Abstract
Purpose
Health related quality of life (HRQoL) is an important outcome in cancer care, although it is not well reported in surgical uro-oncology. Radical cystectomy (RC) with lymph-node dissection is the standard treatment of muscle-invasive bladder cancer and high-risk noninvasive bladder cancer. A wide range of impairments are reported postsurgery. The aims were to evaluate whether a standardized pre- and postoperative physical exercise program and enhanced mobilization can impact on HRQoL and inpatient satisfaction in RC, as defined by the European Organisation for Research and Treatment of Cancer (EORTC).
Materials and methods
Patients were randomized to fast-track RC and intervention (nI=50) or fast-track standard treatment (nS=57). HRQoL and inpatient satisfaction was measured using valid questionnaires: EORTC Quality of Life Questionnaire Core 30 (QLQ-C30) combined with the disease-specific EORTC BLS24 (baseline), and EORTC BLM30 (follow-up), and IN-PATSAT32 inpatient-satisfaction survey at discharge. Efficacy was defined as the differences in HRQoL-scores between treatment groups at the 4-month follow-up.
Results
The intervention group significantly improved HRQoL scores in dyspnea (P≤0.05), constipation (P<0.02), and abdominal flatulence (P≤0.05) compared to the standard group. In contrast, the standard group reported significantly reduced symptoms in sleeping pattern (P≤0.04) and clinically relevant differences in role function, body function, and fatigue. The intervention did not compromise inpatient satisfaction.
Conclusion
We found no overall impact on global HRQoL due to a physical rehabilitation program. However, pre- and postoperative physical rehabilitation can significantly and positively impact on HRQoL aspects related to bowel management and respiratory function (dyspnea) without compromising inpatient satisfaction. These results highlight the role of multimodal rehabilitation, including physical exercises in fast-track RC.
Keywords: rehabilitation, bladder cancer, health-related quality of life, physical exercise, patient-reported outcome (PRO)
Introduction
In 2011, 1,701 new cases of bladder cancer (BC) (1,267 men and 434 women) were diagnosed in Denmark (DK), accounting for approximately 4%–5% of all new cancers. With age standardized to the Danish population in 2000, the incidence rate of BC was 45 per 100,000 among men and 13 per 100,000 among women thereby representing the fourth- and tenth-commonest neoplasms among Danish males and females. The numbers are comparable to Europe and the US.1,2 The disease most commonly occurs above the age of 60 years and peaks around the 70th year of life.3 In high-risk muscle-invasive BC (MIBC) and non-MIBC, radical cystectomy (RC) remains the standard treatment. RC is a surgical removal of the bladder with lymph-node dissection and is considered the most advanced surgical procedure in uro-oncology.4 In 2013, approximately 300 RC cases were performed in DK, of which a third were conducted at Aarhus University Hospital.5 The procedure is followed by high morbidity, and early complications are reported in up 64% in experienced settings,6 and thus it is recommended to be performed in high-volume centers.7,8 The procedure considerably affects a patient’s daily life,4,9–11 and RC-patients have reported significantly worse body functioning compared to the general population.10 A wide range of impairments is reported from loss of the bladder, including complications related to urinary diversion (UD), fatigue, loss of bowel control or constipation, and sexual dysfunction. These aspects have been associated with health-related quality of life (HRQoL) and recovery.10
Multimodal rehabilitation or fast track (FT) is a concept of multifaceted approaches with the main aim of reducing the surgical stress response and postoperative morbidity, to accelerate early recovery and improve patient-reported outcome (PRO).12,13 Standard FT comprises key components involving all three phases of care: preoperative patient education and information, intraoperative (minimally invasive surgery, standardized anesthetic) and postoperative enhanced mobilization, early oral nutrition, and effective pain relief.13
Physical rehabilitation is involved in cancer care; however, physical exercises and standardized enhanced postoperative mobilization and their possible impact on PROs have not been reported in RC.10,14,15 Moreover, current evidence in RC pathways reports HRQoL at a single time point and without a randomized design.16 When introducing physical exercises in RC pathways, a general concern was that the patients might be overwhelmed by the extensive program, leading to stress, exhaustion, and possibly dissatisfaction. These concerns are well known from early studies implementing enhanced recovery programs, and are reported to relate to cultural or clinical tradition.17 However, no studies in FT have provided patients’ assessments of provided care and service, including extended physical exercises.16
Aims and hypothesis
The aims of the study were to evaluate whether pre- and postoperative physical exercises and enhanced mobilization can impact on HRQoL and inpatient satisfaction, as defined by the European Organisation for Research and Treatment of Cancer (EORTC).
Materials and methods
Trial design
The design was a 4-month follow-up study related to a prospective randomized controlled clinical trial (RCT) investigating the efficacy of early rehabilitation on length of stay in RC pathways. The study was approved by the Danish Regional Ethics Committee; it satisfied the requirements stipulated in the Helsinki Declaration, and was registered in the Clinical Trials.gov database (NCT01329107). Data sampling was approved by the Danish Data Protection Agency (2010-41-4306).
