Summary
Adult stem cells (SCs) are important to maintain homeostasis of tissues including several mini-organs like hair follicles and sweat glands. However, the existence of stem cells in minor salivary glands (SGs) is largely unexplored. In vivo histone2B GFP (H2BGFP) pulse chase strategy has allowed us to identify slow cycling, label retaining cells (LRC) of minor salivary glands that preferentially localize in the basal layer of the lower excretory duct with a few in the acini. Engraftment of isolated SG LRC in vivo demonstrated their potential to differentiate into keratin 5 (basal layer marker) and keratin 8 (luminal layer marker) positive structures. Transcriptional analysis revealed activation of TGFβ1 target genes in SG LRC and BMP signaling in SG progenitors. We also provide evidence that minor SGSCs are sensitive to tobacco derived tumor inducing agent and give rise to tumors resembling low grade adenoma. Our data highlight for the first time the existence of minor salivary gland LRCs with stem cells characteristic and emphasize the role of TGFβ pathway in their maintenance.
Introduction
The ability to speak, swallow, taste food, and maintain a healthy oral cavity is heavily reliant on the presence of saliva, an important effect of which on our everyday lives is often unappreciated [1]. Salivary glands (SGs) have an important role not only in oral health but also for general health and well being. Hyposalivation is the most common condition underlying xerostomia, the subjective feeling of dry mouth along with 50% reduction in salivary flow. It is highly common especially in patients undergoing radiotherapy treatment for head and neck cancer, drug usage and patients suffering from Sjogren’s syndrome. Considering the severe impact that xerostomia may have on the patient’s quality of life, there is an unmet clinical need for an efficient treatment. Stem cell therapy could provide an option to prevent and repair damage of tissues induced by degenerative processes due to autoimmune responses, radiation-side effects or other cytotoxic events of the salivary glands.
The salivary gland system consists of the major and minor salivary glands. While the major glands secrete their fluids fully only upon stimulation, the minor mucosal glands function more or less continuously providing ongoing protection to the oral tissues [2] [3]. There are estimated to be 600–1,000 minor salivary glands in humans and these are located in the buccal, labial, distal palatal, and lingual regions of the oral mucosal membrane, although they are occasionally found at other oral sites [2].
Cells with stem/progenitor properties have been detected in major salivary glands, but till date, no data has described their presence within the minor salivary glands [1] [4] [5] [6]. Additionally, very little is known about the molecular regulators of the development, stem cell activity and the regenerative processes of the minor salivary glands. The progenitor cell population isolated from major salivary gland based on the cell surface marker c-Kit has been used to regenerate salivary glands after irradiation. Remarkably, post irradiation stem cell treatment with c-Kit+ adult salivary gland stem cells restored radiation-induced dysfunction [7]. Other groups have used cell surface markers such as Sca-1, CD133, CD24 and CD49f to enrich the epithelial progenitor cells in the major salivary glands but a combination of definitive cell markers for salivary progenitor cells remains to be determined [8]. There have been a few studies that have used genetic lineage tracing experiments in mice to identify progenitor cells in the developing salivary glands. In one such study, an Ascl3+ progenitor population was identified in the ductal compartment of the submandibular gland (SMG), which gave rise to both ductal and acinar cells. Importantly, not all SMG cells were derived from the Ascl3 cells, but only a subpopulation of acinar and ductal cells. The Ascl3+ cells were therefore considered to be progenitor cells [9].
In this report, using previously developed strategy to fluorescently label slow-cycling cells [10] we were able to, for the first time localize and isolate label retaining cells (LRCs) of the minor salivary gland (SGs) in the lower part of its excretory duct. However some myoepithelial LRCs were also detected in the acini. The LRCs in the duct and the acini localized within the keratin 5 positive basal layer and not the keratin 8 positive luminal layers. When these cells were sorted and injected into the salivary glands of the NOD.Cg donor mice, they regenerated some structures containing both the basal and the luminal types of cells. By determining the gene expression profiles of the isolated minor SG LRCs and the non-LRCs in vivo, we have identified that TGFβ1 pathway is active and dominant in the minor SG LRCs. On the other hand, non-LRCs showed high expression of BMP dependent expression of P-SMAD 1, 5, 8. We have also provided evidence that minor SGSCs are sensitive to tobacco derived tumor inducing agent and give rise to tumors resembling low grade adenoma [11]. Our data highlight for the first time the existence of label retaining minor SG stem cells and their primary importance in SG homeostasis. It also emphasizes the role of TGFβ pathway in SGSCs maintenance.
Material and Methods
Mice
Two transgenic mouse lines were used: keratin 5-driven tetracycline repressor mice (K5-tTA) [12] and tetracycline response element-driven histone fusion, H2BGFP transgenic mice (pTRE-H2BGFP) [10]. In this double transgenic line, H2BGFP expression is turned on (“pulse”, no doxycycline treatment) from early embryogenesis. By feeding the animals doxycycline (Dox diet pellets 1mg/g from BioServ for 4 weeks starting at P21–28), H2BGFP expression is turned off for the duration of the treatment (“chase”). In addition two reporter mice line from Jackson Laboratory were used: Tg(TCF/Lef1-HIST1H2BB/EGFP)61Hadj/J 013752 and Gli1tm3(cre/ESR1)Alj/J007913). For CreER induction, Tamoxifen (Sigma) was dissolved in corn oil (20 mg/ml) and injected intraperitoneally (i.p.; 10 mg daily for 3 days). For tumor induction, 4NQO obtained from Sigma-Aldrich was dissolved in propylene glycol (Sigma-Aldrich) as stock solution (4 mg/mL) (1h warm water bath to dissolve), stored at 4°C, and diluted in the drinking water to a final concentration of 50µg/mL. Water was changed every 3 days. All mice work was conducted according to the Institutional Animal Care and Use Committee (IACUC) at the University of Southern California. The protocol No. 11172 was approved by the IACUC Committee. All surgeries were performed under either isoflurane or ketamine anesthesia and all efforts were made to minimize the suffering with analgesics (Buprenex prior and post surgery was administrated).
Immunohistochemistry and Immunofluorescence Staining
All frozen sections were fixed in 4% paraformaldehyde. Tissue sections were stained with hematoxylin and eosin for H&E visualization. For immunofluorescence staining, sections were permeabilized with 0.1% Triton X-100 for 10min and blocked in 0.1% Triton-PBS, 0.25% goat normal serum, 0.25% donkey normal serum and 0.1% BSA for 1h at room temperature. Primary antibodies were incubated in the blocking buffer overnight at 4°C and washed with PBS. Secondary antibodies were incubated in 0.1% BSA for 1h at room temperature.
Isolation of minor SG LRCs
K5TetOff/TreH2BGFP animals were fed 1mg/g doxycycline food for 4 weeks starting around P21–28. H2BGFP+ upper palate were dissected out of 20–30 mice and treated with 1000U/ml Collagenase type I for 1h at 37°C with shaking. SGs were washed with DPBS and digested in 0.25% Trypsin-EDTA for 20min at 37°C with shaking. Trypsin was neutralized and the solution was filtered through a 40um cell strainer to obtain a single cell suspension. For colony formation equal numbers of sorted cells were plated onto mitomycin-treated 3T3 fibroblasts in E-media [13] supplemented with 15% serum and 0.3mM calcium. Number and the colony size were visualized using a fluorescent microscope as the cells expressed H2BGFP.
