyne 3700 System (Abbott Diagnostics, Santa Clara, CA, USA); a differential count was included as part of the complete blood cell count. Serum biochemical parameters were measured using a Beckman Coulter AU 2700 plus (Olympus, Tokyo, Japan).
Polysomnography
Overnight polysomnography, the gold standard diagnostic assessment, was performed using an Embla A-10 data acquisition and analysis system (Medcare Flaga, Reykjavik, Iceland) in the attended setting at a sleep laboratory under baseline conditions. The following physiological parameters were monitored: brain electrical activity on electroencephalography (with electrodes placed at C4-A1, C3-A2, O2-A1, and O1-A2); eye movements on electro-oculography; submental muscle activity on electromyography; ribcage and abdominal effort by respiratory inductive plethysmography (RIP; Xact-Trace, Medcare Flaga); body position by a calibrated sensor; snoring sounds by a piezoelectric sensor; oronasal flow by a nasal pressure cannula (Medcare Flaga); hemoglobin oxygen saturation (SpO2) by pulse oximetry (8000J; Nonin Medical, Plymouth, MN, USA) with the averaging time set at 3 s; and electrical activity of the heart by electrocardiography (lead II) sampled at 512 Hz. Sleep stages and arousals were scored according to standard criteria by a skilled pulmonary physician using the Somnologica Studio software package (Medcare Flaga). Apnea was defined as cessation of airflow for > 10 s and was classified as obstructive in the presence of continued movement on RIP or central in the absence of movement on RIP. Hypopnea was defined as a reduction > 50% in oronasal flow amplitude for > 10 s, accompanied by > 3% desaturation and/or arousal. Hypopnea was classified as obstructive when there was evidence of upper airway resistance, such as snoring, paradoxical motion in the respiratory bands, or inspiratory flow limitation indicated by nasal pressure signals.
Patients underwent a second night of polysomnography with CPAP for titration purposes. The optimal CPAP level was determined in the laboratory under supervision of a technician. It was defined as the lowest pressure at which the lowest number of respiratory events and arousals occurred with the highest sleep efficiency.
CPAP Therapy
All patients with severe OSA (AHI > 30/h) were treated with fixed pressure nasal CPAP. Data from the CPAP machines were downloaded after 6 months to assess treatment adherence. The number of hours logged on the integrated time recorder was evaluated. Patients were considered CPAP compliant if they used CPAP ≥ 4 h/night 5 days per week, and symptoms of snoring and daytime sleepiness were evaluated using the Epworth Sleepiness Scale (ESS).
Statistical Analysis
Data were analyzed with SPSS for Windows software (SPSS Inc., Chicago, IL, USA). All variables were tested for normality of the distribution using the Kolmogorov-Smirnov test. Continuous variables with normal distributions are expressed as means ± standard deviation. Continuous variables with non-normal distributions are summarized as medians (interquartile range, IQR). Categorical variables are expressed as numbers (percentages). Correlations between nonparametric variables were analyzed using Spearman correlation. Correlations between parametric variables were analyzed using Pearson correlation. Comparisons between independent groups for the values that were normally distributed were conducted using Student t-test and between values not normally distributed using the Mann-Whitney U-test. Categorical variables were compared using the χ2 test.
The MPV, PDW, and CRP were not normally distributed, so the Wilcoxon test was used to compare values before and after CPAP treatment. A p < 0.05 indicated statistical significance.
