Abstract
Study Objectives:
Pediatric obstructive sleep apnea (OSA) is associated with hyperactive behavior, cognitive deficits, psychiatric morbidity, and sleepiness, but objective polysomnographic measures of OSA presence or severity among children scheduled for adenotonsillectomy have not explained why. To assess whether sleep fragmentation might explain neurobehavioral outcomes, we prospectively assessed the predictive value of standard arousals and also respiratory cycle-related EEG changes (RCREC), thought to reflect inspiratory microarousals.
Methods:
Washtenaw County Adenotonsillectomy Cohort II participants included children (ages 3-12 years) scheduled for adenotonsillectomy, for any clinical indication. At enrollment and again 7.2 ± 0.9 (SD) months later, children had polysomnography, a multiple sleep latency test, parent-completed behavioral rating scales, cognitive testing, and psychiatric evaluation. The RCREC were computed as previously described for delta, theta, alpha, sigma, and beta EEG frequency bands.
Results:
Participants included 133 children, 109 with OSA (apnea-hypopnea index [AHI] ≥ 1.5, mean 8.3 ± 10.6) and 24 without OSA (AHI 0.9 ± 0.3). At baseline, the arousal index and RCREC showed no consistent, significant associations with neurobehavioral morbidities, among all subjects or the 109 with OSA. At follow-up, the arousal index, RCREC, and neurobehavioral measures all tended to improve, but neither baseline measure of sleep fragmentation effectively predicted outcomes (all p > 0.05, with only scattered exceptions, among all subjects or those with OSA).
Conclusion:
Sleep fragmentation, as reflected by standard arousals or by RCREC, appears unlikely to explain neurobehavioral morbidity among children who undergo adenotonsillectomy.
Clinical Trial Registration:
ClinicalTrials.gov, ID: NCT00233194
Citation:
Chervin RD, Garetz SL, Ruzicka DL, Hodges EK, Giordani BJ, Dillon JE, Felt BT, Hoban TF, Guire KE, O'Brien LM, Burns JW. Do respiratory cycle-related EEG changes or arousals from sleep predict neurobehavioral deficits and response to adenotonsillectomy in children?. J Clin Sleep Med 2014;10(8):903-911.
Keywords: obstructive sleep apnea, polysomnography, child, respiratory cycle-related EEG changes, arousals, cognition, behavior, sleepiness
Neurobehavioral morbidity is among the most prominent manifestations of obstructive sleep apnea (OSA), in adults or children. Exactly what causes adverse cognitive and behavioral outcomes, however, remains incompletely understood. Recommended evaluations for children suspected to have OSA include objective testing, usually by polysomnography.1 However, studies to date have had difficulty showing that polysomnographic results predict the hyperactive behavior, cognitive deficits, psychiatric morbidity, or sleepiness that accompany OSA, or their improvement after OSA is treated.2–8 For example, in a previous cohort of children investigated before and after adenotonsillectomy, usually for suspected OSA, our group showed that standard polysomnographic measures do not provide strong, if any, predictive value for neurobehavioral morbidity associated with OSA, or for resolution of that morbidity after adenotonsillectomy.2 Children without objective evidence for OSA on polysomnography, in comparison to those with OSA, showed at least as much neurobehavioral improvement after adenotonsillectomy.9 A 22-item validated parental questionnaire scale for childhood OSA, contained within the Pediatric Sleep Questionnaire, predicted neurobehavioral outcomes as well or better than did standard polysomnographic measures.10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) in children is associated with neurobehavioral morbidity, but common polysomnographic measures of OSA presence or severity do not predict which children experience such morbidity or improvement in these areas after adenotonsillectomy. This study was performed to assess whether sleep fragmentation—in the form of standardly scored arousals or respiratory cycle-related EEG changes (RCREC) believed to reflect inspiratory microarousals—might improve predictive utility of sleep studies.
Study Impact: Cognition, behavior, sleepiness, and mental health generally improved 6 months after adenotonsillectomy, but rates of arousals and magnitude of RCREC at baseline did not consistently predict outcomes. These measures of sleep fragmentation do not appear to explain the neurobehavioral morbidity of pediatric OSA, or to improve the prognostic utility of polysomnography for these outcomes.
In an effort to improve the predictive value of polysomnography, we developed a computer algorithm to demonstrate and quantify EEG changes that occur in synchrony with the average, non-apneic respiratory cycle.11 The first demonstration of this algorithm proved the existence of respiratory cycle-related EEG changes (RCREC) and suggested in a small sample of 10 children that the new measure could help to explain sleepiness even when the rate of apneic events (the respiratory disturbance index, or RDI) does not.12 Studies in adults showed that RCREC, at least in the sigma EEG frequency ranges, explained substantial amounts of objectively assessed daytime sleepiness, beyond that explained by combinations of the best measures derived from polysomnography.13 Furthermore, the observations that sigma power increased on average during inspiration while delta power simultaneously decreased suggested that the RCREC do reflect inspiratory microarousals, possibly associated with the work of breathing. In our previous Washtenaw Adenotonsillectomy Cohort of children studied before and after adenotonsillectomy, preoperative sigma and beta range RCREC predicted parent-rated daytime sleepiness independently of the apnea-hypopnea index.14 In subsequent studies of adults, correlation of RCREC with esophageal pressure measurements during sleep,15 and reduction of RCREC with application of continuous positive airway pressure,16 provided additional evidence to suggest that RCREC represent subtle, but numerous and consequential inspiratory microarousals.