FACS
For FACS, upper palates were dissected 4 weeks post chase from K5tetH2BGFP mice and labeled with PE conjugated anti-α6 integrin (CD49f) (1:200; BD Pharmingen) and APC conjugated CD44 (1:200); BD Pharminogen) for 30min and sorted using the FACS Aria II cell sorter (BD, Bioscience) for H2BGFP+/CD44+/α6+and H2BGFP−/CD44+/α6+ populations. Cells were collected in RNA protect Cell Reagent (Qiagen) for RNA isolation.
Glandular Injections
The sorted cell populations (H2BGFP+/CD44+/α6+) and (H2BGFP−/CD44+/α6+) were plated in 4-well plates with 3T3 feeder cells. After 14 days in culture, the sorted cells were collected and mixed with matrigel (BD) (1:1). The unsorted dissected clumps were micro dissected directly from 4 weeks chased minor SG tissue. The sorted cells suspension and dissociated unsorted minor SG cell clumps were injected into submandibular gland of immunocompromised NOD.Cg mice. 8 weeks after injection, mice were sacrificed, and transplants were dissected out and analyzed.
RNA Isolation, Microarray, RT-PCR, and qPCR
Total RNAs were purified from FACS-sorted SG LRCs, SG non-LRCs, using Qiagen’s RNeasy Micro kit according to the manufacturer’s instructions. Equal amounts of RNA were reverse transcribed using the Superscript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. cDNAs were amplified by PCR and used in triplicates for each qPCR sample primer set with all primer sets designed to work under the same conditions. Real-time PCR amplification of the particular genes of interest was performed using an Applied Biosystems 7900HT Fast Real-Time PCR System and the fold difference between samples and controls were calculated based on the 2−ΔΔCT method, normalized to β-actin levels. Total RNAs from FACS of minor SG LRCs (H2BGFP+/CD44+/ α6+) and minor SG non-LRCs (H2BGFP−/CD44+/ α6+) were purified using RNeasy Micro Kit (Qiagen), and quantified using Nanodrop. RNA 6000 Pico Assay (Agilent Technologies) was used for RNA quality check. Microarray analysis was performed by the University of Southern California Microarray Core Facility using Affymetrix GeneChip® Mouse 430Plus 2.0 Arrays.
Results
Label retaining cells of minor salivary glands localize predominantly in the basal layer of the lower excretory duct and the myoepithelial layer of acini
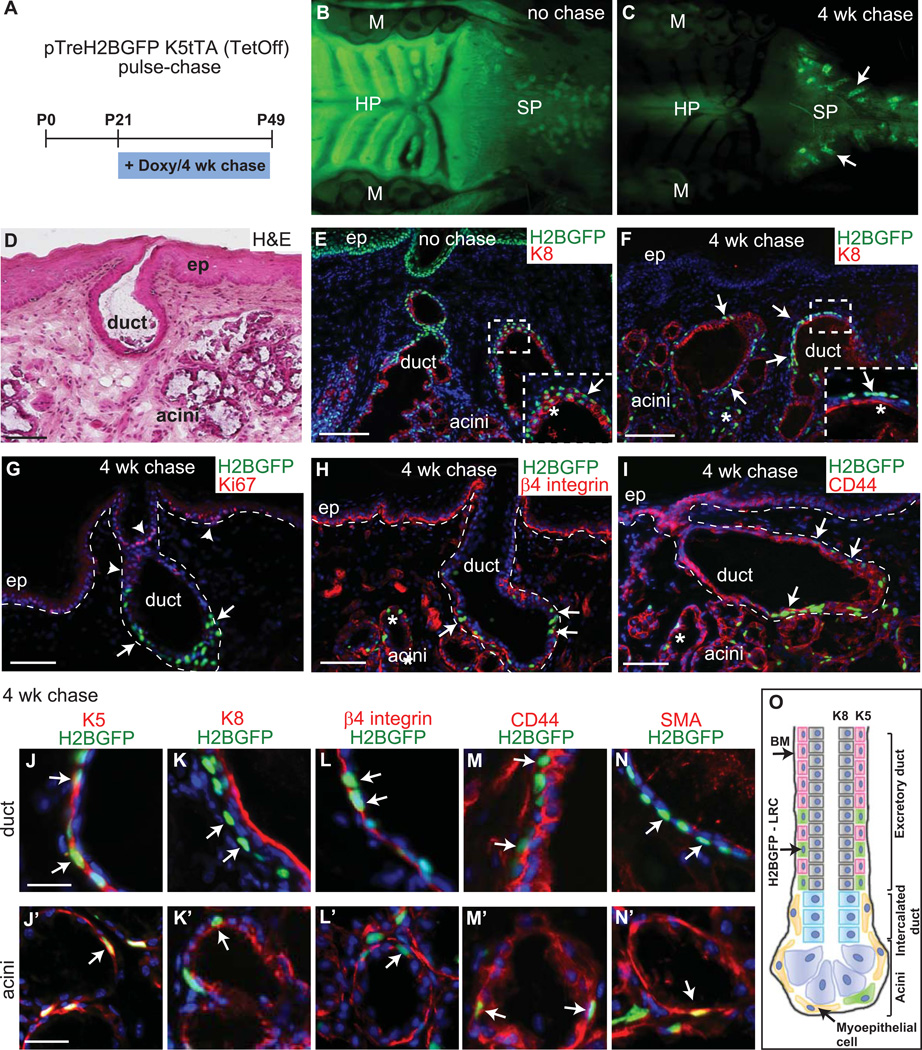
To localize LRCs in the minor salivary glands we used a previously developed system for in vivo detection of infrequently dividing cells (Fig. 1A) [10]. During 4 weeks of “chase”, slow cycling cells retain H2BGFP expression (label retaining cells), whereas rapidly dividing transit amplifying cells dilute out the H2BGFP label upon each division. In the animals before “chase”, H2BGFP expression was uniformly detected in all cells of the oral epithelium including soft palate where high number of minor salivary glands are localized (Figure 1B). All the cells including cells in the ducts and acini were uniformly labeled with H2BGFP (Fig. 1E). After 4 weeks of “chase” by switching off H2BGFP expression with Doxy treatment beginning at 3 to 4 weeks of age, we demonstrated the presence of infrequently dividing LRCs in minor SGs (Fig. 1C, F and O). The pattern of LRC at 4 weeks of chase was similar to pattern seen after 7 weeks of chase (Fig. Sup 1A and B) indicating that there is a slow turnover of minor salivary gland epithelial cells. The H2BGFP expression of LRCs was enriched in the lower excretory duct (Fig. 1F–I), however some LRCs were also observed in the acini (Fig. 1 F–I, arrows). In contrast no GFP positive cells were observed in the soft palate epithelium (Fig 1F–I). All of the LRCs were slow cycling excluding immunofluorescence staining for proliferation marker Ki67 (Fig. 1G and Fig. Sup 1A). Histological localization of LRCs in SGs was confirmed by visualizing serial sections of the soft palate region by hematoxylin and eosin staining (H&E) (Fig. 1D). Immunofluorescence staining with a number of different markers was performed to further define the precise location of SG LRCs. We have demonstrated that the minor SG LRCs are all positive for β4 integrin indicating that they are attached to the basement membrane (Fig. 1L and L’). In addition, these SG LRCs also co-expressed the basal layer marker keratin 5 (K5) (Fig. 1J–J’), whereas the luminal layer marker, keratin 8 (K8) did not overlap with SG LRCs (Fig. 1K and K’). All the epithelial cells, including LRCs were also positive for CD44 (Fig. 1M and M’). However only LRCs within the acini expressed smooth muscle actin (SMA) indicating their myoepithelial characteristic with basal layer localization (Fig. 1N and N’).
Figure 1. Preferential localization of LRC in basal layer of minor SG.
