Abstract
This study investigated the potential causes of anxious people's social avoidance. The classic ultimatum game (UG) was utilized in concert with electroencephalogram (EEG) recording. Participants were divided into two groups according to levels of trait anxiety as identified by a self-report scale. The behavioral results indicate that high-anxious participants were more prone to reject human-proposed than computer-proposed unequal offers compared to their low-anxious counterparts. The event-related potential (ERP) results indicate that the high-anxious group showed a larger feedback-related negativity (FRN) when receiving unequal monetary offers than equal ones, and a larger P3 when receiving human-proposed offers than computer-proposed ones, but these effects were absent in the low-anxious group. We suggest anxious people's social avoidance results from hypersensitivity to unequal distributions during interpersonal interactions.
Keywords: Anxiety, social decision-making, Ultimatum Game, fairness evaluation, equality, event-related potential (ERP), feedback-related negativity (FRN), P3
Introduction
Linking Anxiety with Social Decision-making
Anxiety, an unpleasant emotional state characterized by automatic cognitive bias to threat-related information, plays a significant role in our everyday lives (D. M. Clark, 1999). In its exaggerated form, anxiety interrupts daily function such as social skills (J. V. Clark & Arkowitz, 1975). Patients suffering from anxiety disorders deliver poor performance in interpersonal interactions (Hirsch, Meynen, & Clark, 2004). For example, anxiety disorders are associated with spousal conflict (Whisman, Sheldon, & Goering, 2000). This social impairment may result from decision-making biases that lead anxious individuals to choose avoidance strategies in interpersonal situations (Grecucci et al., 2012). Indeed, levels of anxiety are negatively correlated with the willingness to communicate with strangers (Duronto, Nishida, & Nakayama, 2005; Samochowiec & Florack, 2010). When deciding whether to engage in a risky but potentially rewarding social activity (e.g., dating an unknown person), high-anxious people's expectations are more pessimistic than those of non-anxious people (Wray & Stone, 2005). Investigating the relationship between anxiety and social decision-making may have important implications for the treatment of social dysfunction and thus help improving anxious people's quality of life.
Social Decision-making Paradigm: Ultimatum Game
The Ultimatum Game (UG) is a classic paradigm for social decision-making research (Güth, Huck, & Müller, 2001; Güth, Schmittberger, & Schwarze, 1982). In this game, one of two players (“proposer”) splits an amount of money between the two. The other player (“responder”) may accept the offer, but also has the right to reject it, in which case neither players receive anything. Numerous studies have reported that the acceptance rates significantly decrease when responders receive disadvantageous unequal offers, often interpreted to indicate that people have a taste for fairness (Camerer & Thaler, 1995; see Feng et al., 2013 for the discussion on the relationship between equality and fairness). In addition, acceptance rates of offers from putative human proposers are lower than those from the computer, indicating a motivation to punish nonreciprocators during social interaction (Rilling & Sanfey, 2011; Sanfey, Rilling, Aronson, Nystrom, & Cohen, 2003). In other words, UG responders consider both the equality factor and the social-interaction factor when making decisions.
The processing of both factors might be affected by the feeling of anxiety. Anxious people's stronger emotional reactivity might lead to oversensitive reactions to inequitable distribution (Grecucci et al., 2012). However, anxious people might be less likely to reject unequal offers because they tend to avoid social confrontation with others (Mennin, Heimberg, Turk, & Fresco, 2002). The behavioral results of Grecucci et al. (2012) support the second hypothesis, which highlights the importance of the social-interaction factor. In contrast, our recent electrodermal study suggests that the skin conductance responses to unequal offers were positively correlated with levels of anxiety in the high-anxious group, indicating that the equality factor affected high-anxious people's emotional responses to offers (T. Wu, Luo, Broster, Gu, & Luo, 2013). Despite these discrepancies, both studies reveal that the impact of anxiety on UG performance is significant. It was hypothesized that anxious people's UG behavior manifests a tradeoff between the equality and social-interaction factors, and these two studies may have captured different aspects of this tradeoff. The present study aimed to test this theory.
Electrophysiological Indices
Event-related potentials (ERPs) are an important neuroscience technique to relate behavioral performance and brain electrical activity during cognitive tasks (Rugg & Coles, 1996). By utilizing the ERP, the present study investigated anxious individuals’ fairness considerations when evaluating UG offers. Two ERP components, namely the feedback-related negativity (FRN) and P3, were chosen for the analyses.