As sleep fragmentation—whether visible, in the form of standard 3-second arousals, or in the form of RCREC—may well play an important role in the neurobehavioral morbidity that characterizes childhood OSA, we studied this question in a new cohort of subjects scheduled for adenotonsillectomy for clinical indications. At baseline and 7 months later, about 6 months after surgery, we assessed the subjects for standard arousals and RCREC, along with key neurobehavioral comorbidities at each time point. We tested the hypotheses that arousals and RCREC would predict neurobehavioral morbidity at baseline, and improvement at follow-up. Some of these subjects, members of the Washtenaw County Adenotonsillectomy Cohort II, also provided data for a previous publication that focused on work of breathing, assessed through quantitative esophageal pressure monitoring, as another potential strategy to improve predictive value of laboratory-based pediatric polysomnography.17
METHODS
Overview
Subjects were recruited from the 2 largest otolaryngology practices in Washtenaw County, Michigan, for this institutional review board (IRBMED) approved study. Clinical staff helped to identify families with children, aged 3.0 to 12.9 years, who were scheduled for adenotonsillectomy for any clinical indication, but as usual,18 were not thought to need sleep studies prior to the procedure. Exclusion criteria, detailed previously,17 included medical, mental, or physical conditions that might impede interpretation of EEG or neurobehavioral data; clinicians' need for polysomnography (estimated to exclude < 5% of all potential patients of these surgeons); current or past treatment for OSA; medical conditions or syndromes with high risk of OSA or daytime sleepiness; or imminent expectation of further surgery or family relocation. One aim of this study was to examine a sample of children that overall reflects the mild sleep apnea characteristic of the 500,000-plus who undergo adenotonsillectomy each year in the US.19 Subjects with and without OSA on polysomnography were therefore included.
A parent signed a written informed consent, and each child signed assent. Sleep and neurobehavioral assessments were then completed up to 3 days before the adenotonsillectomy, and again at a date targeted to fall about 6 months thereafter. A mental health professional (child psychiatrist, child psychologist, or behavioral developmental pediatrician) interviewed each family. A full, nocturnal, laboratory-based polysomnogram was followed on the next day by a multiple sleep latency test (MSLT). Between naps, children were given neuropsychological testing. A parent completed behavioral rating scales and a standard socioeconomic survey.20 At each of the 2 major testing periods, children were given a $25 gift certificate to a local toy store, and parents were given $125 for their time and effort.
As described in detail previously,17 pediatric polysomnography conformed to standard recommendations,21 published after the start of this research protocol, except that piezoelectric strain gauges rather than inductance plethysmography were used to monitor thoracic and abdominal excursion. Esophageal pressure was monitored through a water-filled, 6-French pediatric feeding tube.22,23 MSLTs followed standard procedures,24 except that to accommodate these young pediatric research subjects, 4 naps were performed instead of 5, and nap opportunities were lengthened from the adult standard (20 minutes) to 30 minutes.25,26
Scoring
All sleep studies were scored, or in a minority of instances, thoroughly rescored, by a single pediatric-experienced sleep and electroencephalography-registered technologist. To prevent bias and minimize any effect of scoring drift with time, all scoring was performed in batches that each contained the pre- and post-adenotonsillectomy studies of several subjects, all de-identified, and without access to other study measures. Sleep staging followed standard criteria.21 Obstructive apneas (≥ 2 respiratory cycles in duration), hypopneas, and central apneas were scored following pediatric criteria recommended by the American Academy of Sleep Medicine (AASM) in 2007. The apnea-hypopnea index was calculated as the number of pediatric (≥ 2 breaths) apneas and hypopneas per hour of sleep. An apnea-hypopnea index > 1, operationalized more precisely as ≥ 1.5 for this study, was used to identify children with OSA.27 The AASM 2007 manual criteria were also used to identify 3-sec arousals, recommended for children as well as adults. In MSLTs, the mean sleep latency across all nap opportunities provided an objective measure of daytime sleepiness. A relatively low mean sleep latency, in the absence of established cutoffs for an MSLT with 30-min naps, was defined solely for the purpose of current analyses as being within the lower half of values recorded.
Computation of Respiratory Cycle-Related EEG Changes (RCREC)
The RCREC were computed for delta, theta, alpha, beta, and sigma EEG frequency ranges as previously detailed and diagrammed.15 The nasal-oral airflow signal, digitally filtered to pass frequencies between 0.13 Hz (8 cycles per minute) and 0.5 Hz (30 cycles per minute), was used for the respiration signal. The data were bandpass filtered to reduce noisy artifacts observed in the collected data. A computer algorithm implemented in MatLab (Mathworks, Natick, MA) used the filtered nasal-oral airflow signal to divide each respiratory cycle into 4 segments based on peaks, troughs, and mid-line crossings: early inspiration, late inspiration, early expiration, and late expiration. As in our previous studies, to avoid analysis of RCREC during sleep time occupied by apneas, hypopneas, and airflow signal artifacts, only respiration cycles with airflow amplitudes and durations between the 5th and 95th percentile were used in the calculations. The frequency-specific EEG power in the C3-A2 lead was computed during each respiratory cycle segment, and normalized to the frequency-specific EEG power for the entire relevant respiratory cycle. Normalized power for each of the 4 respiratory cycle segments was averaged over all respiratory cycles in the first 3 h of sleep, and the difference between the maximum and minimum segment-specific average normalized power was taken as a measure of the respiratory cycle-related EEG changes (RCREC) for a given subject. The RCREC assess, in short, the extent to which EEG signal power varies in synchrony with the respiratory cycle. As noted above, the RCREC are thought to reflect brief but numerous inspiratory microarousals that are magnified in response to labored breathing through a constricted upper airway.