Chart illustrating the Doxy treatment of pTreH2BGFP/K5tTA mice at p21 for 4 weeks chase (A). View of soft palate of pTreH2BGFP/K5tTA mice before (B) and after (C) 4 weeks Doxy chase, with the slow cycling cells retaining GFP label. Shown are side view sections of soft palate of mice before (E) and after 4-week chase (F–N’). Shown are H&E staining (D) and epifluorescence of H2B-GFP (green) and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with antibodies (Abs) indicated (Red) (E–N’). Schematic representation of minor salivary gland with LRCs. Majority of LRCs is localized within the basal layer (K5) of excretory duct and few within myoepithelial layer of acini (O). Arrows denote GFP positive LRC, asterix indicate acinar part of the gland. Arrowheads in (G) denote Ki67 positive epithelial cells in the gland and soft palate epithelium. Scale bar: D–F 100µm; G–I 50µm; J–N’ 20µm. Abbreviations: HP, hard palate; SP, soft palate; M, molars; ep, epithelium; Doxy, doxycycline
Isolation and regenerative potential of label retaining cells of minor salivary gland
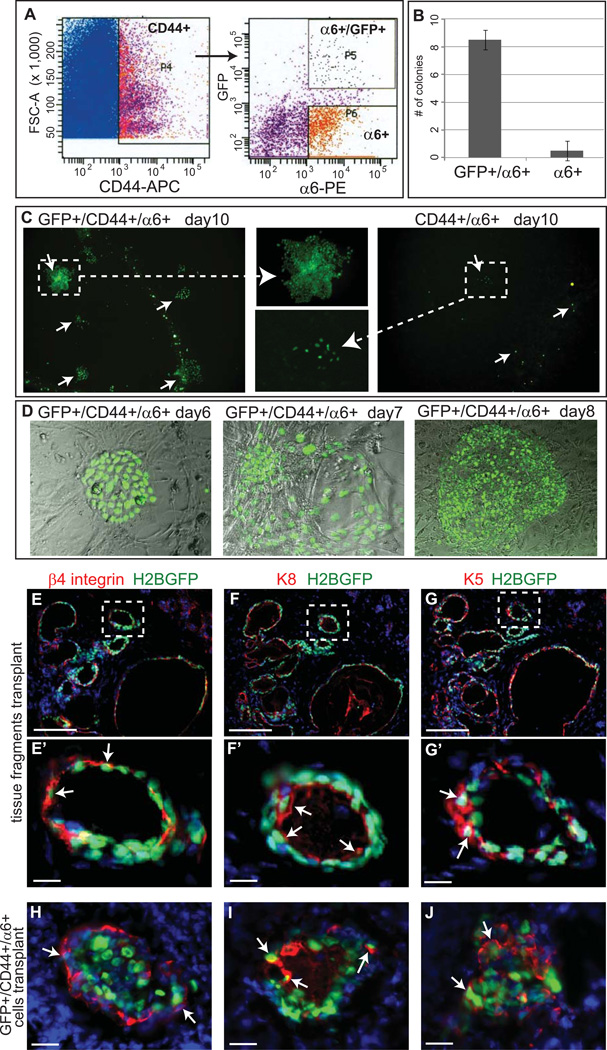
To isolate the minor SG LRCs, we used a combination of surgical dissection with subsequent enzymatic digestions followed by fluorescence-activated cell sorting (FACS). Since minor SG LRCs were attached to the basement membrane (Fig. 1H, L, L’) and all the cells in the minor salivary gland were expressing epithelial marker CD44 (Fig. 1 I, M, M’), we stained these cells with a FACS specific antibody against α6 integrin and CD44 to achieve a high purity of sorted live LRCs and adjacent non-LRC basal cells. FACS analysis revealed that minor SG LRCs accounted for approximately 3% of the epithelial cells enriched by CD44 staining fraction. H2BGFP+ LRCs were highly enriched in the colonies, some of which contained ~400 cells (Fig. 2B, C and D), consistent with the high proliferative capacity documented for hair follicle LRCs [10]. On the other hand non-LRCs (H2BGFP−) had limited potential in colony formation (Fig. 2B and C). To test the potential of H2BGFP+ minor SG LRCs in the reconstitution of the salivary gland, we performed intra-glandular transplantation of the sorted cells as well as dissected pieces of 4 weeks chased minor SG tissue into the salivary glands of NOD.Cg mice (Fig. 2E–J and Fig. Sup. 2A). Eight weeks after the transplantation dominant structures that formed from dissected pieces of minor SG tissue were resembling ducts (Fig. 2 E–G’). A pure population of sorted H2BGFP+ cells that were initially positive only for K5 and negative for K8, after transplantation gave rise to both K5 and K8 positive structures indicating its differentiation potential (Fig. 2H– J). In contrast, we did not observe any structures formed by the non-LRCs (H2BGFP−) in the transplanted salivary glands in three independent experiments. At week eight after initial transplantation the cells within the transplant were still expanding as indicated by positive staining for proliferation marker Ki67 (Fig. Sup. 2B and C). Together the in vitro cell culture and in vivo transplantation experiments indicate that the minor SG LRCs possess stem cell characteristics such as differentiation potential and can self renew..
Figure 2. LRC are clonogenic and have regenerative potential.
Minor salivary glands obtained from pTreH2BGFP/K5tTA mice, 4 weeks after chase were digested and used to sort for LRCs. Epithelial marker CD44, basement membrane marker integrin α6 were used to isolate slow cycling GFP+ CD44+ α6+ LRC population and CD44+ α6+ progenitor population (A). The FACS sorted cells (LRC and progenitors) were placed in culture for clonogenic potential assessment. Colonies were counted at day 10 post sort in two independent experiments (B). The epithelial morphology of the colonies that formed from LRC and progenitors are shown (C, D). Epifluorescence of H2BGFP (green) and indirect immunofluorescence with antibodies as indicated of sections of ductal structures that formed after transplantation of dissected minor salivary glands from soft palate (E–G’) and sorted GFP+ α6+ LRC (H–J) are shown. Scale bar: E–G 100µm; E’–J 20µm. Arrows denote GFP positive cells that formed ductal like structures after transplantation.