Setting and participants
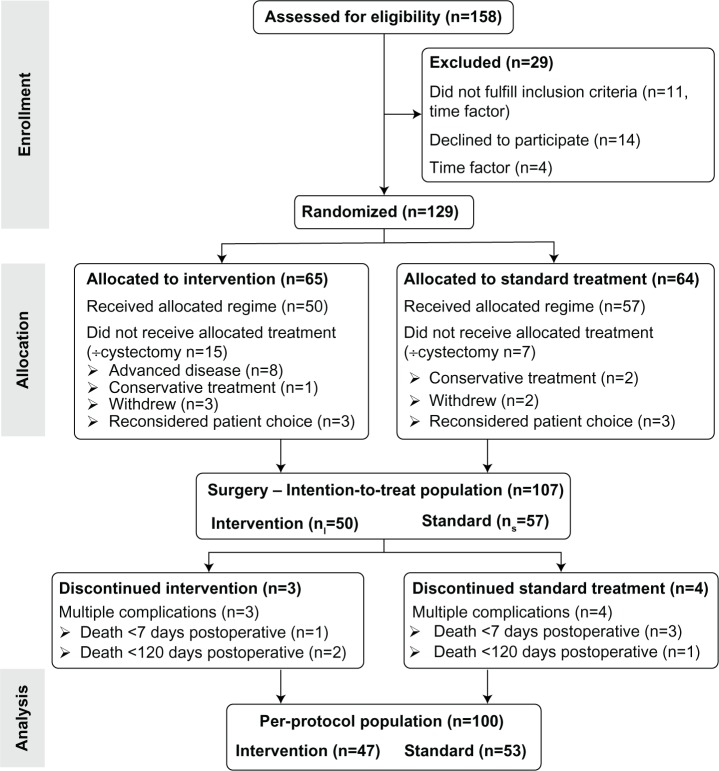
All patients (n=158) recommended for RC because of localized MIBC or non-MIBC from May 2011 to February 2013 at Aarhus University Hospital (DK) were assessed for eligibility. Patients with mental or cognitive disorders and continence issues like painful bladder and neuromuscular diseases were excluded. Fifteen patients did not meet the inclusion criteria (four because of changes in time slots), and 14 patients declined to participate in the study (Figure 1). In total, 129 patients were randomized to receive intervention: a pre- and postoperative rehabilitation program (physical exercise and enhanced postoperative mobilization) or standard FT treatment and care. Following the randomization, 22 patients were rediagnosed or reconsidered their treatment choice, which resulted in 50 patients being allocated to the intervention group (nI=50) and 57 patients to the standard group (nS=57).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart. Efficacy of a rehabilitation program in patients undergoing radical cystectomy, Aarhus University Hospital, 2011–2013.
Notes: Adapted from Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.48 The “÷” symbol signifies no cystectomy.
Randomization
When selected for surgery, patients underwent web-based block randomization using two, four, or six blocks provided by the Institute of Health at Aarhus University. The randomization was blinded to staff and investigators. Written and verbal information was provided, and informed consent was obtained.
Standard treatment
Basic components in FT pathways were standard procedure.12,13 A minilaparotomy RC was performed with/without robot assistance based on department capacity, patient characteristics, and patient preferences.18 All patients underwent nutritional screening and counseling, and in case of risk the patients recieved oral nutritional supplements according to the best European practices.19 Behavioral recommendations concerning smoking and alcohol intake were given. Anamnesis of pain, nausea, and bowel function was obtained, and comorbidity was addressed. Patients and relatives were consulted by the multidisciplinary team (MDT). Verbal and written information concerning hospitalization and the choice of UD was provided for optimal patient decision making. The rectal ampulla was emptied the evening prior to surgery. Patients fasted after midnight, and were offered sweet juice 4 hours before surgery.20,21 A single-dose infection prophylaxis was given, and venous thromboembolism prophylaxis (Fragmin injections; Eisai, Tokyo, Japan), including compression stockings, was administered postsurgery.22,23 All patients received standardized anesthesia and analgesia throughout the perioperative period. Surgery was performed using sevoflurane as a sedative. Perioperative bupivacaine and Ultiva (GlaxoSmithKline, London, UK) infusions were used for pain management. Analgesia within the first 72 hours postoperation was attained via subfascial continuous infusion of bupivacaine through a soaker catheter installed upon closure of the wound.24 If the postoperative hemoglobin was <6.0 mmol/L or symptoms of clinical anemia were observed during mobilization, a blood transfusion was given. Early postoperative nutritional intake was encouraged, aiming for a daily intake of 6,300 kJ,25 and if it was not successful, parenteral supplementation was initiated at day 4 combined with enteral nutrition. Postoperative mobilization was encouraged at least twice a day by the staff and supported by a physiotherapist once a day. Due to the total public health care system, discharge was not cost-driven and dependent only on standardized criteria.
Intervention
The intervention group received standard FT and an exercise-based intervention that involved both pre- and postoperative exercises, as described in Table 1. Following randomization, the intervention group was instructed in using a step trainer for home use. Moreover, the physiotherapist introduced a home-based daily exercise program consisting of six different exercises with repetitions. Patients were encouraged to perform both activities twice a day. Daily achievements were documented in a personal diary by the patient.
Table 1.
The exercise-based rehabilitation program. Efficacy of a multidisciplinary rehabilitation program for patients undergoing radical cystectomy, Aarhus University Hospital, 2011–2013, Denmark
| Preoperative outpatient optimization from inclusion to surgery (−14 days) | Postoperative in-hospital optimization Day 0–7+ |
|---|---|
| Information • Information about standard goals for patient involvement concerning mobilization, exercise training and managing urinary diversion. • Standardized information about the interactions among lifestyle aspects, alcohol and smoking, nutritional status, and physical activity. • Provision of supplementary written information. • Discussion of mutual expectations and motivation. |
Mobilization • Instructions for getting out of bed. • Aggressive and progressive standardized mobilization plans, including: – Scheduled time out of bed increasing from 3 hours on day 1 after surgery to 8 hours on the fourth postoperative day. – Walking distance increasing from 125 m on the day after surgery to 1000 m on the fourth postoperative day. • Encouragement to follow fixed standard goals for mobilization and walking. • Registration of daily mobilization and walking activities in a patient diary. • Evaluation of ability to perform personal activities of daily living using the Katz score.34 |
| Exercise-based prehabilitation program • A standardized written exercise program was introduced and distributed by the MDT-physical therapist. Patients were instructed to perform the exercise training program twice daily. • The exercise program included the following: – Step training on a step trainer (15 minutes per training session). The step trainer was delivered from the hospital. – Six different muscle strength and endurance exercises. The number of repetitions was individualized, and patients were encouraged to progress through the training program by increasing the number of exercise repetitions. • A patient diary was distributed, and patients were instructed to record the number of training sessions and number of exercise repetitions daily. • Evaluation. |
Exercise-based rehabilitation program • Physical therapy was provided twice per day for the first 7 postoperative days. • The physical therapy sessions included the following: – Respiratory and circulatory exercises. – Mobilization in and out of bed. – Walking. – Supervised standardized progressive muscle strength and endurance training. • Evaluation. |
| Follow-up • A proactive telephone call after 1 week to ensure adherence to the program. • In case of questions, patients could contact the MDT. |
Follow-up • Discharged with a home training exercise program. • In case of questions, patients could contact the MDT. |
Abbreviation: MDT, multidisciplinary team.