Neurobehavioral Outcomes
Standardized, well-validated assessments were used to identify DSM-IV diagnoses, behavioral problems, and cognitive deficits long considered to reflect the most important morbidity in childhood SDB.28–34 Psychiatric assessments included the Computerized Diagnostic Interview Schedule for Children–Parent,35–37 and the Children's Psychiatric Rating Scale.38–40 The final categorical diagnostic outcome variable, however, was presence or absence of a DSM-IV-defined disruptive behavior disorder—attention deficit hyperactivity disorder, conduct disorder, or oppositional-defiant disorder—as determined by the interviewing clinician.
A composite behavioral hyperactivity index2 (mean 50; SD 10) was constructed from the average of the attention deficit hyperactivity disorder T-scores produced by each of two validated parental rating scales: the Conners' Parent Rating Scales41 and the Child Symptom Inventory-442 (or the Early Childhood Inventory-443 for children between 3 and 5 years). On the resulting behavioral hyperactivity index, higher scores indicated more significant symptoms. Although the 2 component scales cover similar domains of behavior, item presentation and content is somewhat different in each; their combined use was planned, as in our past research,2 to strengthen construct validity.
Finally, cognitive testing for a total of about 2 hours included the NEPSY,44 a developmental neuropsychological test battery created for children aged 3-12 years. From the NEPSY, the Memory and Learning Score and the Attention/Executive Functions Score (each with normal population mean 100, SD 15) were averaged to create a composite Cognitive Index, for which higher scores indicate better performance. These NEPSY subtests were selected in part because similar tests of word list learning, memory for faces, and executive functioning were found to improve after AT in our previous research.9 These NEPSY subtests also correlated with some measures of sleep architecture in a previous study of hyperactive children.4 Additional cognitive tests included the Stanford Binet 5th Edition (full-scale intelligence quotient, with mean 100, SD 15);45 the WIAT-II (average of reading comprehension and math reasoning age-base standard scores, mean 100, SD 15 for each);46 and the Continuous Performance Test-Second Edition (CPT-II47 or Kiddie CPT48 for children aged 3 or 4 years; average of omissions t score, commissions t score, and variability t score, each with mean 50, SD 10, higher scores being less desirable).
Analyses
For the current analyses, data were used for any subject who had complete baseline polysomnography with total sleep time > 6 h, behavioral ratings, cognitive testing with the NEPSY, psychiatric assessments, and MSLTs. Ninety-five percent of these subjects had follow-up assessments, and among subjects with follow-up, missing data for each individual measure were minimal. The pre-specified primary explanatory variables were the arousal index (arousals per hour of sleep) and delta RCREC, which had shown initial promise as a predictor of improvements in both MSLT results12 and parent rating scales for hyperactive behavior49 after adenotonsillectomy in children. The primary outcome variable was the behavioral hyperactivity index. As outcomes were highly similar for children with and without OSA, main results are presented for analyses on the entire group, as originally planned, to maximize power and range of explanatory variables. Secondary analyses were computed within subsamples of children with baseline AHI ≥ 1.5 and AHI ≥ 5.0.
Baseline associations were assessed with general linear regression models when neurobehavioral measures showed normal distributions. When these measures were non-normally distributed, they were dichotomized and logistic regression models were used. Similar approaches were then used to examine polysomnographic predictors of post-adenotonsillectomy changes in each neurobehavioral outcome measure. All models of baseline outcomes and their changes were adjusted for several pre-specified potential confounders at baseline, including age, gender, body mass index z-score, socioeconomic class, and periodic leg movement index. The level of significance was set at p < 0.05. Results were not adjusted for multiple comparisons, to maintain sensitivity for associations in this early-stage effort to detect any possible relationships between measures of sleep fragmentation and neurobehavioral outcomes. All computations were performed with SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Subjects
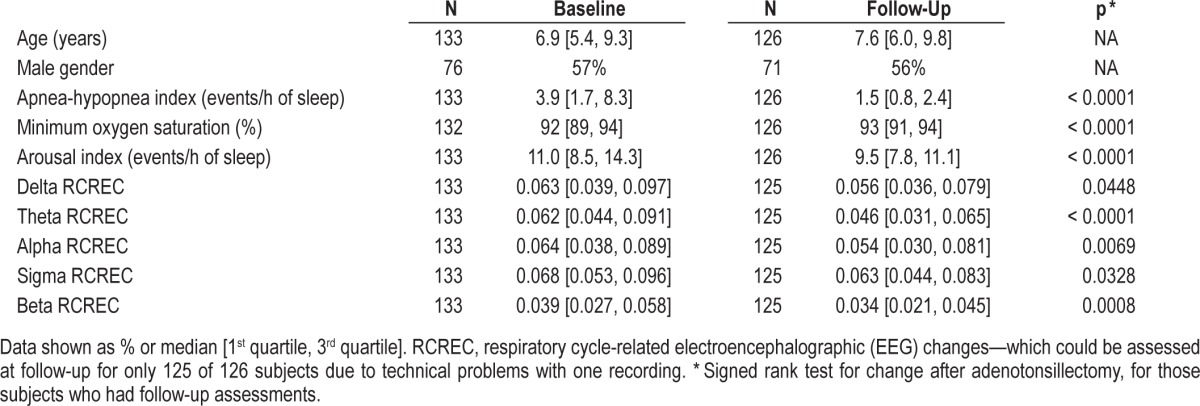
Recruitment and identification of subjects for this research is summarized in Figure 1. For the 133 subjects whose data form the basis for this report, demographic and polysomnographic explanatory variables at baseline are shown in Table 1. At baseline, 109 of 133 subjects (82%) had OSA, with a mean apnea-hypopnea index of 8.3 ± 10.6 (SD). This reflects a mild to moderate level, though all severities were represented and the apnea-hypopnea index ranged up to 81 events per hour of sleep. The 24 subjects without OSA showed a mean apneahypopnea index of 0.9 ± 0.3. Subjects with OSA, in comparison to those without OSA, had more frequent arousals and demonstrably worse (larger) RCREC in beta frequency bands, but not delta, theta, alpha, or sigma.