Defining the molecular characteristic of minor salivary gland label retaining cells
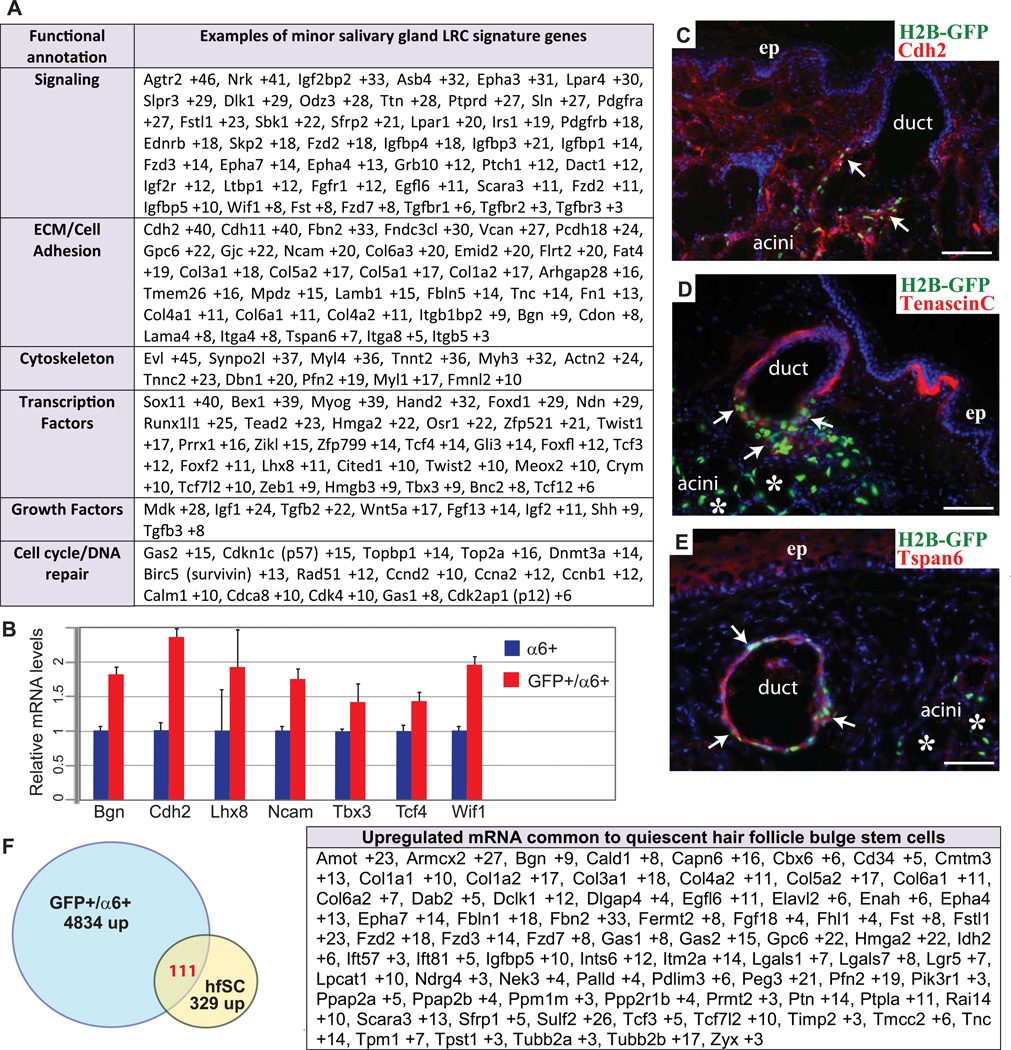
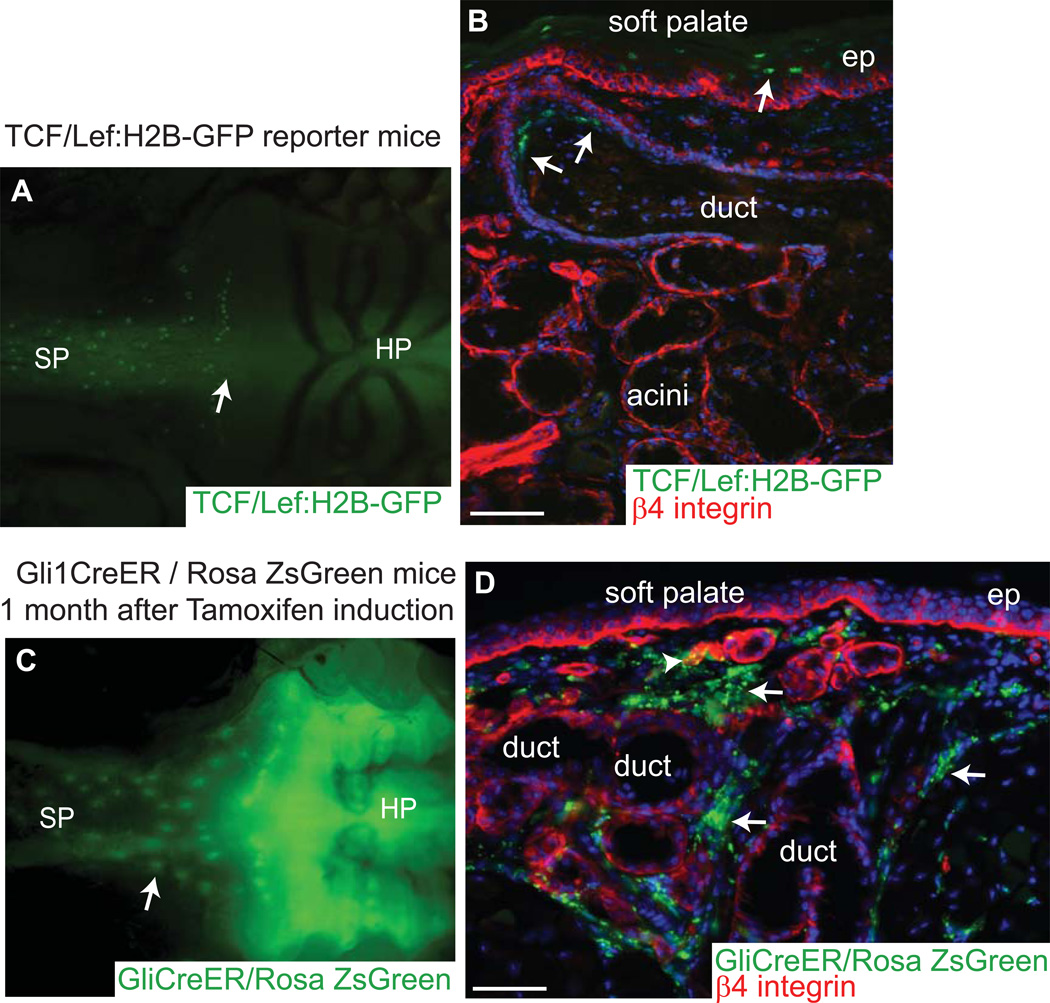
Using microarray analyses, we obtained the transcriptional profiles for the two populations: GFP+/α6+/CD44+ (minor SG LRCs) and GFP−/α6+/CD44+ adjacent basal layer cells (minor SG non-LRCs), and high-stringency analyses uncovered the distinguishing features of the minor SG LRCs (Table S1). There were 4834 mRNAs up-regulated and 1889 mRNAs down-regulated by at least 2.5 – fold (Table S1). Functional annotations revealed up-regulation of a number of mRNAs in LRCs that are involved in cell adhesion, signaling and transcription (Fig. 3A). Some of the genes were validated through real-time PCR using separately isolated biological samples and immunofluorescence staining (Fig. 3B and 3C–E). Preferential ductal localization of Ncadherin (Cdh2), tenascin (Tnc) and tetraspanin 6 (Tspan6), which are up-regulated in the array data for LRCs indicates that the sorted fraction was enriched in ductal minor SG LRCs (Fig.3C–E). Up-regulated LRC mRNAs encoded members of the transforming growth factor beta (TGFβ) signaling pathway known to inhibit epithelial cells proliferation, like Tgfb2, Tgfb3 and Ltbp1 necessary for latent TGFβ activation. Although LRCs expressed some of the cyclins involved in cell cycle progression, they also expressed high levels of cell cycle inhibitors (Cdkn1c and Cdk2ap1). Inhibitors of Wnt pathway including Sfrp2, Dab2, Dact1, Tcf3, and Wif1, and the Wnt receptors Fzd3, Fzd7, and Fzd2 were also up regulated (Fig. 3A and Table S1). Consistent with the array data the Wnt signaling appeared to be repressed as we didn’t observe activation of β-catenin-dependent signaling, using transgenic reporter mice (Fig. 4A and B) [14]. We also observed that genes of Sonic hedgehog (Shh) pathway (Shh, Ptch1, Gli3 (Fig. 3A and Table S1) were upregulated in the LRC, which is associated with maintenance of various adult epithelial stem cells [15], [16, 17]. To determine which cells respond to Shh signaling we used Gli1-CreERT2; ROSA26LoxP-STOP-LoxP-ZsGreen1 mice (Gli1-CreER; Zsgreen), injected with tamoxifen at 4–6 weeks of age. 1 month after Tamoxifen induction we detected ZsGreen+ cells near the minor salivary gland ducts (Fig. 4C). Analysis of the sections of the upper palate revealed sporadic presence of Shh responsive cells in the most upper duct of the minor SG, however majority of the cells responding to Shh are present in the stroma surrounding the minor SG (Fig. 4D). Interestingly this observation is consistent with the model proposed by A.L. Joyner group [18] where Shh from basal epithelial cells of adult prostate signals to the surrounding stroma. Many of the up-regulated mRNAs in the SG LRCs encode for secretory or integral membrane proteins (Fig. 3A), which suggests the ability of these LRCs to organize their niche and respond to their special environment. Many of the extracellular matrix and cell adhesion proteins involved in niche formation/interaction like tenascin (Tnc), biglycan (Bgn), collagens (Col) and laminins (Lam) were also up regulated. Interestingly when minor SG LRCs were compared with the signature gene list of LRC of quiescent hair follicle (HF) stem cells [10] [19], minor SG LRC were found to express almost 30% of mRNAs present in hair follicle LRCs (Fig. 3F).