The FRN represents a rapid evaluation of the subjective value of current events along a “good-no good” dimension, with “no good” events elicit a larger FRN than “good” events (Holroyd, Hajcak, & Larsen, 2006; Holroyd, Larsen, & Cohen, 2004). During the UG, the FRN amplitude is more pronounced in response to unequal offers than to equal offers (Alexopoulos, Pfabigan, Lamm, Bauer, & Fischmeister, 2012; Boksem & De Cremer, 2010; Campanha, Minati, Fregni, & Boggio, 2011; Van der Veen & Sahibdin, 2011).
The P3 component also plays an important role in the evaluative process; its amplitude responds to all the major properties of an event. For instance, many studies suggest that during monetary gambling, the FRN responds specifically to outcome valence while the P3 encodes both valence and magnitude (Gu et al., 2011; Philiastides, Biele, Vavatzanidis, Kazzer, & Heekeren, 2010; Y. Wu & Zhou, 2009). In the same vein, social decision-making literature suggests that P3 amplitude is sensitive both to equality considerations and to social context, such that unequal offers elicit a larger P3 response than equal offers (Hewig et al., 2011), and the same is true when contrasting other-relevant and self-relevant properties (Y. Wu, Hu, van Dijk, Leliveld, & Zhou, 2012). Consequently, the FRN and P3 were chosen to detect the brain processing of the equality and social-interaction factors.
Previous research has linked FRN and P3 amplitudes to anxiety. High-anxious participants show a smaller FRN elicited by negative feedback during monetary gambling (Gu, Ge, Jiang, & Luo, 2010; Gu, Huang, & Luo, 2010). In response to novel stimuli, the P3 became larger as anxiety levels increased (Grillon & Ameli, 1994), which might be linked with an altered mechanism of attention allocation (see Polich, 2007 for the relationship between the P3 and attentional resources). The neurotransmitter activities underlying the FRN and P3 components, including dopamine (Holroyd & Coles, 2002) and norepinephrine (Polich & Criado, 2006), are related to anxiety symptoms (Goddard et al., 2010; Yoshioka, Matsumoto, Togashi, & Saito, 1996). These findings support our idea that both the FRN and P3 would be sensitive to anxiety during social decision-making.
Experimental Hypotheses
In the current study, the classic UG paradigm was utilized with electroencephalogram (EEG) recording. Due to the characteristics of the FRN and P3 described above, we predicted that both components would evidence the influence of anxiety on the equality factor, such that the main effect of equality would be more prominent in the high-anxious group than in the low-anxious group. Further, we predicted that the influence of anxiety on the social-interaction factor would be evidenced on the P3, such that the main effect of social interaction would be more prominent in the high-anxious group than in their low-anxious counterparts.
Method
Participants
Two hundred and fifty-nine undergraduate students (all Chinese) were recruited from the campus of Beijing Normal University. They completed the Chinese version of the Trait form of Spielberger's State-Trait Anxiety Inventory (STAI-T), which has demonstrated good internal consistency reliability, convergent validity, and discriminate validity (Shek, 1993; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). In this sample, individuals with STAI-T scores in the lower 25% of the distribution were assigned to the low-trait anxiety (LTA) group, whereas individuals in the upper 25% of the distribution were assigned to the high-trait anxiety (HTA) group (Gu, Ge et al., 2010; T. Wu et al., 2013). From those who were suitable for these criteria, we randomly chose 40 students and asked them to participate in the formal experiment, with both the LTA group and the HTA group consisting of 20 participants (LTA group: 8 females, mean age: 22.7 ± 2.0 years; HTA group: 12 females, mean age: 20.4 ± 2.2 years). An independent-samples t-test confirmed significantly lower levels of trait anxiety among LTA participants (31.2 ± 3.9; [23, 36]) compared to HTA participants (57.0 ± 6.6; [48, 69]) (t(38) = 15.13, p < .001). Participants had normal or corrected-to-normal vision and had no history of psychiatric, medical, or neurological illness. All were right-handed. All participants provided written informed consent prior to the experiment.
In appreciation of the strong relationship between anxiety and depression (L. A. Clark & Watson, 1991; Stavrakaki & Vargo, 1986), the Chinese version of Zung's self-rating depression scale (SDS) was used to assess self-reported symptoms of depression in order to control the potential effect of depression during statistical analysis. Both clinical and non-clinical studies have established excellent reliability and validity of the SDS (Shu, 1993; Zung, Richards, & Short, 1965). An independent-samples t-test revealed that the two groups differed significantly in SDS scores (LTA: 29.0 ± 6.0, HTA: 40.0 ± 6.0; t(38) = 5.71, p < .001).