RESULTS
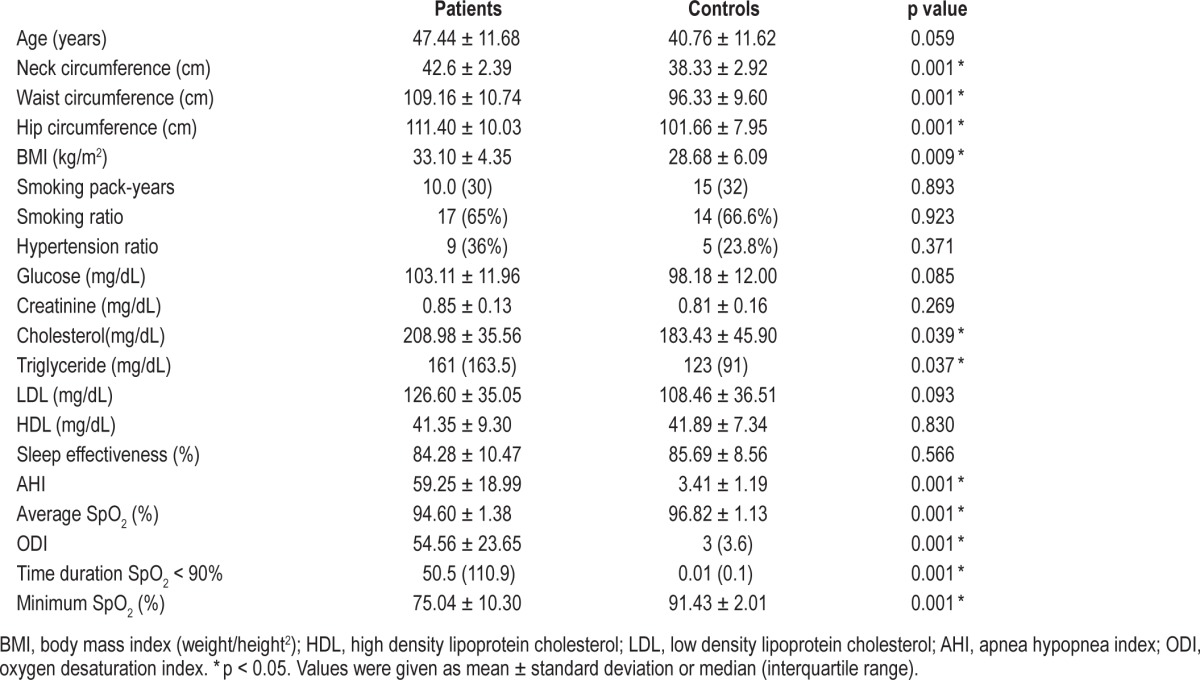
The characteristics of the study subjects and controls are shown in Table 1. No significant differences were observed between the controls and severe OSA patients in terms of smoking ratio, pack-year smoking history, hypertension ratio, glucose, creatinine, HDL, or LDL. However, BMI was significantly higher in the study group than that in the control group (p = 0.009). Fixed CPAP pressures used by the patients ranged from 6 to 12 cm H2O (mean 9 ± 1.53 cm H2O).
Table 1.
Properties of study cases and controls
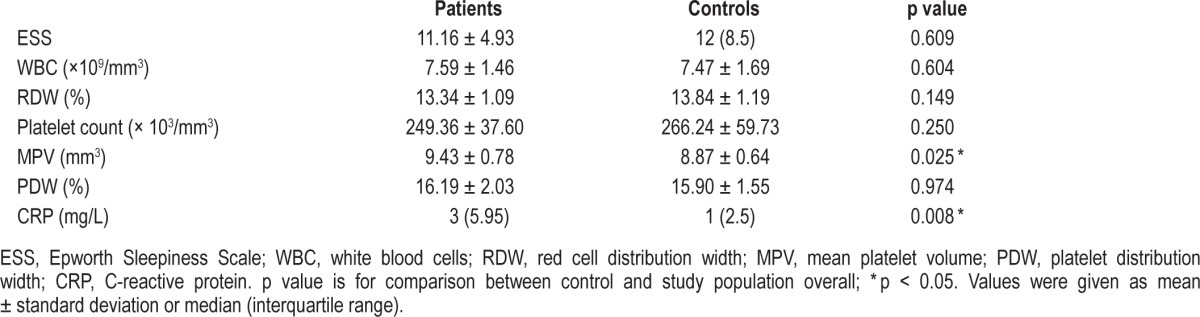
No significant difference was observed between controls and OSA patients in terms of ESS, RDW, platelet number, or PDW at the beginning of the study, but a significant difference existed between MPV and CRP in the 2 groups at admission (p = 0.025, p = 0.008) (Table 2).
Table 2.