Figure 3. Transcriptional profiling of minor SG LRCs.
Functional annotation of genes up-regulated (>2.5×) in minor SG LRCs (A). Real-time PCR confirmation of selected signature genes of the minor SG LRCs obtained in array analysis (B). Side view sections of soft palate of mice after 4-week chase with H2BGFP (green) and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with antibodies (Abs) for Cdh2, TnC, Tspan6 as indicated (Red) (C–D). Comparisons of minor SG LRCs and hair follicle LRCs profiles. Venn diagram showing similarities between the minor SG LRCs and hair follicle LRCs molecular signature (F). Table in (F) highlights several of the key similarly expressed genes in minor SG LRCs and hair follicle LRCs. Scale bar: C–E 50µm.
Figure 4. Wnt pathway is not active in minor SG LRCs, whereas Sonic hedgehog signals predominantly to stromal cells.
View of soft palate of Tcf/Lef:H2B-GFP reporter mice (A) and Gli1CreER/RosaZsGreen mice 1 month after Tamoxifen induction (C). Side view sections of soft palate of Tcf/Lef:H2B-GFP reporter mice (B) and Gli1CreER/RosaZsGreen mice 1 month after Tamoxifen induction (D). Shown are epifluorescence of H2B-GFP (green) or ZsGreen and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with antibody as indicated (Red). Arrows in A and B denote Tcf/Lef1:H2BGFP positive cells localized in the upper duct of minor salivary gland and epithelium of soft palate. Arrows in C and D denote Shh responsive cells in the stroma surrounding the minor SG, whereas arrow head indicate Shh responsive cells localized in the upper duct of minor SG. Scale bar: B and D 50µm. Abbreviations: ep, epithelium; SP, soft patale; HP, hard palate.
Activation of TGFβ signaling pathway in minor salivary glands LRCs and BMP pathway in early progenitors
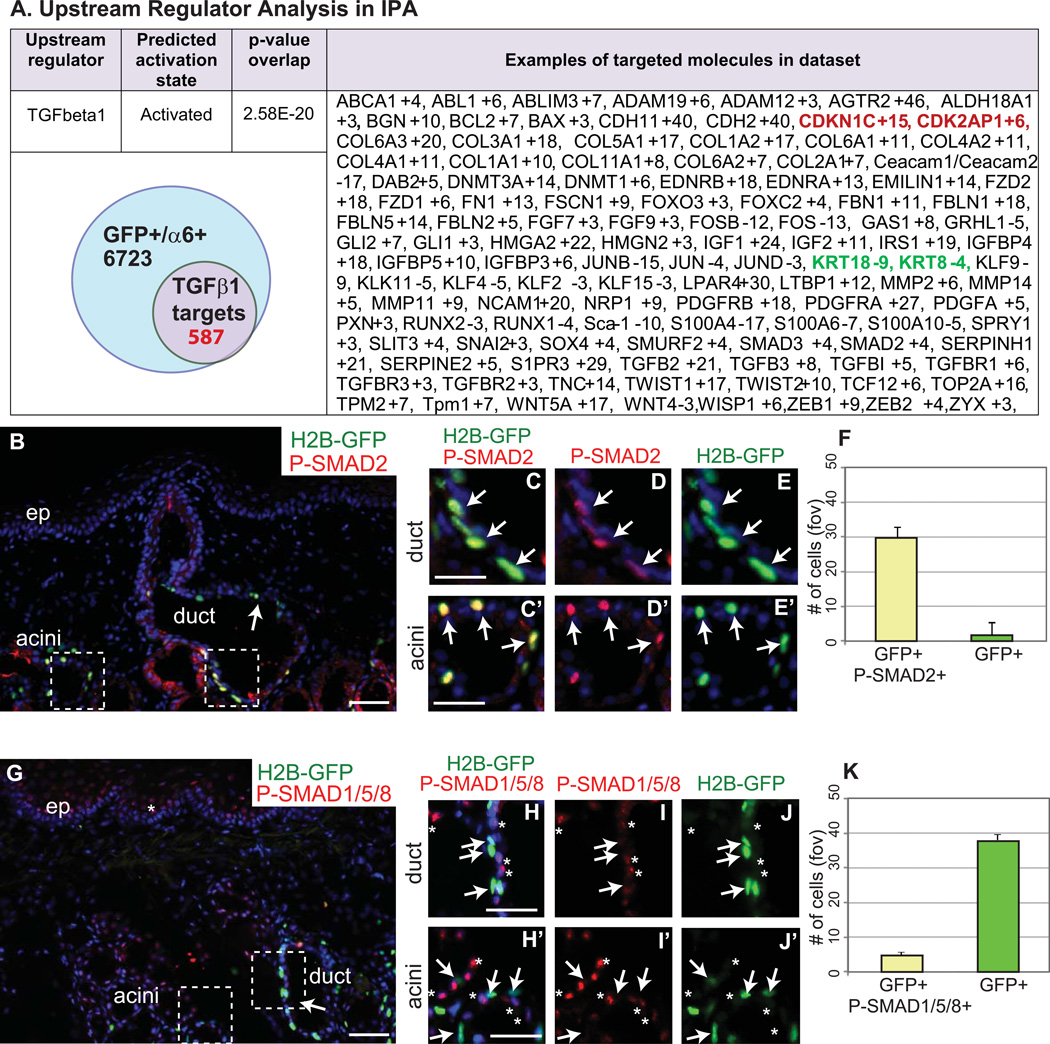
Consistent with their slow-cycling properties, minor SG LRCs expressed elevated transcripts encoding cell cycle inhibitors: Cdkn1c (p57) and Cdk2Ap1 (p12) that are known targets of TGFβ signaling pathway [20] [21]. TGFβ-was previously shown to induce cell cycle arrest in human hematopoietic stem cells through the up-regulation of p57/KIP2 [20]. It is well established that at high levels, TGFβ often inhibits cell proliferation in a reversible manner [22]. Increasing evidence suggests that the TGFβ constitutes as an integral component in the intercellular crosstalk between stem cells and their microenvironment along with maintaining the balance between active proliferation and reversible cell cycle exit in reservoirs of stem cells [23]. Therefore we further tested if TGFβ pathway is activated preferentially in the minor SG LRCs. Indeed we observed that the prevalent TGFβ receptor activation by nuclear phospho-Smad2 immunoreactivity in the H2BGFP LRCs when compared to the adjacent progeny (Fig. 5B–F). We used Upstream Regulator Analysis in Ingenuity Pathway Analysis (IPA) software to identify the cascade of upstream transcriptional regulators that can explain the observed gene expression changes in our dataset, which can help illuminate the biological activities occurring in the minor SG LRCs. Consistent with our initial observation TGFβ was the top growth factor - upstream regulator (Fig. 5A). Minor SG LRC had 587 activated TGFβ target genes within all up/down (+/− 2.5) genes including cell cycle inhibitors Cdkn1c (p57) and Cdk2Ap1 (p12), known stem cell genes ABCA1, ALDH18A1, Hmga2 and also components of TGFβ pathway, Smad2, Smad3, Smurf2, TGFβ2, TGFβ3, TGFBI, TGFBR1 and TGFBR2 and TGFBR3. Targets of TGFβ involved in niche regulation included tenascin, biglycan and collagens (Fig. 5A and Table S2). Since BMP signaling pathway was previously shown to be required to maintain a quiescent state in hair follicle stem cells [24] we also checked the status of BMP pathway activation in minor SG LRCs. Surprisingly indicative of BMP receptor activation, nuclear phospho-Smad1,5,8 immunoreactivity was prevalent in the non-LRC adjacent progenitor cells but not in LRCs (Fig. 5G–K). Consistently the array data showed down-regulation of known BMP target genes Id2 and Id4 in the LRCs when compared to the non-LRCs (Table S1).