Figure 1. Identification of n = 133 subjects whose data were used for baseline analyses, and n = 126 children for whom assessments were repeated at follow-up.
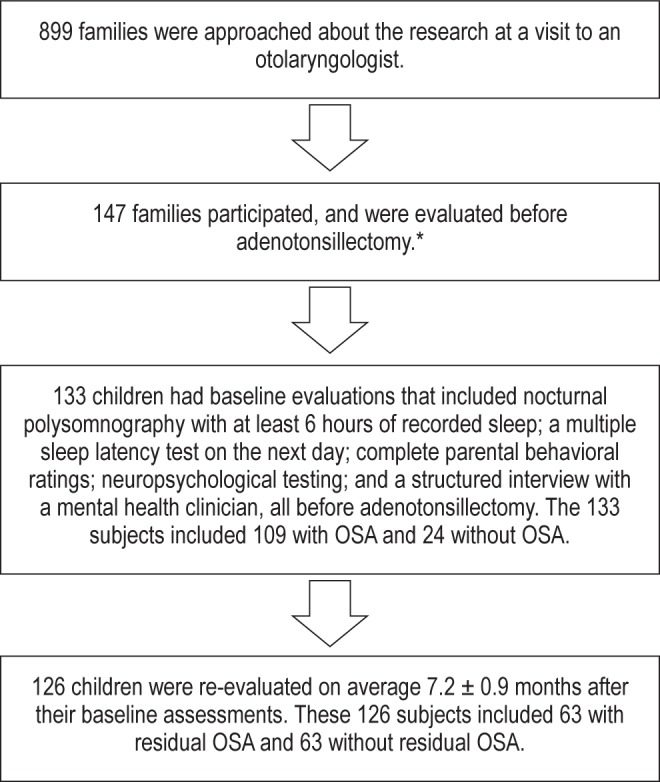
*The most common reasons that families cited when they declined to participate were lack of time, desire to avoid any additional stress, or lack of interest in research.
Table 1.
Demographic and polysomnographic data for 133 subjects with and without OSA (apnea-hypopnea index ≥ 1.5) at baseline.
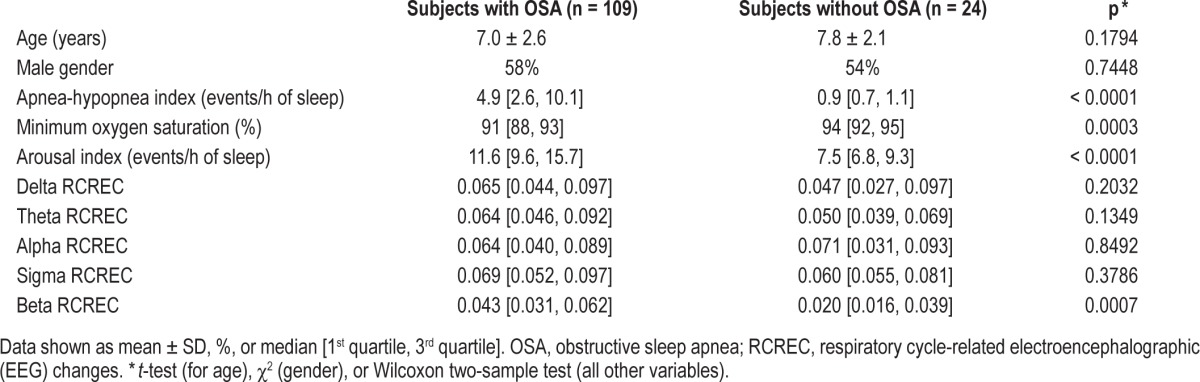
Table 2 shows the same demographic and polysomnographic variables at baseline and follow-up for the entire sample. Both the apnea-hypopnea index and minimum oxygen saturation clearly improved by follow-up, 7.2 ± 0.9 (SD) months after initial assessments. At follow-up, 63 of 126 retested subjects (50%) still qualified for OSA (p < 0.0001 for change in frequency after surgery). Arousals improved by follow-up to a small but significant extent (p < 0.0001), and RCREC also improved significantly in each EEG frequency range.
Table 2.
Demographic and polysomnographic data for all participants, at baseline and follow-up.
Among children with OSA, arousals and RCREC in each frequency range improved (each p < 0.05), whereas among children without OSA, none of these variables improved (each p > 0.10). However, the differences in extent of improvement after surgery, between children with and without OSA, differed only for arousals (p = 0.0002) and beta RCREC (p = 0.0134, Wilcoxon two sample tests).
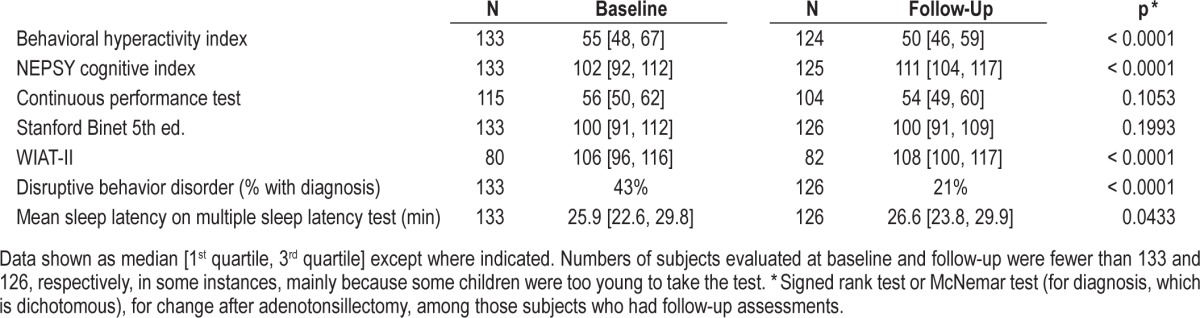
Table 3 shows outcome measures at baseline and follow-up for all subjects. The behavioral hyperactivity index was half of a standard deviation above the anticipated average at baseline, but normalized by follow-up. The NEPSY Cognitive Index (executive functioning and memory) improved, from baseline values that were at the population average, to about one standard deviation higher at follow-up. The Continuous Performance Test and Stanford-Binet intelligence quotient did not change, but the WIAT II (reading comprehension and math) scores did. The frequency of DSM-IV-consistent disruptive behavior disorder diagnoses was high (43%) at baseline, but at follow-up, the frequency of any of these diagnoses was more than halved (21%). The mean sleep latency on the MSLT improved slightly but significantly at follow-up.