Comparison of control cases and OSA patients at baseline
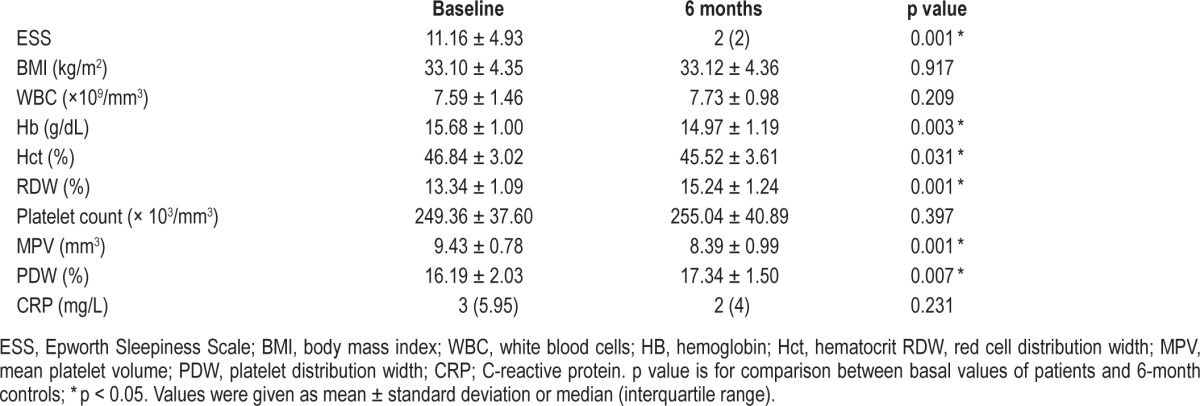
The ESS of the patients before treatment was 11.16 ± 4.93, whereas that of the control group was 12 (IQR 8.5; p = 0.609). After CPAP treatment, the ESS was 2 (IQR 2) in the severe OSA group, a statistically significant difference from the initial value (p = 0.001). CPAP therapy resulted in a significantly lower MPV but increased PDW and RDW in patients with severe OSA after 6 months (p = 0.001; p = 0.007; p = 0.001). The effects of 6 months of CPAP treatment on hemoglobin, hematocrit (Hct), RDW, MPV, platelet count, PDW, and CRP are shown in Table 3.
Table 3.
Effects of 6 months of CPAP treatment on severe OSA patients
DISCUSSION
In the present study, we investigated the effects of nasal CPAP therapy on blood count parameters, including MPV, RDW, PDW, and platelet count. No difference was found between RDW and PDW when the severe OSA group was compared with the control group, whereas MPV increased in the OSAS group. We found that 6 months of CPAP therapy significantly reduced MPV while increasing the RDW and PDW in patients with severe OSA.
New indices related to erythrocytes and platelet counts have been investigated and MPV and PDW and their association with RDW have gained importance.16 All of these parameters are useful clinical markers of a variety of cardiovascular and thrombotic diseases.13,14,17
The mechanism of platelet activation in patients with OSA is controversial. The most probable mechanism is that hypoxia and repetitive arousal from sleep increase sympathetic activity, and the resulting elevated circulating catecholamines cause concentration-dependent platelet activation.18–20 Another likely mechanism is a direct effect of chronic intermittent hypoxia on platelet activation. MPV, an indicator of platelet activation, was significantly higher in patients with severe OSA than that in control subjects, and MPV is correlated with AHI and the desaturation index.13,14 The association between MPV and the risk and prognosis of cardiovascular diseases has been demonstrated in a previous study.21 CPAP is known to decrease cardiovascular risks by decreasing ambulatory blood pressure and arterial stiffness and increasing the sensitivity of the arterial baroreflex.22 Additionally, CPAP relieves airway obstruction and hypoxia, as well as decrease airway and systemic inflammation and the levels of OSA induced inflammatory mediators.23 CPAP treatment decreased platelet activation. Adenosine diphosphate-induced platelet aggregability increased significantly in patients with moderate-to-severe OSA compared to that in controls; CPAP treatment improves platelet aggregability in these patients.24 Similar to our results one study found that 6 months of CPAP therapy significantly reduced MPV values in patients with severe OSA.15 The decreased MPV could be explained by decreased hypoxia and inflammation, as postulated in that study, which could help to decrease the frequency of cardiovascular complications in OSA patients. Varol et al. reported that platelet counts were significantly lower in patients with severe OSA than those in a control group, and that platelet counts after 6 months of CPAP were significantly higher than baseline values.15 In contrast, we found no difference in platelet number between controls and the OSA group after 6 months of CPAP treatment, but the decrease in MPV was marked.