Figure 5. Activation of TGFβ signaling pathway in minor salivary glands LRCs and BMP pathway in early progenitors.
Upstream regulator analysis in IPA. Venn diagram showing known TGFβ targets (IPA database) that are differentially expressed in minor SG LRCs, examples of those genes are shown in table (A). Active TGFβ signaling is found in minor SG LRCs indicated by positive phospho-Smad2 expression (B–F). Active BMP signaling is found in minor SG non-LRCs indicated by positive phospho-Smad1/5/8 expression (G–K). The number of phospho-Smad2 and phospho-Smad1/5/8 cells and overlap with GFP positive cells is shown in F and K, respectively. Scale bar: B and G 50µm; C–E’ and H–J’ 20µm. Arrows denote GFP positive LRC, asterisk indicate non-LRCs. Abbreviations: ep, epithelium; fov, field of view.
Minor salivary glands are sensitive to a carcinogenic tobacco compound and give rise to a low-grade adenoma
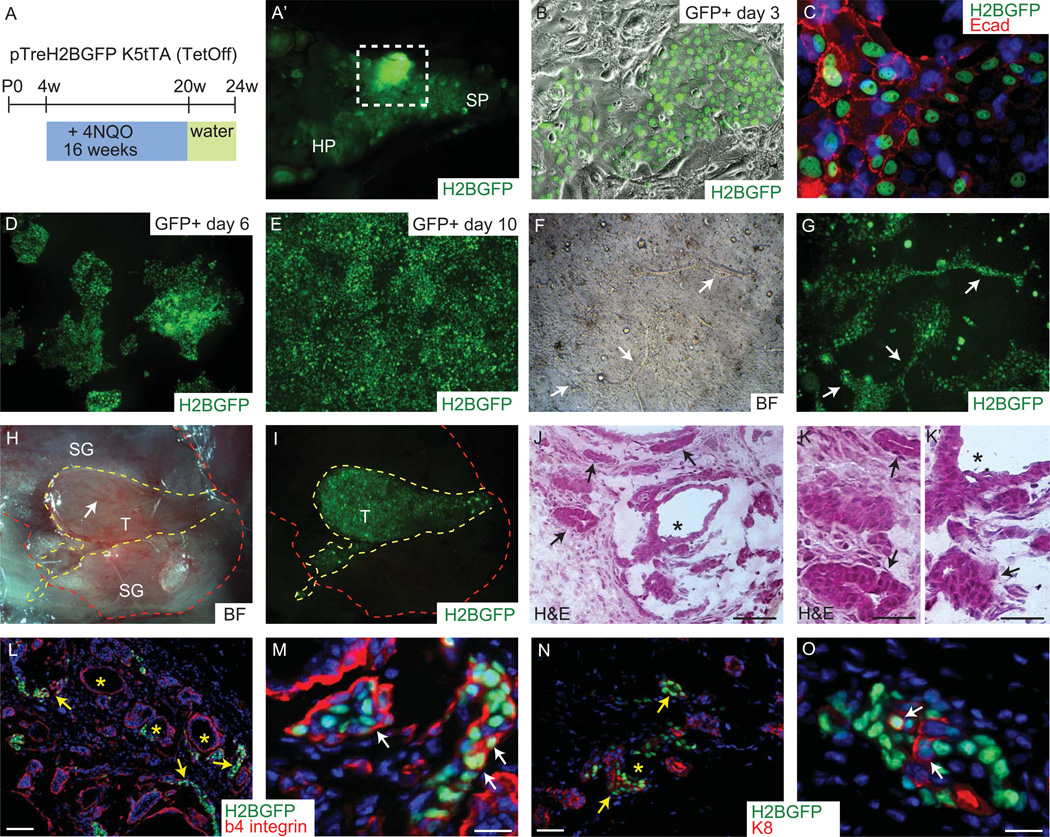
The analysis of tumor cell of origin requires a better understanding in all tissue cell types and their position in the lineage hierarchy. In particular, stem cells are often considered to be excellent candidate cells of origin for cancer, given their inherent ability to self-renew. To test the tumorigenic potential of K5 driven H2BGFP positive cells of the minor salivary glands we employed a previously published mouse model of 4NQO-induced oral tumors [25] on transgenic K5-tTA/pTRE-H2B-GFP mouse line. Mice were administered for 16 weeks with 4-nitroquinoline-oxide (4NQO, synthetic water-soluble organic compound that forms DNA adducts, serving as a surrogate of tobacco exposure) to induce oral cancer (Fig. 6A) [25]. At 24 weeks majority of the mice developed squamous cell carcinoma of the tongue as expected [25]. In addition, small tumors on the soft palate were also observed (Fig. 6A’). Since the tumors were very small in size excluding possibility of chase and FACS sorting we’ve established H2BGFP positive cell lines from dissected primary tumors to further test their tumorigenic potential. The minor SG derived tumor cells were highly clonogenic and could be maintained in culture for long period of time (Fig. 6D, E). When grown in 3D matrices the cells were prone to form tubular-like structures resembling ducts (Fig. 6F, G). To test the tumorigenic potential of those cells we injected the H2BGFP positive cells into the salivary gland of NOD.Cg donor mice. After 5 weeks the cells formed well-vascularized, visible tumors (Fig. 6H, I), which were still GFP positive (Fig. 6I). The morphology of the tumors that formed resembled low-grade adenoma. Histology revealed that the tumor had different growth patterns of ductal or tubular like structures (arrows in Fig. 6J and K), solid (arrows in Fig. 6K’) and microcystic with central lumina formed by one layer of cubic cells (asterisk in Fig. 6J and K’). The tumors that formed after the injection of established H2BGFP positive cell line expressed basal layer marker beta4-integrin (Fig. 6L and M), some of the cells also stained positive for luminal marker keratin 8 (Fig. 6N and O).
Figure 6. Tumorigenic potential of minor SG basal layer cells.