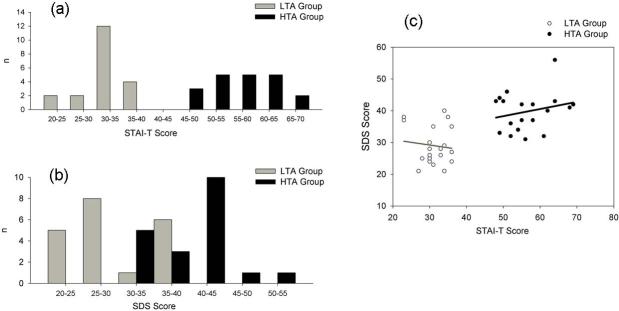
See Figure 1 for the distributions of the STAI-T and SDS scores. The correlation between STAT-T and SDS was not significant in either the LTA group (r = -.109, p = .649) or the HTA group (r = .247, p = .293).
Figure 1.
The distributions of the STAI-T (a) and SDS (b) scores, as well as their relationship (c), in the two groups. HTA: high-trait anxiety; LTA: low-trait anxiety.
Procedure
The task procedure replicated that of Wu et al. (2013). To reinforce the social nature of the task, participants were told that they would play the UG together with three other anonymous college students, but in fact no other people were playing the game. Participants received no further information about the identities of the other supposed players. Before the task, each participant was instructed about the rules in the UG and was informed that his/her payment depended on his/her choice in the task; the higher the score a participant earned, the higher payment s/he would receive at the end of the experiment.
Figure 2 illustrates the experimental procedure of an exemplar trial. Each trial began with the presentation of a central fixation cross for 1.5 - 2 s (randomized across trials). Afterwards, an offer proposed by a pseudo-player or the computer was presented for 2 s. Participants played as responders and decided whether or not to accept the offer by pressing the “F” or “J” buttons on the keyboard with their left or right index fingers (the button assignments were counterbalanced across participants). After participants finished their decisions, they waited for 0.8-1.2 s and received the feedback which informed about the results of the current trial.
Figure 2.

Single-trial settings. A central fixation cross was presented for 1.5 - 2 s, which was followed by an offer (made by a pseudo-player or the computer) presented for a time window of 2 s. Immediately afterwards, participants had to decide whether to accept the proposal or not. After a short delay (0.8 – 1.2 s), a feedback screen informed participants about the results of the current trial.
There were six blocks in the task. In three of the blocks (human-proposer blocks), participants were told that the offers were proposed by the other three players, whereas in the other three blocks (computer-proposer blocks), the offers were proposed randomly by the computer. Each block consisted of 50 trials (300 trials in total), with each block separated by a short interval. Before the start of the task, participants were told that the offers in human-proposer blocks were randomly selected from the other three players’ proposals, that they would be unable to identify which proposer suggested the offer in a given trial, and that the “proposers” would not be able to know whether the participants accepted or rejected their offers (Boksem & De Cremer, 2010). Unbeknownst to the participants, all offers were actually proposed by the computer in predetermined pseudorandom sequences. Each block included 20 equitable trials (10 offers of 50:50 and 10 offers of 40:60), 20 inequitable trials (10 offers of 10:90 and 10 offers of 20:80), and 10 moderate inequitable offers (30:70).
Electrophysiological Recording and Measures
The electroencephalogram (EEG) was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (NeuroScan Inc.) with an online reference to the left mastoid and off-line algebraic re-reference to the average of the left and right mastoids. The electrooculogram (EOG) was recorded for the purpose of artifact correction. Horizontal EOG was recorded from electrodes placed at the outer canthi of both eyes. Vertical EOG was recorded from electrodes placed above and below the left eye. All inter-electrode impedance was maintained at < 5 kΩ. EEG and EOG signals were amplified with a 0.05 – 100 Hz online band-pass filter and continuously sampled at 500 Hz/channel.
During the offline analysis, ocular artifacts were removed from the EEG signal using a regression procedure implemented with Neuroscan software (Semlitsch, Anderer, Schuster, & Presslich, 1986). After 30 Hz low-pass digital filtering through a zero phase shift, the EEG data were epoched time-locked following the onset of each offer. Separate EEG epochs of 1000 ms were baseline-corrected by subtracting from each sample the average activity of that channel during the −200 – 0 ms baseline period. Any trial in which EEG voltages exceeded a threshold of ± 100 μV during the recording epoch was excluded from further analysis. As a result, 15.3 ± 14.0% of the epochs were rejected across participants.