Standardized postoperative enhanced mobilization and exercises were supervised twice a day (2×30 minutes) by the MDT-physiotherapist in addition to standard care. Differences and similarities between the intervention and the standard group are illustrated in Table 2.
Table 2.
Intervention compared to standard fast track (FT). Efficacy of a multidisciplinary rehabilitation program for patients undergoing radical cystectomy. Aarhus University Hospital, 2011–2013, Denmark
| Intervention FT, n= 50 | Standard FT, n=57 | |
|---|---|---|
| Preoperative (2 weeks prior to surgery) | Prehabilitation (exercise program) and Standard FT treatment |
• Nutritional screening and counseling, supportive oral supplements when recommended • Patient education: lifestyle issues (alcohol, smoking) and postoperative care • Optimizing comorbid conditions • Counseling on choice of urinary diversion • The evening before surgery, the rectal ampulla was emptied • Fasting from midnight, carbohydrate loading 4 hours before surgery |
| Perioperative | • Infection prophylaxis (single doses) • Minilaparotomy or robot-assisted radical cystectomy • Standardized anesthesia and analgesia throughout surgery using sevoflurane (sedative) and bupivacaine and Ultiva for pain management |
• Infection prophylaxis (single doses) • Minilaparotomy or robot-assisted radical cystectomy • Standardized anesthesia and analgesia throughout surgery using sevoflurane (sedative) and bupivacaine and Ultiva for pain management |
| Postoperative | Postrehabilitation (exercise program and enhanced mobilization) and Standard FT treatment |
• Analgesia within the first 72 hours – subfascial Pain-buster providing continuous infusion of bupivacaine; peripheral pain treatment – oral paracetamol • Prevention of nausea • Thromboembolism prophylaxis: compression stockings and Fragmin (Pfizer, New York City, NY, USA) injections • Early oral intake: daily goals – minimum 6,300 kJ, protein 1.2 g/kg/day, including oral supplements • Standard mobilization: walking activity in every ward shift and supervised by a physiotherapist once a day • Early removal of intravenous and urinary catheters |
| Discharge | Standardized discharge criteria | |
Measurements
HRQoL
All patients underwent the same demographic and clinical assessments at baseline (Table 3). HRQoL was measured using the EORTC Quality of Life Core Questionnaire 30 (QLQ-C30).26 The EORTC QLQ-C30 was used in combination with the bladder symptom-specific EORTC BLS24 preoperatively and the EORTC BLM30 postoperatively.26 The EORTC QLQ-C30 and EORTC BLS2426 questionnaires were administered at baseline 14–17 days before the scheduled surgery, and follow-up was conducted 4 months postsurgery (EORTC QLQ-C30 + EORTC BLM30). The EORTC IN-PATSAT-3226,37 inpatient-satisfaction survey was administered the day before discharge.
Table 3.
Clinical and demographic covariates in 107 patients undergoing radical cystectomy at Aarhus University Hospital (Denmark), 2011–2013. To analyze and test for statistical differences between groups, the following tests were used: rank-sum test (Wilcoxon) for categorical variables, Pearson’s two-sided χ2 test for proportions, and Student’s t-test for continuous variables
| Intervention n=50 |
Standard n=57 |
Difference | P-value | |
|---|---|---|---|---|
| Sex | 0.38 | |||
| Men, n (%) | 39 (78) | 40 (70) | ||
| Women, n (%) | 11 (22) | 17 (30) | ||
| Age, years | 0.48 | |||
| Mean (95% CI) | 69 (66–72) | 71 (68–73) | −2 (−5.0 to 2.4) | |
| Range | 46–85 | 47–91 | ||
| Maximum tumor stage, n (%) | 0.57 | |||
| T1 | 10 (20) | 14 (25) | ||
| T2 | 29 (58) | 25 (44) | ||
| T3 | 10 (20) | 14 (24) | ||
| T4 | 1 (2) | 4 (7) | ||
| Urinary diversion, n (%) | 0.61 | |||
| Ileal conduit | 44 (88) | 48 (84) | ||
| Orthotopic neobladder | 5 (10) | 7 (12) | ||
| Continent cutaneous reservoir | 1 (2) | 2 (4) | ||
| Surgical procedure, n (%) | 0.64 | |||
| Open surgery | 41 (82) | 44 (77) | ||
| Robot-assisted | 9 (18) | 13 (23) | ||
| Time (minutes) for surgical procedure | ||||
| Open surgery, mean (95% CI) | 257 (232–282) | 258 (236–281) | −1 (−35 to 32) | 0.90 |
| Robot-assisted, mean (95% CI) | 448 (341–555) | 426 (392–461) | 22 (−67 to 111) | 0.60 |
| Transfer pack SAG-M, mean (95% CI) | 3.24 (2.47–4.01) | 2.69 (2.0–3.41) | 0.50 (−0.49 to 1.6) | 0.30 |
| Pain, VAS 1–10, n (%) | 0.22 | |||
| 0, n (%) | 36 (72) | 45 (79) | ||
| 1–3 | 6 (12) | 8 (14) | ||
| 4–5 | 5 (10) | 4 (7) | ||
| ≥6 | 3 (6) | 0 | ||
| Comorbidity index score (age-adjusted), n (%) | 0.82 | |||
| 0, no comorbidity | 1 (2) | 0 | ||
| 1–2, low | 16 (32) | 14 (25) | ||
| 3–4, high | 23 (46) | 31 (54) | ||
| ≥5, severe | 10 (20) | 12 (21) | ||
| Nutritional risk score (NRS 2002), n (%) | 0.26 | |||
| ≥3, at risk | 14 (28) | 9 (16) | ||
| <3 | 36 (72) | 48 (84) | ||
| Body mass index | 0.77 | |||
| Mean (95% CI) | 26 (25–27) | 26 (25–27) | 0 (−2 to 1) | |
| Nutritional intake (preoperative) | ||||
| Energy (kJ), mean (95% CI) | 8897 (8,294–9,501) | 8,818 (8,111–9,986) | −79 (−846 to 1,004) | 0.85 |
| Protein (g), mean (95% CI) | 87 (81–93) | 86 (82–92) | −6 (−7 to 10) | 0.76 |
| Bowel function (Bristol 1–6)35, n (%) | 0.25 | |||
| 1–3 | 11 (22) | 15 (25) | ||