As elevated inflammatory markers have been reported in OSA patients, inflammation25 is one of the postulated links between OSA and increased cardiovascular morbidity. CPAP is known to reduce inflammation in OSA patients. Inflammation is strongly related to ineffective erythropoiesis and inflammatory cytokine production, which desensitizes bone marrow erythroid progenitors to erythropoiesis, inhibits RBC maturation, and promotes anisocytosis.26 Accordingly, RDW increases in many inflammatory diseases and also in OSA.11,12 The RDW is positively related to markers such as CRP, erythrocyte sedimentation rate, interleukin-6, soluble transferin receptor, soluble tumor necrosis factor (TNF) receptor I, and soluble TNF receptor II.17 In the present study, RDW and PDW levels were similar in the control and severe OSA groups, and both RDW and PDW levels increased, albeit not significantly, in the severe OSAS group, following 6 months of CPAP. MPV is the best known of the platelet parameters, whereas PDW is less documented and derived from direct flow cytometric measurements of platelet cell volume. Because PDW is a quantitative assessment of platelet size and volume, it has limited use.22 However, unlike our previous study in which we found that RDW was high in patients with OSA, this finding was not verified in this small group of patients, which could be ascribed to the small sample size in this study.12
The RDW was higher in OSA patients as a result of either ineffective erythropoiesis due to chronic inflammation or enhanced erythropoiesis stimulated by increased production of erythropoietin (EPO), as hypothesized in coronary artery disease patients.27,28 Although inflammation parameters decrease after treatment, as reported by Zamarron et al., some (such as endothelin, von Willebrand factor, and e-selectin values) were unchanged after 1 year of CPAP therapy.29 Similar to these parameters, PDW and RDW also did not decrease after treatment. This could explain why these parameters remained at the same level. The explanation for the increases in PDW and RDW may be activation of hypoxia inducible factor 1 (HIF-1), which is activated by sustained hypoxia, resulting in increased expression of erythropoietin.30 Yuan et al. reported that more severe intermittent hypoxia and reoxygenation cycles result in HIF-1 activation in a manner dependent on activation of Ca2+ calmodulin kinase.31 Ryan et al. found no significant difference in serum erythropoietin levels between patients and control subjects or between OSA patients before and after CPAP therapy, while a significant difference in TNF-α levels was identified. Ryan et al. presented this as evidence for selective activation of nuclear factor kappa beta over HIF-1 and production of TNF-α over EPO.32 If EPO levels do not decrease after CPAP therapy in patients with OSA, then RDW could remain high, as observed in the present study.
The severity of OSA is significantly associated with increased hematocrit, even after controlling for possible confounding factors, but does not usually lead to clinical polycythemia.33 In a study conducted by Khan et al., hematocrit changed significantly in both sexes after 1 year of CPAP treatment. Hematocrit declined from 40.7% to 39.1% and from 38.1% to 37.6% in males and females, respectively, after treatment.34 In our patient groups, hematocrit decreased significantly after treatment. Among the entire cohort, anemia did not occur before OSA treatment but did after OSA treatment.34 Similar to their study, we found that hematocrit values decreased after 6 months of CPAP therapy. When the balance between inflammation and hypoxia-induced erythropoiesis was tipped toward hypoxia-induced erythropoiesis, PAP treatment attenuated hypoxia and EPO levels, and hematocrit declined. The increase in RDW and PDW could simply be a surrogate marker for the decrease in hematocrit levels.
BMI was a predictor of blood viscosity, likely due to its correlation with plasma viscosity and red blood cell aggregation.35 In a study carried out by Irace et al., plasma viscosity was directly associated with LDL cholesterol and inversely associated with HDL cholesterol, whereas triglycerides did not seem to have important effects unless values were greater than 400 mg/dL. However, the contribution of LDL and HDL cholesterol to plasma viscosity seems quite limited.36 These recent data suggest that both BMI and abdominal adiposity induce hyper-viscosity, whereas the effect of cholesterol and triglycerides is limited. Thus, the effect of hyperviscosity on MPV, RDW, and PDW in OSA patients is more complex then estimated previously. However, this could have affected the comparison between our OSA patients and controls. Because BMI did not change significantly in the study group after therapy, this could not explain the change in blood parameters; therefore, further studies on this subject are needed.