According to previous literature, there are essentially two ways to calculated the FRN amplitude, that is, either using the grand-averaged waveforms or creating difference wave between “error” and “correct” trials (Holroyd, Pakzad-Vaezi, & Krigolson, 2008). The latter approach is based on the assumption that the FRN appears only on “error” trials, but this has been criticized because the differential ERPs could be caused by neural activity associated with either condition (Foti, Weinberg, Dien, & Hajcak, 2011; Holroyd et al., 2008). In addition, we are interested in the group effect on the FRN in both the unequal condition and the equal condition. Thus in the current study, we directly measured the grand-averaged waveforms rather than the difference wave so as to observe the ERPs in two conditions separately.
FRN amplitudes were measured as the mean values within the 300 – 350 ms time window following offer presentation. According to visual inspection of grand-averaged waveforms and their scalp distributions, the FRN was maximal in the frontal area. Hence, six frontal electrodes in this area (F1, Fz, F2, FC1, FCz, FC2) were chosen for further analysis of the FRN. P3 amplitudes were measured as the mean values within the 400 – 600 ms time window. The P3 was maximal in the parietal-occipital area. Hence, four parietal-occipital midline electrodes (CPz, Pz, POz, Oz) were chosen for analysis of the P3.
Data Analysis
Acceptance rates were analyzed using three-way Equality (equal vs. unequal) × Proposer (human vs. computer) × Anxiety (LTA vs. HTA) ANOVA test, while the Electrode was added into the analyses on the FRN and P3 amplitudes as the fourth factor. As mentioned above, the Electrode factor included six levels for the FRN and four levels for the P3. The acceptance rates for equal (i.e., 40:60 and 50:50) and unequal (i.e., 10:90 and 20:80) offers were calculated respectively (Harle & Sanfey, 2007; Hewig et al., 2011). Moderate unequal offers (30:70) were excluded from analysis, since previous studies suggest that UG players hold diverse opinions about whether such offers should be regarded as fair (Halko, Hlushchuk, Hari, & Schurmann, 2009; Hewig et al., 2011; van't Wout, Chang, & Sanfey, 2010).
For all the analyses, the significance level was set at .05. Greenhouse-Geisser correction for ANOVA tests was used when appropriate. Post-hoc comparisons were evaluated using the Newman–Keuls method. Partial eta-squared () values were provided to demonstrate effect size where appropriate, such that 0.05 represents a small effect, 0.10 represents a medium effect, and 0.20 represents a large effect (Cohen, 1973).
Results
Behavioral Results
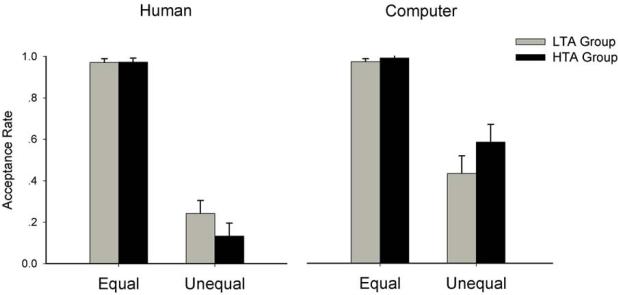
See Figure 3 for the illustration of behavioral data. A significant main effect of the Equality factor was confirmed (F(1, 38) = 182.75, p < .001, ), with the acceptance rate being lower for unequal offers (34.9 ± 4.6%) than equal offers (97.7 ± 1.1%). The main effect of the Proposer factor was also significant (F(1, 38) = 39.35, p < .001, ), with the acceptance rate being lower when participants received offers in the human-proposer condition (58.0 ± 2.3%) than those in the computer-proposer condition (74.5 ± 3.1%). The Proposer × Equality interaction was significant (F(1, 38) = 36.55, p < .001, ). The Proposer × Anxiety interaction was significant (F(1, 38) = 6.86, p = .013, ). The Proposer × Equality × Anxiety interaction was significant (F(1, 38) = 5.57, p = .022, ); while both the LTA and the HTA group accepted fewer unequal offers proposed by human than by computer, this effect appeared to be weaker in the LTA group (F(1, 38) = 6.71, p = .013, ) than in the HTA group (F(1, 38) = 35.51, p < .001, ). Finally, when adding depression as a covariate in the ANOVA test, the Proposer × Equality × Anxiety interaction (F(1, 37) = 5.58, p = .024, ) remained significant, indicating that the influence of anxiety on UG performance was not attributable to the effect of depression.