| 4–5 | 37 (74) | 39 (65) | ||
| ≥6 | 2 (4) | 3 (10) | ||
| Smoker, n (%) | 0.38 | |||
| Never | 10 (20) | 9 (16) | ||
| <5 years | 16 (32) | 12 (21) | ||
| ≥5 years | 5 (10) | 15 (26) | ||
| Present | 15 (30) | 18 (32) | ||
| Missing | 4 (8) | 3 (5) | ||
| Marital status, n (%) | 0.56 | |||
| Living with a partner | 31 (62) | 32 (56) | ||
| Living alone | 16 (32) | 21 (37) | ||
| Missing | 3 (6) | 4 (7) | ||
Abbreviations: CI, confidence interval; SAG-M, saline, adenine, glucose, mannitol; VAS, visual analog scale; T, tumor.
The EORTC QLQ-C30 is a 30-item cancer-specific questionnaire that measures HRQoL on a global health scale, five functional scales (physical, role, emotional, cognitive, and social functioning), three symptom scales (fatigue, pain, and nausea/vomiting) and six single-item scales that evaluate different aspects of cancer care (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). According to manual the items were scored on a 4-point Likert scale ranging from 1 (not at all) to 4 (very much). The two items measuring global health status were scored on a modified 7-point linear analog scale. The EORTC BLS24 and the EORTC BLM30 address specific aspects before and after surgery, and they share several items and scales, including those assessing urinary symptoms, bowel symptoms, and sexual functioning. All data were reported according to the recommendations in the CONSORT (Consolidated Standards of Reporting Trials) PRO Extension.27
Interpretation of HRQoL scores
The multi-item scale scores result from the summation of the single-item scores in each scale. The scores are then transformed to a linear scale according to an equation defined by the EORTC. The transformation provides a linear expression such that all scales range from 0 to 100.26,28–30 A high score on the global health and function scales and a low score on the symptom scales indicates good QoL.26 Clinically relevant differences are recommended to be interpreted with respect to any changes perceived by the patient or within a group, and not only with respect to statistically observed differences.31 The magnitude of changes in HRQoL is relatively small in most clinical situations. A recent meta-analysis provided recommendations for interpretation of efficacy sizes.28 In general, a difference of less than 4 percentage points (P) (0–<4 P) is considered “trivial”, 4–≤ 9 P is considered “small”, >9–≤15 P is considered “medium”, and >15 P is considered “large”. Although the differences (efficacy sizes) varied for different scales, the 95% confidence interval (CI) calculated for each category had some overlap.28–30 Consensus among experts have stated differences of 5–10 P are clinically relevant and should be considered along with the P-value.28,30–32
Pre- and postoperative variables
Preoperative comorbid status and nutritional risk were measured using the age-adjusted Charlson Comorbidity Index score33 and the Nutrition Risk Screening 2002 tool.19 To evaluate the intervention, the personal activities of daily living variable was measured daily using the Katz score.34 Enhanced mobilization was logged and expressed as “hours out of bed”, and the exact walking distance in meters was logged using patient diaries and documented by the staff. Habitual bowel function was measured using the Bristol scale.35 The time to restore bowel function was reported as the number of postoperative days. Information regarding pain and nausea was obtained at baseline and daily before and after any activity measured by the visual analog scale.36 End points were global HRQoL and HRQoL scale scores related to physical activity and enhanced mobilization.
Statistics and sample size
EORTC QLQ-C30, EORTC BLS24, and EORTC BLM30 scores were calculated according to the manual.26 Absolute differences within and between treatment groups from baseline to follow-up were calculated. Mean differences of the transformed linear score were reported with a 95% CI. Null hypotheses were tested using the nonparametric Wilcoxon rank-sum test with respect to the original Likert scale. Statistical significance was considered to be P≤0.05. Missing data were handled according to the manual.26,27
Results
In total, 107 patients were included (Figure 1). Overall responsiveness at baseline (EORTC QLQ-C30 + EORTC BLS24) was 96%, except for single items related to sexuality. During the follow-up period, seven patients died, and others were referred to further treatment. Therefore, the basis for measuring differences at follow-up revealed a responsiveness of 84% (nI=42) and 86% (nS=50), respectively. In aspects of sexuality, the responsiveness was 72% in both groups (nI=36, nS=41). At baseline, there was no statistically significant difference in any items between treatment groups, except for financial difficulties, which was omitted from further analysis.
EORTC QLQ-C30 global health/QLQ, functional, and symptom scales
There was no difference in the global health score or in the scores of the five functional scales between the treatment groups. A clinically relevant difference was observed in role function (absolute difference −10.5, P=0.11) and cognitive function (absolute difference −6.1, P=0.37) (Table 4).
Table 4.