Schematic of pTreH2BGFP/K5tTA mice with 4NQO treatment (A). View of mouse soft palate with developed tumors in the minor SG (A’). Tumor cells were placed in culture and formed epithelial colonies (B–E), as indicated by E-cadherin presence (C). Minor SG tumor cells formed tubular-like structures when placed in 3D matrigel (arrows in F–G). Injected minor SG tumor cells into the gland of NOD.Cg mice formed secondary tumors that are highly vascularized (arrow in H) and could be traced due to the GFP label (I). Shown is H&E for tumor morphology (J, K and K’) and epifluorescence of H2BGFP (green) and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with antibodies as indicated (Red) (L–O). Scale bar: L and N 100µm; M and O 20µm. Arrows in J, K, L, and N indicate tubular-like structures, arrows in K’ show solid pattern and asterisk in J, K’, L and N indicate microcystic structures. Arrows in (M) indicate basal layer localization of GFP positive cells; arrows in (O) indicate K8 expression in GFP positive cells. Abbreviations: HP, hard palate; SP, soft palate; SG, salivary gland; T, tumor.
Discussion
Hundreds of minor salivary glands are present in the lining of the mucosa in the aerodigestive tract that provide protection to the oral tissues day and night [2] [3]. The cells with stem/progenitor properties have been detected in major salivary glands, however their precise characterization and function has not been addressed so far. In addition limited data is available about the existence of stem cells within the minor salivary glands [1] [4] [5] [6]. Our studies provide for the first time an insight into the stem cells biology of the minor salivary glands.
Preferential localization of LRCs in the excretory duct of the minor salivary gland and regenerative potential
The minor salivary glands are simpler in structure than the major SGs. They consist of small clusters of secretory cells with intercalated duct and the striated duct either less developed or not present and shorter excretory duct that delivers the saliva product onto the surface of the mucosa [2]. Accumulating data indicates that like other glandular tissues such as mammary gland and prostate, the cells with stem/progenitor properties are also present in the major salivary glands, however till date, the existence of stem cells in the minor salivary glands of soft palate has not been addressed. Here, we demonstrate that cells with slow cycling characteristic are present in minor salivary gland as a population localized in the basal layer of the excretory duct. The LRC were always present in the basal layer adjacent to K8 positive luminal layer suggesting that the LRC of minor salivary glands are only present in the excretory duct but not intercalated duct. Some of the LRC were also present in the acinar part of the gland bottom and we demonstrate their myoepithelial characteristic by SMA co-expression (Fig. 1). Previous studies on major salivary glands imply that cells capable of proliferation and differentiation reside only within the ducts of SGs and may represent potent stem cells [1]. Studies have also demonstrated that acinar cells themselves display a limited degree of proliferative ability, and the total ablation of acinar cell function in ligation experiments suggests that acinar cell proliferation is unlikely to account for the rescue of salivary gland function [1]. In our transplantation experiments we used a sorted fraction of GFP+/CD44+/α6+ cells but we were not able to distinguish between ductal versus acinar LRC. Further studies are needed to address the importance of ductal and acinar LRC in the minor salivary glands homeostasis.
TGFβ pathway governs the gene network regulation to maintain quiescent homeostasis in minor salivary glands
Recent discoveries suggest that the quiescent state is not just a passive state but, instead, actively regulated by different intrinsic mechanisms. Quiescent stem cells can sense environmental changes and respond by re-entering the cell cycle for proliferation [26]. To respond to those changes rapidly, a quiescent stem cell would maintain the expression of all necessary components that are required for activation and proliferation [26]. Our results indicate that slow cycling cells of minor salivary glands maintain the expression of some of the cyclins involved in cell cycle progression and at the same time consistent with their slow-cycling properties express high levels of cell cycle inhibitors Cdkn1c (p57) and Cdk2ap1 (p12). Many cyclin-dependent kinase inhibitors, including p21, p27 and p57, are expressed in quiescent stem cells and promote cell cycle arrest by inhibiting cyclin-dependent kinases. In double-knockout mice lacking both p57 and p27, HSC quiescence is severely impaired [27]. These studies suggest that cyclin-dependent kinase inhibitors are functionally important for the maintenance of stem cell quiescence. Some of the cyclin-dependent kinase inhibitors were shown to be directly regulated by TGFβ that at high levels TGFβ often inhibits cell proliferation in a reversible manner [20] [22]. Our analyses suggest that TGFβ might play a crucial role in maintaining the minor salivary stem cells in quiescent state by regulating genes important for cell cycle of stem cells but also by maintaining the proper microenvironment to balance active proliferation and reversible cell cycle exit in reservoirs of stem cells [23]. In hair follicle stem cells another member of TGF signaling family, namely BMP was shown to be crucial to maintain the hair follicle stem cells in quiescent state [24]. In addition intra-stem cell antagonistic competition, between BMP and Wnt signaling turned out to be crucial for maintaining balance in stem cell activity [28]. In the hair follicle SC niche, Wnt signaling and beta-catenin stabilization transiently activates Lef1/Tcf complexes and promotes their binding to target genes that promote transiently amplifying cell conversion and proliferation [29]. Consistent with those observations our data indicate that minor salivary gland LRC show activation of TGFβ pathway whereas Wnt signaling appears to be repressed, as we didn’t observe activation of β-catenin-dependent signaling, using transgenic reporter mice. Moreover minor SG LRCs have high expression levels of Wnt pathway inhibitors including Sfrp2, Dab2, Dact1, Tcf3, and Wif1. We also observed genes of Sonic hedgehog (Shh) pathway to be upregulated in LRC, which is associated with the maintenance of various adult epithelial stem cells [15], [16, 17]. Interestingly using Gli1 reporter mice we have learned that cells responding to Shh signaling are present in the stroma surrounding the minor SG. This observation is consistent with the model proposed by A.L. Joyner group [18] where Shh from basal epithelial cells of adult prostate signals to the surrounding stroma.
Tumorigenic potential of minor salivary gland basal cells
Minor salivary gland neoplasm represents a heterogeneous group with diverse morphologies, tumor biology and consequently, varied clinical behavior. Tumors that develop from these glands have the same histopathologic heterogeneity seen in those that arise from the parotid or submandibular glands. However, in contrast to major salivary gland tumors, which are predominantly benign, most minor salivary gland neoplasm tend to be malignant [30]. The etiology of these tumors remains unknown. There is limited association with conventional risk factors associated with squamous cell carcinoma, such as smoking and alcohol intake [30]. Interestingly our results showed that treatment of mice with 4NQO which is mimicking tobacco exposure resulted in formation of tumors that resembled low-grade adenoma with mixed pattern indicating that K5 derived cells from minor salivary glands give rise to both cribriform areas–and double-layered ductal like structures with K5 and K8 positive cells. Unfortunately with our current system where K5 marks both basal cell population within the duct and myoepithelial population within the acini we can’t distinguish if one of those populations can give rise preferentially to different structures within the tumor or they participate equally.
So far the knowledge on minor salivary gland development and presence of stem cells in those glands was relatively limited compared with what was known about major gland. In this current report for the first time we localize and characterize label-retaining cells with stem cell properties in the minor salivary glands. Regeneration or repair of salivary glands requires understanding of the spatial and temporal interactions of the various cell types within the gland as it develops and during homeostasis. The signaling systems in the minor SG stem cells and their niche will need to be incorporated into current models of salivary bioengineering as well as regenerative therapies for a successful outcome. Our data serve to emphasize the importance of some of those signaling systems in the development and maintenance of minor SG stem cells.
Supplementary Material
Side view sections of soft palate of pTreH2BGFP/K5tTA mice after four (A) and seven (B) weeks of Doxy chase, with the slow cycling cells retaining GFP label. Shown is epifluorescence of H2B-GFP (green) and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with Ki67 antibody (Red). Scale bar: A and B 50µm. Arrows denote GFP positive LRC. Abbreviations: ep, epithelium.