An increase in blood viscosity in OSA patients and a decrease following CPAP treatment was demonstrated in previous studies.37,38 The relationship between MPV and blood viscosity and the decrease in both MPV and blood viscosity values with CPAP treatment may influence protection against the cardiovascular complications that can develop in association with OSAS.39
This study was designed to show the effect of CPAP treatment on MPV, RDW, and PDW, which are significantly different in severe OSA patients. It was reported in an earlier study that subjects with poor adherence most frequently overestimate their CPAP use time.40 Thus, instead of relying on patient reports of usage, we used card data from the device as an objective measure of adherence to the therapy, and more than 4 hours use/night for at least 5 days/week was accepted as compliance with the therapy.40
A limitation of our study was the small sample size. Another limitation is that cases compliant with CPAP treatment were not compared with those who were not compliant. In addition, our study design was unable to explain the increase in RDW and PDW values observed after CPAP treatment. Unlike those in the study of Varol et al., our patients used fixed-level, not auto-CPAP therapy.
We demonstrated that 6 months of CPAP therapy significantly lowered MPV values but increased RDW and PDW in patients with severe OSA. Our results suggest that OSA may lead to platelet changes and that CPAP treatment may lower the MPV. This result shows that a reduction in the MPV is a measure of the cardioprotective effect of CPAP in patients with OSAS. CPAP treatment should be investigated further in terms of its hematologic effects and potential implications.
DISCLOSURE STATEMENT
This was not an industry supported study. The study took place in Yedikule Chest Disease and Thoracic Surgery Training and Research Hospital, Sleep Laboratory, Istanbul, Turkey. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 2.Romero AJ, Carbia CD, Ceballo MF, Diaz NB. Red cell distribution width (RDW): its use in the characterization of microcytic and hypochromic anemias. Medicina. 1999;59:17–22. [PubMed] [Google Scholar]
- 3.Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–8. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 4.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol. 2011;178:475–81. doi: 10.1016/j.resp.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Kuwahira I, Shimizu M, et al. Significant relationship between platelet activation and apnea-hypopnea index in patients with obstructive sleep apnea syndrome. Tokai J Exp Clin Med. 2011;36:79–83. [PubMed] [Google Scholar]
- 6.Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9:177–90. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets. 2002;13:301–6. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 8.Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–94. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lattimore JD, Celermajer DS, Wilcow I. Obstructive sleepapnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–37. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Ozsu S, Abul Y, Gulsoy A, Bulbul Y, Yaman S, Ozlu T. Red cell distribution width in patients with obstructive sleep apnea syndrome. Lung. 2012;190:319–26. doi: 10.1007/s00408-012-9376-x. [DOI] [PubMed] [Google Scholar]
- 12.Sökücü SN, Karasulu L, Dalar L, Seyhan EC, Altın S. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med. 2012;8:521–5. doi: 10.5664/jcsm.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varol E, Ozturk O, Gonca T, et al. A Mean platelet volume is increased in patients with severe obstructive sleep apnea. Scand J Clin Lab Invest. 2010;70:497–502. doi: 10.3109/00365513.2010.520733. [DOI] [PubMed] [Google Scholar]
- 14.Nena E, Papanas N, Steiropoulos P, Zikidou P, Zarogoulidis P, Pita E. Mean platelet volume and platelet distribution width in non-diabetic subjects with obstructive sleep apnoea syndrome: New indices of severity? Platelets. 2012;23:447–54. doi: 10.3109/09537104.2011.632031. [DOI] [PubMed] [Google Scholar]
- 15.Varol E, Ozturk O, Yucel H, et al. The effects of continuous positive airway pressure therapy on mean platelet volume in patients with obstructive sleep apnea. Platelets. 2011;22:552–6. doi: 10.3109/09537104.2011.578182. [DOI] [PubMed] [Google Scholar]