Table 3.
Neurobehavioral outcome measures at baseline and follow-up.
Among OSA subjects alone, changes in neurobehavioral measures after AT closely paralleled those observed for the entire group, except that improvement in mean sleep latency only showed a trend (p = 0.07). Among subjects without OSA at baseline, improvements also (as anticipated2) paralleled those seen for the entire group, except that in the smaller subsample the 4-point median improvement in WIAT-II scores did not reach significance (p = 0.24), and mean sleep latency on the MSLT did not improve (median change = 0.0 minutes, p = 0.37). No differences in neurobehavioral improvements (for continuous measures), between subjects with and without OSA, reached significance.
Do arousals and RCREC at baseline explain concurrent neurobehavioral morbidity?
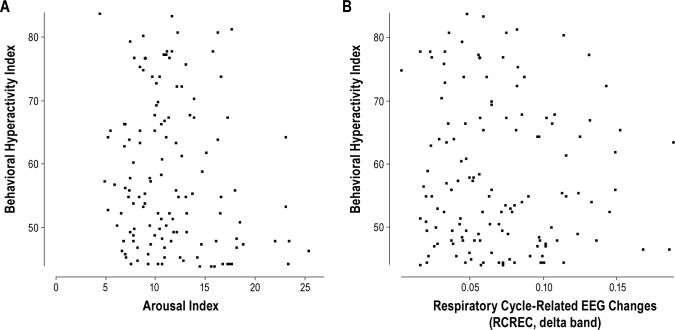
Almost uniformly, in the entire sample of children, neither arousals nor RCREC in any EEG frequency range showed associations (all p > 0.05, with linear or logistic regression as appropriate) with a high behavioral hyperactivity index (> 60; Figure 2); the NEPSY Cognitive Index (executive functioning/ memory); full scale intelligence quotient on the Stanford Binet 5 th Edition; reading comprehension/math reasoning on the WIAT-II; scores on the Continuous Performance Test; a DSMIV-consistent disruptive behavior disorder; or a lower mean sleep latency (< median of 25.9 min) on the 30-min MSLTs. The only exception was that alpha RCREC was associated paradoxically with higher scores on the Stanford Binet (beta = 82, SE = 32, t = 2.6, and p = 0.011).
Figure 2. The baseline behavioral hyperactivity index is plotted against (A) the baseline arousal index, and (B) the baseline respiratory cycle-related EEG changes in the delta frequency range (delta RCREC).
In (A) two outliers with arousal indices of 41 and 34 events per hour of sleep are not shown.
Few associations became newly significant after the sample was restricted to include only the 109 subjects with OSA (baseline AHI ≥ 1.5). The only exceptions were that the baseline arousal index did show weak associations with lower mean sleep latencies on the MSLT (beta = 0.10, SE = 0.05, t = 4.7, and p = 0.029); paradoxically with lower likelihood of a disruptive behavior disorder (OR = 0.90, 95% CI [0.82, 1.00]; and paradoxically with lower Behavioral Hyperactivity Indices (OR = 0.91 [0.82, 1.00]). After the sample was restricted to include only the 54 subjects who had at least moderate OSA at baseline (AHI ≥ 5.0; mean AHI 14 ± 13), no associations emerged that were not apparent in the entire sample.
Do arousals and RCREC at baseline explain improvement in neurobehavioral morbidity at follow-up?
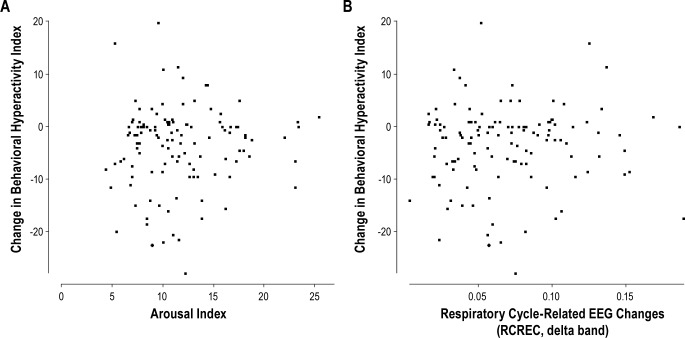
For the most part, neither baseline arousals nor RCREC in any EEG frequency range showed associations (all p > 0.05, by linear regression) with changes (follow-up minus baseline) in scores for the behavioral hyperactivity index (Figure 3); NEPSY cognitive index; Stanford Binet; WIAT-II; continuous performance test; or MSLT. Similarly, neither baseline arousals nor RCREC tended to show association with resolution (transition from present to absent) of a DSM-IV-consistent disruptive behavior disorder. Disparate exceptions were that improvement in the behavioral hyperactivity index was predicted paradoxically by lower baseline beta RCREC (beta = 52, SE = 22, t = 2.4, and p = 0.017); improved Stanford Binet scores were predicted paradoxically by lower baseline alpha RCREC (beta = -69, SE = 28, t = -2.45, and p = 0.016); and resolution of a disruptive behavior disorder was predicted by increased baseline sigma RCREC (beta = 13.7, SE = 6.4, t = 4.6, and p = 0.032).