Health-related quality of life (HRQoL) according to the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and the disease-specific EORTC BLS24 (at baseline) and BLM30 (at follow-up) in 107 patients undergoing radical cystectomy at Aarhus University Hospital, 2011–2013.
| Intervention Difference % (95% CI) Follow-up to baseline nI=50 | Standard Difference % (95% CI) Follow-up to baseline nS=57 | Difference (95% CI) | P-value | |
|---|---|---|---|---|
| Overall HRQoL (score 0–100), % | ||||
| Global health status | 1.8 (−5.2 to 8.7) | 4.2 (−4.0 to 12.3) | −2.4 (−13.2 to 8.4) | 0.60 |
| Functional scales (score 0–100), % | ||||
| Physical functioning | −6.8 (−13.3 to −0.4) | −4.4 (−7.9 to −0.9) | −2.4 (−9.3 to 4.6) | 0.59 |
| Role functioning | −10.2 (−23.3 to 2.9) | 0.3 (−10.4 to −10.9) | −10.5† (−26.9 to −5.9) | 0.11 |
| Emotional functioning | 7.1 (−0.5 to 14.8) | 11.3 (5.7–16.9) | −4.2† (−13.4 to 5.0) | 0.35 |
| Cognitive functioning | −4.4 (−12.0 to 3.0) | 1.6 (−5.3 to 3.2) | −6.1† (−14.6 to 3.0) | 0.37 |
| Social functioning | −7.3 (−15.2 to 0.6) | −5.9 (−11.7 to −0.1) | −1.4 (−10.9 to 8.0) | 0.93 |
| Symptom scales (score 0–100), % | ||||
| Fatigue | 7.3 (−2.5 to 17.2) | 0.9 (−5.9 to 7.7) | 6.5† (−5.0 to 17.9) | 0.18 |
| Nausea and vomiting | 0.8 (−7.1 to 8.7) | 7.5 (−3.9 to 3.9) | 0.8 (−7.5 to 9.1) | 0.36 |
| Pain | 0.8 (−9.8 to 11.4) | −1.3 (−7.8 to 5.2) | 2.1 (−9.7 to 13.9) | 0.73 |
| Dyspnea | −7.0 (−15.5 to 1.6) | 2.6 (−4.4 to 9.6) | −9.6† (−20.4 to −1.2) | 0.05* |
| Insomnia | 6.4 (−4.5 to 17.2) | −7.8 (−17.4 to 1.6) | 14.2† (0.1–28.3) | 0.04* |
| Appetite loss | 0.8 (−9.4 to 10.9) | −2.0 (−11.2 to 7.3) | 2.7 (−10.8 to 16.3) | 0.78 |
| Constipation | 1.6 (5.7–8.8) | 14.4 (6.4–22.4) | −12.8† (−23.7 to −1.9) | 0.02* |
| Diarrhea | 1.6 (7.3–10.5) | 0.6 (1.0–7.3) | 0.9 (−9.8 to 11.7) | 0.81 |
| Disease-specific scales (BLM30, BLS24, score 0–100), % | ||||
| Future perspectives (worries) | −26.6 (−35.6 to −17.6) | −29.3 (−37.7 to 20.9) | 2.7 (−9.4 to 14.8) | 0.55 |
| Abdominal flatulence | 4.3 (−3.6 to 12.1) | 10.7 (3.0–18.3) | −6.4† (−17.4 to −4.4) | 0.05* |
| Sexual interest | 2.0 (−9.0 to 13.6) | −5.3 (−11.7 to 1.2) | 7.3† (−4.9 to 19.7) | 0.16 |
| Sexual activity | −4.4 (−15.0 to 6.9) | −10.5 (−18.2 to −2.8) | 7.4 (−6.4 to 19.4) | 0.47 |
| Single time point postsurgery (score 0–100), % | ||||
| Body image | 28.9 (19.7–37.9) | 19.1 (12.6–25.5) | 9.8† (−0.9 to 20.5) | 0.11 |
| Urinary problems (only neobladders) | 18.1 (5.3–30.9) | 38.3 (19.4–57.0) | −20‡ (−40.4 to 0) | 0.05* |
| Catheter problems (only neobladders) | 38.8 (−2.0 to 79.8) | 8.3 (−11.4 to 28.0) | 30.5‡ (−5.8 to 66.9) | 0.06 |
| Stoma problems (only ileal conduit) | 18.2 (12.75–23.8) | 13.5 (9.2–17.7) | 4.7† (−1.9 to 11.5) | 0.17 |
Notes: Increases in the function subscale scores represent an improvement in the HRQoL, whereas increases in the symptom subscale scores represent a deterioration. The null hypothesis was tested using the Wilcoxon signed-rank test.
P≤0.05;
clinically relevant difference in small–medium efficacy;
clinically relevant difference in large efficacy.
Abbreviation: CI, confidence interval; QLQ-C30, Quality of Life Questionnaire Core 30.
Statistically significant efficacy of the intervention was reported for reduced symptoms of dyspnea (absolute difference −9.6, P≤0.05), constipation (absolute difference −12.8, P≤0.02), and abdominal flatulence (absolute difference −6.4, P≤0.05).
The standard group reported a significant reduction in symptoms of insomnia compared to the intervention group (absolute difference 14.2, P=0.04), and a clinically relevant reduction in fatigue symptoms (absolute difference 6.5, P=0.18), though these were not statistically significant. Other differences were not significant or clinically relevant, and thus considered to be “trivial” (Table 4).
Differences in disease-specific subscales (EORTC BLS24 and BLM30)
Clinically relevant improvement of 7% was observed in the standard group regarding overall sexual interest and activity, though it was not significant (Table 4). In those dimensions where scores were only measured at a single time point (postoperative), higher symptoms were measured in the intervention group concerning body image, catheter care, and urostomy. Patients with an incontinent UD reported a “trivial” nonsignificant difference in stoma care at follow-up. However, the 15% of the patients with a continent reservoir (Table 3) reported significantly fewer urinary symptoms in the intervention group (P=−20; P≤0.05).