Intraglandular transplantation of minor SG.
Sorted minor SG cells: GFP+ CD44+ α6+ (LRCs) and CD44+ α6+ (progenitor population) after expansion in culture were mixed with matrigel and injected into submandibular salivary gland of NOD.Cg mouse (A). The unsorted dissected clumps were microdissected directly from 4 weeks chased minor SG tissue and inserted into submandibular gland of immunocompromised NOD.Cg mice. 8 weeks after injection/insertion, mice were sacrificed, and transplants were dissected out and analyzed. Epifluorescence of H2B-GFP (green) and indirect immunofluorescence with antibodies as indicated of sections of ductal structures that formed after transplantation of dissected minor salivary glands from soft palate (B) and sorted GFP+ CD44+ α6+(LRCs) (C) are shown. Scale bar: B and C 20µm. Arrows denote GFP positive cells that formed ductal like structures after transplantation and overlapped with Ki67.
DEG list (2.5 fold change) of minor SG LRCs compared to non-LRCs.
Minor SG LRC contain 587 activated TGFβ target genes within all up/down (+/− 2.5) genes.
Acknowledgments
We thank Dr. Tudorita Tumbar (Cornell University Ithaca, NY, USA) for her help with H2BGFP mice model optimization. We thank Dr. Anita B. Roberts (National Cancer Institute, NCI) for floxed-Smad1 and Dr. An Zwijsen (University of Leuven) for Smad5 mice. We thank Dr. Yang Chai and Dr. Hu Zhao (University of Southern California) for GliCreER/RosaZsgree samples. We thank Dr. Colin Jamora (University of California, San Diego, CA, USA) for the sharing of K5 antibody. The TROMA-I (K8) monoclonal antibody developed by Philippe Brulet and Rolf Kemler was obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was partially supported by the NIH/NIDCR grant R215351568360 (to AK). KK is supported by NIH/NIAMS Grants R01-AR061552 and R03-AR061028. This work was supported in part also by award number P30CA014089 from the NCI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Authors contribution: HZ: conception and design, collection and/or assembly of data, KB: conception and design, collection and/or assembly of data, EK: collection of data, KK: data analysis and interpretation, YC: microarray data analysis, SZ: collection of data, RK: collection of data, US: data analysis and interpretation, A.K: conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript.
References
- 1.Pringle S, Van Os R, Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2012;31:613–619. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 2.Hand AR, Pathmanathan D, Field RB. Morphological features of the minor salivary glands. Arch Oral Biol. 1999;44(Suppl 1):S3–S10. doi: 10.1016/s0003-9969(99)90002-x. [DOI] [PubMed] [Google Scholar]
- 3.Riva A, Puxeddu R, Uras L, et al. A high resolution sem study of human minor salivary glands. Eur J Morphol. 2000;38:219–226. doi: 10.1076/0924-3860(200010)38:4;1-o;ft219. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz S, Rotter N. Human salivary gland stem cells: isolation, propagation, and characterization. Methods Mol Biol. 2012;879:403–442. doi: 10.1007/978-1-61779-815-3_25. [DOI] [PubMed] [Google Scholar]
- 5.Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17:445–449. doi: 10.1111/j.1601-0825.2010.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanduri LS, Maimets M, Pringle SA, et al. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 7.Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David R, Shai E, Aframian DJ, et al. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng Part A. 2008;14:331–337. doi: 10.1089/tea.2007.0122. [DOI] [PubMed] [Google Scholar]
- 9.Bullard T, Koek L, Roztocil E, et al. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol. 2008;320:72–78. doi: 10.1016/j.ydbio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer NG, Kim L, Nixon IJ, et al. Factors predicting outcome in malignant minor salivary gland tumors of the oropharynx. Arch Otolaryngol Head Neck Surg. 2010;136:1240–1247. doi: 10.1001/archoto.2010.213. [DOI] [PubMed] [Google Scholar]
- 12.Diamond I, Owolabi T, Marco M, et al. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 13.Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer-Vaquer A, Piliszek A, Tian G, et al. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 2010;10:121. doi: 10.1186/1471-213X-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 16.Wang LC, Liu ZY, Gambardella L, et al. Regular articles: conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol. 2000;114:901–908. doi: 10.1046/j.1523-1747.2000.00951.x. [DOI] [PubMed] [Google Scholar]
- 17.Brownell I, Guevara E, Bai CB, et al. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–565. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng YC, Levine CM, Zahid S, et al. Sonic hedgehog signals to multiple prostate stromal stem cells that replenish distinct stromal subtypes during regeneration. Proc Natl Acad Sci U S A. 2013;110:20611–20616. doi: 10.1073/pnas.1315729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scandura JM, Boccuni P, Massague J, et al. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci U S A. 2004;101:15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu MG, Hu GF, Kim Y, et al. Role of p12(CDK2-AP1) in transforming growth factor-beta1-mediated growth suppression. Cancer Res. 2004;64:490–499. doi: 10.1158/0008-5472.can-03-2284. [DOI] [PubMed] [Google Scholar]
- 22.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshimori N, Fuchs E. The harmonies played by TGF-beta in stem cell biology. Cell Stem Cell. 2012;11:751–764. doi: 10.1016/j.stem.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobielak K, Stokes N, de la Cruz J, et al. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–10068. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czerninski R, Amornphimoltham P, Patel V, et al. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009;2:27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 26.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou P, Yoshihara H, Hosokawa K, et al. p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70. Cell Stem Cell. 2011;9:247–261. doi: 10.1016/j.stem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Kandyba E, Leung Y, Chen YB, et al. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc Natl Acad Sci U S A. 2013;110:1351–1356. doi: 10.1073/pnas.1121312110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowry WE, Blanpain C, Nowak JA, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzzo M, Locati LD, Prott FJ, et al. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. 2010;74:134–148. doi: 10.1016/j.critrevonc.2009.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Side view sections of soft palate of pTreH2BGFP/K5tTA mice after four (A) and seven (B) weeks of Doxy chase, with the slow cycling cells retaining GFP label. Shown is epifluorescence of H2B-GFP (green) and 4’,6’-diamidino-2-phenylindole (DAPI) (blue), and indirect immunofluorescence with Ki67 antibody (Red). Scale bar: A and B 50µm. Arrows denote GFP positive LRC. Abbreviations: ep, epithelium.
Intraglandular transplantation of minor SG.
Sorted minor SG cells: GFP+ CD44+ α6+ (LRCs) and CD44+ α6+ (progenitor population) after expansion in culture were mixed with matrigel and injected into submandibular salivary gland of NOD.Cg mouse (A). The unsorted dissected clumps were microdissected directly from 4 weeks chased minor SG tissue and inserted into submandibular gland of immunocompromised NOD.Cg mice. 8 weeks after injection/insertion, mice were sacrificed, and transplants were dissected out and analyzed. Epifluorescence of H2B-GFP (green) and indirect immunofluorescence with antibodies as indicated of sections of ductal structures that formed after transplantation of dissected minor salivary glands from soft palate (B) and sorted GFP+ CD44+ α6+(LRCs) (C) are shown. Scale bar: B and C 20µm. Arrows denote GFP positive cells that formed ductal like structures after transplantation and overlapped with Ki67.
DEG list (2.5 fold change) of minor SG LRCs compared to non-LRCs.
Minor SG LRC contain 587 activated TGFβ target genes within all up/down (+/− 2.5) genes.