- 16.Wiwanitkit V. Plateletcrit, mean platelet volume, platelet distribution width: ıts expected values and correlation with parallel red blood cell parameters. Clin Appl Thromb Hemost. 2004;10:175–8. doi: 10.1177/107602960401000208. [DOI] [PubMed] [Google Scholar]
- 17.Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011;50:635–41. doi: 10.1515/cclm.2011.831. [DOI] [PubMed] [Google Scholar]
- 18.Vizioli L, Muscari S, Muscari A. The relationship of mean platelet volume with the risk and prognosis of cardiovascular diseases. Int J Clin Pract. 2009;63:1509–15. doi: 10.1111/j.1742-1241.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler MG, Nelesen R, Mills P, Ancoli-Israel S, Kennedy B, Dimsdale JE. Sleep apnea, norepinephrine release rate, and daytime hypertension. Sleep. 1997;20:224–31. doi: 10.1093/sleep/20.3.224. [DOI] [PubMed] [Google Scholar]
- 20.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 22.Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med. 1993;13:937–50. [PubMed] [Google Scholar]
- 23.Karamanlı H, Ozol D, Uğur KS, et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. 2014;18:251–6. doi: 10.1007/s11325-012-0761-8. [DOI] [PubMed] [Google Scholar]
- 24.Oga T, Chin K, Tabuchi A, et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J Atheroscler Thromb. 2009;16:862–9. doi: 10.5551/jat.2188. [DOI] [PubMed] [Google Scholar]
- 25.Park Y, Schoene N, Haris W. Mean platelet volume as anindicator of platelet activation: Methodological issues. Platelets. 2002;13:301–6. doi: 10.1080/095371002220148332. [DOI] [PubMed] [Google Scholar]
- 26.Lattimore JD, Celermajer DS, Wilcow I. Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol. 2003;41:1429–37. doi: 10.1016/s0735-1097(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 27.Macdougall IC, Cooper A. The inflammatory response and epoetin sensitivity. Nephrol Dial Transplant. 2002;17(Suppl 1):48–52. doi: 10.1093/ndt/17.suppl_1.48. [DOI] [PubMed] [Google Scholar]
- 28.Fukut H, Ohte N, Mukai S, et al. Elevated plasma levels of B-type natriuretic peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J. 2009;50:301–12. doi: 10.1536/ihj.50.301. [DOI] [PubMed] [Google Scholar]
- 29.Zamarron C, Riveiro A, Gude F. Circulating levels of vascular endothelial markers in obstructive sleep apnoea syndrome. Effects of nasal continuous positive airway pressure. Arch Med Sci. 2011;6:1023–8. doi: 10.5114/aoms.2011.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schofield CJ, Ratcliffe PJ. Oxygen sensing HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 31.Yuan G, Nanduri J, Bhasker RC, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–8. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 32.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 33.Choi JB, Loredo JS, Norman D, et al. Does obstructive sleep apnea increase hematocrit? Sleep Breath. 2006;10:155–60. doi: 10.1007/s11325-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 34.Khan AM, Ashizawa S, Hlebowicz V, Appel DW. Anemia of aging and obstructive sleep apnea. Sleep Breath. 2011;15:29–34. doi: 10.1007/s11325-010-0326-7. [DOI] [PubMed] [Google Scholar]
- 35.Brun JF, Varlet-Marie E, Raynaud de Mauverger E, Mercier J. Both overall adiposity and abdominal adiposity increase blood viscosity by separate mechanisms. Clin Hemorheol Microcirc. 2011;48:257–63. doi: 10.3233/CH-2011-1418. [DOI] [PubMed] [Google Scholar]
- 36.Irace C, Carallo C, Scavelli F, et al. Influence of blood lipids on plasma and blood viscosity. Clin Hemorheol Microcirc. 2013 Feb 27; doi: 10.3233/CH-131705. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Dikmenoğlu N, Çiftçi B, Ileri E, et al. Erythrocyte deformability, plasma viscosity and oxidative status in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2006;7:255–61. doi: 10.1016/j.sleep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Tazbirek M, Slowinska L, Skoczynski S, Pierzchala W. Short-term continuous positive airway pressure therapy reverses the pathological influence of obstructive sleep apnea on blood rheology parameters. Clin Hemorheol Microcirc. 2009;41:241–9. doi: 10.3233/CH-2009-1175. [DOI] [PubMed] [Google Scholar]
- 39.Senen K, Topal E, Kilinc E, et al. Plasma viscosity and mean platelet volume in patients undergoing coronary angiography. Clin Hemorheol Microcirc. 2010;44:35–41. doi: 10.3233/CH-2010-1249. [DOI] [PubMed] [Google Scholar]
- 40.Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnea: implications for future interventions. Indian J Med Res. 2010;131:245–58. [PMC free article] [PubMed] [Google Scholar]