EORTC IN-PATSAT-32: inpatient satisfaction at discharge
Nurse availability was higher rated but not significant in the intervention group, with a small clinically relevant difference (absolute difference −4.5, P=0.13). There was no difference in availability of physicians. Inpatient satisfaction did not differ between treatment groups with respect to the skills and information of physicians and nurses. Similarly, there was no significant disparity in such items as service, general information, and comfort (Table 5).
Table 5.
Inpatient satisfaction with treatment, care, and service measured at discharge using the European Organisation for Research and Treatment of Cancer (EORTC) IN-PATSAT32 in 107 patients undergoing radical cystectomy, Aarhus University Hospital, 2011–2013.
| Scales | Intervention score (95% CI) nI=50 | Standard score (95% CI) nS=57 | P-value |
|---|---|---|---|
| Doctors (score 0–100) | |||
| Interpersonal skills | 85.0 (79.9–90.0) | 88.7 (84.5–92.8) | 0.26 |
| Technical skills | 85.7 (81.7–89.8) | 87.8 (83.9–91.7) | 0.45 |
| Information provisions | 82.3 (77.8–86.8) | 84.9 (79.9–89.8) | 0.44 |
| Availability | 76.2 (69.6–82.9) | 78.3 (73.5–83.08) | 0.62 |
| Nurses (score 0–100) | |||
| Interpersonal skills | 88.6 (84.2–93.1) | 88.6 (84.5–92.8) | 0.99 |
| Technical skills | 87.5 (83.1–91.89) | 86.7 (82.3–91.3) | 0.82 |
| Information provisions | 86.0 (81.9–90.1) | 83.5 (78.5–88.5) | 0.44 |
| Availability | 86.00 (81.3–90.6) | 81.4 (74.8–86.0) | 0.13 |
| Service (score 0–100) | |||
| Other personnel | 82.3 (77.8–86.8) | 81.4 (76.3–86.5) | 0.79 |
| Waiting time | 72.4 (66.2–78.7) | 72.1 (64.5–79.6) | 0.94 |
| Access | 59.2 (51.94–66.56) | 63.4 (57.56–69.32) | 0.37 |
| Single items (score 0–100) | |||
| Exchange of information | 79.0 (73.7–84.2) | 81.6 (75.8–87.3) | 0.13 |
| Comfort/cleanness | 63.5 (55.6–71.4) | 66.9 (59.8–74.1) | 0.51 |
| General satisfaction | 87.5 (83.1–91.8) | 87.7 (83.3–92.1) | 0.99 |
Notes: Linear scores (0–100 points) were calculated according to the EORTC manual. Statistically significant differences between groups were analyzed and tested using Student’s t-test.
Abbreviation: CI, confidence interval.
Discussion
This study is the first RCT to evaluate whether pre- and postrehabilitation, including physical exercises and enhanced mobilization, could impact on HRQoL and inpatient satisfaction with validated questionnaires.16,37 According to the EORTC, most studies in HRQoL research report small (4–≤9 P) to medium (>9–≤15 P) efficacy sizes, even when comparing distinct groups of patients and studies of patients over time. Our results confirm that the relatively small efficacy size in HRQoL studies is similar in RC and consistent with the available literature.28,31
At the 4-month follow-up, there was no difference in global health/QoL scale score between the treatment groups. This can be explained by a change of needs during the follow-up period. Another reasonable explanation may be the need of a more specifically diagnosed evaluation of PROs, as advocated in the EORTC concept of measuring HRQoL.26 However, we found clinically relevant or significant differences in more than half of the dimensions, indicating that HRQoL has an important role in RC.
The intervention group reported a significant decrease in symptoms, and thus an improved HRQoL, in the single-item scales of constipation and abdominal flatulence (Table 4). Our results are supported by a Swedish population-based study that reported postsurgery defecation problems occurring in up to 30% of patients, indicating bowel problems may have a significant impact on HRQoL.38 Moreover, the value of the exercise-based rehabilitation intervention was a 10% significant decrease in symptoms of dyspnea in the intervention group compared with the standard group. This result was encouraging, considering the relatively short duration of intervention and the fragile preoperative condition with respect to age, comorbidities, the high proportion of smokers, and possible lack of motivational factors when living alone (Table 3).39,40
Patient-perceived HRQoL corresponds to the well-known principle that enhanced mobilization improves clinical outcome.12 Future attempts to improve the HRQoL in RC pathways may benefit from including extended preoperative patient education and information related to abdominal disturbances and consequences of immobility. Moreover, increased supervision and awareness of exercise programs may further improve HRQoL.
Surprisingly, the standard group presented a 14% significant decrease in symptoms related to sleep disturbances (14.2%, P=0.04). It has been documented that sleep disturbances generally have an impact on self-assessed HRQoL in individuals undergoing RC.41 Moreover, men with incontinent UD are reported to have worse outcomes compared to men with continent UD.10 In contrast, continent diversion symptoms are hypothesized to be related to insomnia, because patients usually have to evacuate urine “by the clock”, thereby causing nocturnal disturbances during the initial months following discharge.41 However, considering the low proportion of patients with continent diversion in this study, it is likely that other factors may have a role. This result requires further investigation on which factors may impact on sleeping patterns following RC.