Figure 3. The improvement at 6 months in the behavioral hyperactivity index (follow-up minus baseline) is plotted against (A) the baseline arousal index, and (B) the baseline respiratory cycle-related EEG changes in the delta frequency range (delta RCREC).
In (A) two outliers with arousal indices of 41 and 34 events per hour of sleep are not shown.
When the analysis was restricted to include only subjects who had OSA at baseline (AHI ≥ 1.5), the only new finding was that a higher baseline arousal index (in addition to lower alpha RCREC) also predicted improvement in the Stanford Binet (beta = 0.44, SE = 0.20, t = 2.3, and p = 0.025); beta RCREC no longer predicted changes in the behavioral hyperactivity index; and sigma RCREC no longer predicted resolution of a disruptive behavior disorder. After the analysis was restricted to include only subjects who had at least moderate OSA at baseline (AHI ≥ 5.0), no baseline measures of arousal or RCREC predicted improvement in the outcome measures to a statistically significant extent (all p > 0.05).
Do quantitative changes in arousals or RCREC, from baseline to follow-up, explain improvement in neurobehavioral morbidity?
For exploratory purposes, the extent of change in each neurobehavioral measure was regressed on changes in arousal indices and RCREC (separately), with adjustment for potential confounders at baseline or their change scores where appropriate (BMI and periodic leg movement index). These models failed to reveal any consistent or robust associations that were informative beyond per-protocol models, described above, based on baseline arousal indices and RCREC (data not shown).
DISCUSSION
This prospective cohort study of 133 children aged 3 to 12 years represents a substantive effort to examine whether sleep fragmentation, either in the form of standard arousals or RCREC, is likely to explain the neurobehavioral morbidities that are widely considered to be some of the most frequent and consequential results of untreated pediatric OSA. The findings overall were solidly negative. Neither baseline arousal indices nor RCREC in delta or any of the other physiologic frequency ranges consistently predicted the behavioral hyper-activity index, several cognitive measures, DSM-IV psychiatric diagnoses, or excessive daytime sleepiness as determined by an MSLT. Exceptions were infrequent, emerged in unpredictable directions, and generally achieved only marginal significance, despite absence of adjustment for multiple comparisons in an effort to maintain sensitivity to any possible associations. The sizeable sample of adenotonsillectomy patients with and without OSA—children previously shown to have indistinguishable cognitive and behavioral morbidity and treatment outcomes2—provided a good opportunity to assess whether detailed analysis of sleep fragmentation might provide the missing polysomnographic link. Our inability to demonstrate stronger associations between measures of sleep fragmentation and salient neurobehavioral outcomes carries implications about underlying mechanisms, and should help to inform clinical practice.
Previous examinations of the standard arousal index among children with and without OSA showed no difference,2,50 and improvement in this metric after treatment for OSA has generally been limited in magnitude,2 as it was in the present study, where an initial arousal index of 11.0 diminished on follow-up by only about 14%, to 9.5. In the first Washtenaw County Adenotonsillectomy Cohort, the baseline arousal index failed to predict baseline behavioral, cognitive, and psychiatric measures or their improvement one year after surgery, but did predict sleepiness on MSLTs and its postoperative improvement.2 For the current study, we originally anticipated that RCREC might perform better, in prediction of relevant behavioral and cognitive outcomes. Many children with OSA show few frank apneas or hypopneas, yet can be demonstrated by esophageal pressure monitoring to exert continuous abnormal levels of effort to breathe during the night, through a chronically rather than intermittently constricted upper airway.33 The RCREC algorithm was designed to assess for breath-to-breath effects of increased work of breathing on cortical arousal, hypothesized to fluctuate subtly but repeatedly with the respiratory cycle.11 Previous evidence, from both children and adults, has in fact been encouraging that the RCREC do reflect inspiratory micro-arousals and offer insight into subjectively and objectively measured sleepiness.12–16
An explanation, therefore, for why RCREC did not prove more useful in the current study is not completely clear. The main difference between the current cohort and the previous Washtenaw cohort, in which RCREC showed more utility,14 is that the current sample did not include asymptomatic subjects. The new sample however was constructed to be most relevant for examination of clinical usefulness, as in practice clinicians do not evaluate normal subjects. Clinicians need better tools to assess for the presence of consequential and treatable OSA.
Another possibility to consider is that the first described association between delta RCREC and mean sleep latency on the MSLT focused on 10 subjects, specifically selected to include severe OSA that was represented, but not common in the current sample.12 Although the original sample was suitable for an initial test of a biological hypothesis, the current, larger sample better reflects the population of children who routinely undergo adenotonsillectomy for OSA. Similarly, previous samples that demonstrated associations between sleepiness and RCREC focused on children aged 6-10,12 or 5-12,14 whereas the current study included younger ages in part to achieve a more representative sample of children likely to undergo adenotonsillectomy. Among the 36 children aged 9 years or older in the current sample, baseline sigma RCREC was again correlated with mean sleep latency on MSLTs (rho = -0.35, p = 0.037, data not shown) as previously reported for adult patients.13
The differences between the current vs. past study methods and targeted samples suggest that negative findings at present should not negate positive findings, under different circumstances, of several previous studies on the function and potential physiologic significance of RCREC. However, current results from a prospective study designed specifically to address questions about sleep fragmentation are the best to date to answer the question of whether arousals or RCREC can improve insight into neurobehavioral morbidity among children scheduled to be treated for OSA. The results do not suggest that either measure is helpful in this important setting to understand concurrent morbidity physiologically, or to prognosticate about response to therapy.