Across the functional subscales, the intervention group demonstrated a clinically relevant decrease compared to the standard group concerning role function and cognitive function, although these differences were not significant (Table 4). These findings could be explained by the intensive patient focus given initially with continuous support from the MDT pre- and postoperatively, where the patient was focused on the eradication of the tumor.42 The subsequent lack of daily encouragement and motivation following discharge may have resulted in a loss of belief in self-efficacy for different reasons. The lack of daily physical goals when discharged may have changed focus toward a phase where recognizing that the UD, lack of sexual function, and related problems would have a lasting functional everyday impact for the rest of their lives. A recent study confirmed a shift in unmet needs along the treatment trajectory from the time of diagnosis and during the early period of survivorship, suggesting difficulty in adjusting to changes in daily living, involvement, and thereby maintenance of HRQoL.43 The potential gap of clinical awareness in the intervention group after discharge and a shift of focus may have influenced self-perceived cognitive and role function. A supervised postdischarge exercise program for RC patients may be beneficial, as suggested in a previous study.44
A commonly held belief is that continent UD has a higher HRQoL outcome due to an almost similar urinary anatomic function. In this study, we did not stratify for UD, since most of the patients underwent an ileal conduit procedure (85%, Table 3). Among the 15% of patients with a continent reservoir (neobladder and pouch procedures), the intervention group reported a significant decrease in urinary symptoms (P≤0.05). The standard patients, however, reported a relevant decrease in catheterization problems (P=0.06). Irrespective of the randomization, these results may be interpreted with caution, due to few patients undergoing a continent procedure. This is also reflected in the wide CIs. We found no difference in HRQoL aspects related to stoma care.
Inpatient satisfaction
We found no significant difference in general inpatient satisfaction concerning treatment and service provided, although availability of nurses was rated 4.5% higher in the intervention group. This result could indicate that adjustments of mutual expectations concerning postoperative awareness and side effects, service, and availability already exist as standard procedure, and the intervention did not compromise inpatient satisfaction (Table 5). No earlier experience or evidence has been documented in the literature on RC, and further experience with EORTC IN-PATSAT-32 is warranted.
Our results document that early physical rehabilitation has a role in RC and positively impacts on HRQoL, and increased awareness on physical interventions and patient involvement in RC is requested. Sleep disturbance following RC has been reported in two independent studies, and should be further explored. Proactive patient education should integrate new evidence; physical exercises can reduce functional dyspnea and bowel symptoms and improve HRQoL in the early postoperative period, and should be mandatory. Implementing physical exercises may improve the patient journey in RC, and long-term effects should be monitored.
Limitations
An RCT is the gold standard to evaluate efficacy. However, the methodology is reported to have some challenges in FT pathways due to blinding of the staff and patients in daily clinical practice. These challenges are almost impossible to avoid, and performance bias may occur to some degree.13,45 Moreover, changes over time, such as in surgical technique (improvements of the minilaparotomy and robotic procedures), anesthetics, and postoperative pain management may likely have influenced the results. Some components of the intervention may have been incorporated into standard practice, such as enhanced mobilization, which may have influenced early recovery and thereby perceived HRQoL. Moreover, both treatment groups were placed on the same ward, and standard patients might have been encouraged by patients allocated to the intervention.
The EORTC disease-specific questionnaires (BLS24 and BLM30) are in the final phase of validation, and efficacy should be interpreted with caution.46 Although this study was a single-center study, it is considered to be applicable to MIBC patients undergoing RC surgery at departments with high adherence to FT recommendations and comparable health care organizations.47
Conclusion
We found no impact of physical rehabilitation on global HRQoL. However, exercise-based interventions in a multidisciplinary setting can significantly and positively impact on HRQoL. Aspects related to bowel management and mobilization and hence respiratory function (dyspnea) were improved without compromising inpatient satisfaction. These results highlight that rehabilitation including physical exercises can improve HRQoL following RC.
Acknowledgments
This study was carried out in a public health care system. The study was supported by Aarhus University Hospital (Denmark), the Dansac Foundation (Denmark), the Foundation of Inge Eriksen (Denmark), Nutricia (Denmark), the ML Joergensen Foundation (Denmark), Helse-Fonden (Denmark), the Novo-Nordic Nursing Research Foundation, and the Danish Cancer Research Foundation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 2.Statens Serum Institut Cancerregisteret: tal og analyser [The Danish Cancer Registry: annual national statistics on incidence and survival] 2011. [Accessed May 1, 2014]. Available from: http://www.ssi.dk/Sundhedsdataogit/Registre/~/media/Indhold/DK%20-%20dansk/Sundhedsdata%20og%20it/NSF/Registre/Cancerregisteret/Cancerregisteret%202011.ashx. Danish.
- 3.Johansen LS, Christensen TH, Bendixen A, Nordling J, Jensen KM, Kehlet H. Cystectomy in Denmark 2000–2005. Ugeskr Laeger. 2008;170(4):215–217. Danish. [PubMed] [Google Scholar]
- 4.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Actas Urol Esp. 2012;36(8):449–460. doi: 10.1016/j.acuro.2011.11.001. Spanish. [DOI] [PubMed] [Google Scholar]
- 5.Dansk Urologisk Cancer Gruppe DaBlaCa (Dansk Blærecancer Udvalg) [Danish Bladder Cancer Group] 2013. [Accessed February 6, 2014]. Available from: http://ducg.dk/dablaca-blaerecancer. Danish.
- 6.Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol. 2009;55(1):164–176. doi: 10.1016/j.eururo.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 7.Froehner M, Brausi MA, Herr HW, Muto G, Studer UE. Complications following radical cystectomy for bladder cancer in the elderly. Eur Urol. 2009;56(3):443–454. doi: 10.1016/j.eururo.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Hautmann RE, Hautmann SH, Hautmann O. Complications associated with urinary diversion. Nat Rev Urol. 2011;8(12):667–677. doi: 10.1038/nrurol.2011.147. [DOI] [PubMed] [Google Scholar]
- 9.Shih C, Porter MP. Health-related quality of life after cystectomy and urinary diversion for bladder cancer. Adv Urol. 2011;2011:715892. doi: 10.1155/2011/715892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer S, Ziegler C, Schwalenberg T, Hinz A, Götze H, Schulte T. Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Support Care Cancer. 2013;21(5):1383–1393. doi: 10.1007/s00520-012-1680-8. [DOI] [PubMed] [Google Scholar]
- 11.Wright JL, Porter MP. Quality-of-life assessment in patients with bladder cancer. Nat Clin Pract Urol. 2007;4(3):147–154. doi: 10.1038/ncpuro0750. [DOI] [PubMed] [Google Scholar]
- 12.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101(3):172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 14.Jack S, West M, Grocott MP. Perioperative exercise training in elderly subjects. Best Pract Res Clin Anaesthesiol. 2011;25(3):461–472. doi: 10.1016/j.bpa.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Singh F, Newton RU, Galvao DA, Spry N, Baker MK. A systematic review of pre-surgical exercise intervention studies with cancer patients. Surg Oncol. 2013;22(2):92–104. doi: 10.1016/j.suronc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Brédart A, Coens C, Aaronson N, et al. Determinants of patient satisfaction in oncology settings from European and Asian countries: preliminary results based on the EORTC IN-PATSAT32 questionnaire. Eur J Cancer. 2007;43(2):323–330. doi: 10.1016/j.ejca.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Kehlet H. Multimodal approach to postoperative recovery. Curr Opin Crit Care. 2009;15(4):355–358. doi: 10.1097/MCC.0b013e32832fbbe7. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JB, Pedersen KV, Olsen KØ, Bisgaard UF, Jensen KM. Mini-laparotomy approach to radical cystectomy. BJU Int. 2011;108(7):1125–1130. doi: 10.1111/j.1464-410X.2010.09958.x. [DOI] [PubMed] [Google Scholar]
- 19.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31(6):783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Svanfeldt M, Thorell A, Hausel J, et al. Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg. 2007;94(11):1342–1350. doi: 10.1002/bjs.5919. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen MS, Jørgensen LN, Wille-Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009;(1):CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva A, Dalton M, Amaragiri SV, Lees T. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. 2010;(7):CD001484. doi: 10.1002/14651858.CD001484.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Gwak MS, Choi SJ, et al. Prospective, randomized study of ropivacaine wound infusion versus intrathecal morphine with intravenous fentanyl for analgesia in living donors for liver transplantation. Liver Transpl. 2013;19(9):1036–1045. doi: 10.1002/lt.23691. [DOI] [PubMed] [Google Scholar]
- 25.Ernaeringsterapi.info [website on the Internet] [Accessed May 1, 2014]. Available from: http://www.ernaeringsterapi.info.
- 26.EORTC Quality of Life Group EORTC guidelines for assessing quality of life in EORTC clinical trials. [Accessed May 1, 2014]. Available from: http://groups.eortc.be/qol/sites/default/files/archives/clinical_trials_guidelines_qol.pdf.
- 27.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 28.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713–1721. doi: 10.1016/j.ejca.2012.02.059. [DOI] [PubMed] [Google Scholar]
- 29.Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer. 2008;44(13):1793–1798. doi: 10.1016/j.ejca.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 30.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5(6):555–567. doi: 10.1007/BF00439229. [DOI] [PubMed] [Google Scholar]
- 31.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 32.Martinelli F, Quinten C, Maringwa JT, et al. Examining the relationships among health-related quality-of-life indicators in cancer patients participating in clinical trials: a pooled study of baseline EORTC QLQ-C30 data. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):587–599. doi: 10.1586/erp.11.51. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 34.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 35.Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech Coloproctol. 2001;5(3):163–164. doi: 10.1007/s101510100019. [DOI] [PubMed] [Google Scholar]
- 36.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 37.Bredart A, Kop JL, Griesser AC, et al. Assessment of needs, health-related quality of life, and satisfaction with care in breast cancer patients to better target supportive care. Ann Oncol. 2013;24(8):2151–2158. doi: 10.1093/annonc/mdt128. [DOI] [PubMed] [Google Scholar]
- 38.Thulin H, Kreicbergs U, Onelöv E, et al. Defecation disturbances after cystectomy for urinary bladder cancer. BJU Int. 2011;108(2):196–203. doi: 10.1111/j.1464-410X.2010.09815.x. [DOI] [PubMed] [Google Scholar]
- 39.Jensen BT, Laustsen S, Petersen AK, et al. Preoperative risk factors related to bladder cancer rehabilitation: a registry study. Eur J Clin Nutr. 2013;67(9):917–921. doi: 10.1038/ejcn.2013.120. [DOI] [PubMed] [Google Scholar]
- 40.Witjes JA, Compérat E, Cowan NC, et al. Guidelines on Muscle-Invasive and Metastatic Bladder Cancer. Arnhem, Netherlands: European Association of Urology; 2013. [Accessed May 1, 2014]. Available from: http://www.uroweb.org/gls/pdf/07_Bladder%20Cancer_LRV2.pdf. [Google Scholar]
- 41.Thulin H, Kreicbergs U, Wijkström H, Steineck G, Henningsohn L. Sleep disturbances decrease self-assessed quality of life in individuals who have undergone cystectomy. J Urol. 2010;184(1):198–202. doi: 10.1016/j.juro.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Lee CT. Quality of life following incontinent cutaneous and orthotopic urinary diversions. Curr Treat Options Oncol. 2009;10(3–4):275–286. doi: 10.1007/s11864-009-0110-8. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed NE, Chaoprang Herrera P, Hudson S, et al. Muscle invasive bladder cancer: examining survivors’ burden and unmet needs. J Urol. 2014;191(1):48–53. doi: 10.1016/j.juro.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karvinen KH, Courneya KS, Venner P, North S. Exercise programming and counseling preferences in bladder cancer survivors: a population-based study. J Cancer Surviv. 2007;1(1):27–34. doi: 10.1007/s11764-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 45.Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr. 2013;32(6):879–887. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Aaronson N. Bladder cancer: EORTC QLQ-BLS24, EORTC QLQ-BLM30. [Accessed January 19, 2013]. Available from: http://groups.eortc.be/qol/bladder-cancer-eortc-qlq-bls24-eortc-qlq-blm30.
- 47.Karl A, Buchner A, Becker A, et al. A new concept for early recovery after surgery in patients undergoing radical cystectomy for bladder cancer – results of a prospective randomized study. J Urol. 2014;191(2):335–340. doi: 10.1016/j.juro.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]