Limitations of the current study do include the fact that it was not designed to assess all possible measures of sleep or sleep fragmentation as predictors of neurobehavioral outcomes. Arousals could potentially have been characterized with more refinement by scoring for cyclic alternating pattern (CAP), recently reported to help explain some cognitive measures in children.51 Similarly, distinction between respiratory arousals and spontaneous arousals conceivably also could have clarified the role of an arousal index more closely related to apnea severity.52 The current study was longitudinal, but followed children for only about 7 months. Some data suggest that the effect of OSA on the brain and behavior becomes apparent several years later, and may exert potent irreversible consequences as early as the first year of life.53,54 Finally, the research MSLTs conducted in this study were modified to include 30-minute nap attempts and also applied to children as young as 3 years old, both with some published precedent,25 but without formal validation.
To the extent that measures of sleep fragmentation in childhood OSA do fail to predict salient neurobehavioral morbidity, concurrently or in the future, then the blame for such morbidity must shift toward other aspects of OSA pathophysiology. In particular, intermittent hypoxia,55 with attendant oxidative stress and inflammation,56 remains highly suspect. Cytokines affected by sleep apnea may explain a large proportion of the variance in associated sleepiness.57,58 Biomarkers may eventually replace polysomnographic measures for clinically significant OSA. However, a satisfying mechanistic explanation for how sleep disruption leads to daytime sleepiness and behavioral changes could still depend in part on innovative research into the neurophysiology of arousals, awakenings, and disruptions to sleep continuity.59
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chervin has received research grants from the NIH and the University of Michigan; is on the Board of Directors for American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, Associated Professional Sleep Societies, and the International Pediatric Sleep Association; is on the advisory board (volunteer) for Sweet Dreamzzz (not-for-profit community organization); has been a consultant for Proctor & Gamble through a contract established with the University of Michigan; is a consultant to MC3 and Zansors; developed a questionnaire licensed by Zansors through the University of Michigan; is an editor for UpToDate; is a Cambridge University Press book editor; and is named in copyrighted material and patents (including one for RCREC discussed in this report) held by the University of Michigan, for screening, assessment, and treatment of sleep disorders. Dr. Burns has received NIH and Department of Defense research grants and is named in patents (including one for RCREC discussed in this report) held by Michigan Technological University, for screening or assessment of sleep disorders. The other authors have indicated no financial conflicts of interest. The work was performed at the University of Michigan, Ann Arbor, Michigan, and Michigan Technological University, Ann Arbor, Michigan. Support was from National Institutes of Health (HL080941, HL105999). There was no off-label or investigational drug use.
ACKNOWLEDGMENTS
The investigators are grateful to the patients and families who volunteered considerable time and effort, and to the surgeons and staff who identified these participants at the clinics of the University of Michigan Division of Pediatric Otolaryngology and the Michigan Otolaryngology Surgery Associates. Surgeons who assisted at the University of Michigan included Drs. Charles Koopmann, Marci Lesperance, Glenn Green, Marc Thorne, and Susan Garetz. Surgeons who assisted at the Michigan Otolaryngology Surgery Associates included Drs. Thomas Weimert, Ronald Bogdasarian, Paul Hoff, and Laurence Ho.
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117:e769–78. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chervin RD, Archbold KH. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep. 2001;24:313–20. doi: 10.1093/sleep/24.3.313. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien LM, Holbrook CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics. 2003;111:554–63. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145:458–64. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Melendres CS, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW, Wells CT, Jeffries J, Chini B, Kalra M, Amin R. Neuropsychological effects of pediatric obstructive sleep apnea. J Int Neuropsychol Soc. 2004;10:962–75. doi: 10.1017/s135561770410708x. [DOI] [PubMed] [Google Scholar]
- 8.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160:203–10. doi: 10.1001/archpedi.160.2.203. [DOI] [PubMed] [Google Scholar]
- 9.Giordani B, Hodges EK, Guire KE, et al. Neuropsychological and behavioral functioning in children with and without obstructive sleep apnea referred for tonsillectomy. J Int Neuropsychol Soc. 2008;14:571–81. doi: 10.1017/S1355617708080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133:216–22. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 11.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Method for detection of respiratory cycle-related EEG changes in sleep-disordered breathing. Sleep. 2004;27:110–5. doi: 10.1093/sleep/27.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep. 2004;27:116–21. doi: 10.1093/sleep/27.1.116. [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171:652–8. doi: 10.1164/rccm.200408-1056OC. [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Weatherly RA, Ruzicka DL, et al. Subjective sleepiness and polysomnographic correlates in children scheduled for adenotonsillectomy vs. other surgical care. Sleep. 2006;29:495–503. [PMC free article] [PubMed] [Google Scholar]
- 15.Chervin RD, Malhotra RK, Burns JW. Respiratory cycle-related EEG changes during sleep reflect esophageal pressures. Sleep. 2008;31:1713–20. doi: 10.1093/sleep/31.12.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chervin RD, Shelgikar AV, Burns JW. Respiratory cycle-related EEG changes: response to CPAP. Sleep. 2012;35:203–9. doi: 10.5665/sleep.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chervin RD, Ruzicka DL, Hoban TF, et al. Esophageal pressures, polysomnography, and neurobehavioral outcomes of adenotonsillectomy in children. Chest. 2012;142:101–10. doi: 10.1378/chest.11-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weatherly RA, Mai EF, Ruzicka DL, Chervin RD. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: a survey of practice patterns. Sleep Med. 2003;4:297–307. doi: 10.1016/s1389-9457(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 19.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingshead AB. Two factor index of social position. New Haven: Yale Press; 1965. [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Kushida CA, Giacomini A, Lee MK, Guilleminault C, Dement WC. Technical protocol for the use of esophageal manometry in the diagnosis of sleep-related breathing disorders. Sleep Med. 2002;3:163–73. doi: 10.1016/s1389-9457(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 23.Chervin RD, Ruzicka DL, Wiebelhaus JL, et al. Tolerance of esophageal pressure monitoring during polysomnography in children. Sleep. 2003;26:1022–6. doi: 10.1093/sleep/26.8.1022. [DOI] [PubMed] [Google Scholar]
- 24.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 25.Gozal D, Wang M, Pope DW., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108:693–7. doi: 10.1542/peds.108.3.693. [DOI] [PubMed] [Google Scholar]
- 26.Golan N, Suraya S, Pillar G. Daytime sleepiness in children with attention deficit hyperactive disorder. Sleep. 2003;26:A127. (Abstract Suppl) [Google Scholar]
- 27.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 28.Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung. 1981;159:275–87. doi: 10.1007/BF02713925. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes SK, Shimoda KC, Wald LR, et al. Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. J Pediatr. 1995;127:741–4. doi: 10.1016/s0022-3476(95)70164-8. [DOI] [PubMed] [Google Scholar]
- 30.Weissbluth M, Davis AT, Poncher J, Reiff J. Signs of airway obstruction during sleep and behavioral, developmental, and academic problems. Dev Behav Pediatr. 1983;1983:119–21. doi: 10.1097/00004703-198306000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr. 1996;155:56–62. doi: 10.1007/BF02115629. [DOI] [PubMed] [Google Scholar]
- 32.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68:360–6. doi: 10.1136/adc.68.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring–evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139:165–71. doi: 10.1007/BF01377349. [DOI] [PubMed] [Google Scholar]
- 34.Hansen DE, Vandenberg B. Neuropsychological features and differential diagnosis of sleep apnea syndrome in children. J Clin Child Psychol. 1997;26:304–10. doi: 10.1207/s15374424jccp2603_9. [DOI] [PubMed] [Google Scholar]
- 35.Halperin JM, Newcorn JH, Kopstein I, et al. Serotonin, aggression, and parental psychopathology in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:1391–8. doi: 10.1097/00004583-199710000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Shaffer D, Fisher P, Dulcan MK, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3: Description, acceptability, prevalence rates, and performance in the MECA study. J Am Acad Child Adolesc Psychiatry. 1996;35:865–77. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Swanson JM, Wigal S, Greenhill LL. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37:519–26. [PubMed] [Google Scholar]
- 38.Overall JE, Campbell M. Behavioral assessment of psychopathology in children: infantile autism. J Clin Psychol. 1988;44:708–16. doi: 10.1002/1097-4679(198809)44:5<708::aid-jclp2270440507>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 39.Spencer EK, Alpert M, Pouget ER. Scales for the assessment of neuroleptic response in schizophrenic children: specific measures derived from the CPRS. Psychopharmacol Bull. 1994;30:199–202. [PubMed] [Google Scholar]
- 40.National Institute of Mental Health. Children's Psychiatric Rating Scale -- NIMH. Psychopharmacol Bull. 1985;21:770. [Google Scholar]
- 41.Conners CK. Conners' Rating Scales - Revised. North Tonawanda, NY: Multi-Health Systems Publishing; 1997. [Google Scholar]
- 42.Gadow KD, Sprafkin J. Child Symptom Inventory-4. Stony Brook, NY: Checkmate Plus; 1994. [Google Scholar]
- 43.Sprafkin J, Gadow KD. Early Childhood Symptom Inventory Manual. Stony Brook, NY: Checkmate Plus; 1996. [Google Scholar]
- 44.Korkman M, Kirk U, Kemp S. A Developmental Neuropsychological Assessment. San Antonio: Harcourt Brace Jovanovich; 1998. [Google Scholar]
- 45.Roid GH. The Stanford-Binet, 5th Edition (SB5) Itasca, IL: Riverside Publishing Company; 2003. [Google Scholar]
- 46.The Psychological C. Wechsler Individual Achievement Test. San Antonio: The Psychological Corporation; 1992. [Google Scholar]
- 47.Conners CK. Conners' CPT II for Windows. North Tonawanda, NY: Multi-Health Systems; 2000. [Google Scholar]
- 48.Conners CK. Conners' Continuous Performance Test for Windows: Kiddie Version (K-CPT) North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- 49.Chervin RD, Burns JW, Subotic NS, et al. Respiratory cycle-related EEG changes (RCREC) predict neurobehavioral outcomes in childhood OSA. Sleep. 2004;27:A99. (Abstract Suppl) [Google Scholar]
- 50.Goh DYT, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682–6. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 51.Bruni O, Kohler M, Novelli L, et al. The role of NREM sleep instability in child cognitive performance. Sleep. 2012;35:649–56. doi: 10.5665/sleep.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep. 2004;27:279–82. doi: 10.1093/sleep/27.2.279. [DOI] [PubMed] [Google Scholar]
- 53.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–90. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- 54.Bonuck K, Freeman K, Chervin RD, Xu L. Sleep-disordered breathing in a population-based cohort: behavioral outcomes at 4 and 7 years. Pediatrics. 2012;129:e857–65. doi: 10.1542/peds.2011-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–16. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:143–50. doi: 10.1210/jc.2009-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 59.Chervin RD, Fetterolf JL, Ruzicka DL, Thelen BJ, Burns JW. Sleep stage dynamics differ between children with and without obstructive sleep apnea. Sleep. 2009;32:1325–32. doi: 10.1093/sleep/32.10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]