Figure 3.
The illustration of the Proposer × Equality × Anxiety interaction on the acceptance rate. Error bars indicate 1 SE. HTA: high-trait anxiety; LTA: low-trait anxiety.
ERP Results
The FRN component
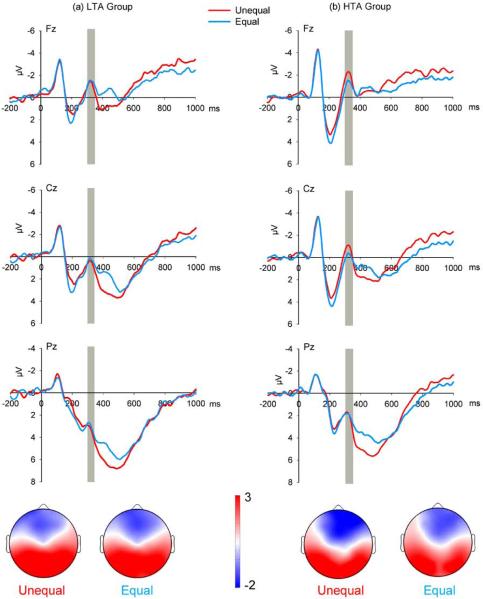
See Figure 4 for the FRN results in each group. The main effect of Electrode was significant (F(5, 190) = 13.77, p < .001, ), with the FRN being larger at Fz (−1.43 μV) and F2 (−1.30 μV) than other electrodes selected (p values < .05), but these two sites showed no significant difference with each other (p = .132). No significant main effect of the Equality factor (F(1, 38) = 1.90, p = .176, ), the Proposer factor (F(1, 38) = 0.94, p = .338, ), or the Anxiety factor (F(1, 38) = 0.27, p = .604, ) was detected. A significant interaction of Equality × Anxiety was observed (F(1, 38) = 4.74, p = .036, ); no FRN difference reached significance in the LTA group (F(1, 38) = 0.32, p = .575, ), but the FRN was larger in response to unequal offers (−1.60 μV) compared to equal offers (−0.89 μV) in the HTA group (F(1, 38) = 6.31, p = .016, ). No other significant interaction was found (F values < 2.94, p values > .05). When adding depression as a covariate in the ANOVA test, the Equality × Anxiety interaction remained significant (F(1, 37) = 4.73, p = .036, ), indicating that the influence of anxiety on the FRN was not attributable to the effect of depression.
Figure 4.
The effect of the Equality factor on grand-average ERPs evoked by offers in the low-trait anxiety (LTA) group (a) and the high-trait anxiety (HTA) group (b) at mid-line recording sites Fz, Cz, and Pz. The time point “0” indicates offer presentation onset. The gray shaded areas indicate the 300–350 ms time window for the calculation of the mean value. The scalp topographies of each condition are presented beneath. Note: The waveforms in this figure are presented to provide a comparison of the midline scalp topographies for the FRN results. The electrodes used in the ANOVA were F1, Fz, F2, FC1, FCz, and FC2.
The P3 component
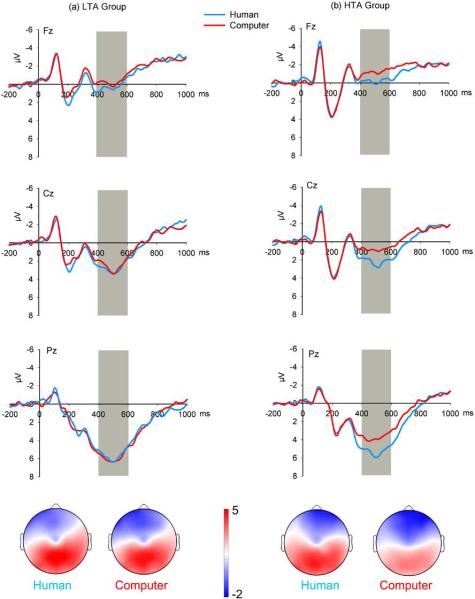
See Figure 5 for the P3 results in each group. The main effect of Electrode was significant (F(3, 114) = 14.62, p < .001, ), with the P3 being larger at Pz (5.14 μV) and POz (5.06 μV) than other electrodes selected (p values < .05), but these two sites showed no significant difference with each other (p = .075). The main effect of Equality was significant (F(1, 38) = 12.85, p = .001, ), indicating that the P3 was larger in response to unequal offers (4.87 μV) than to equal offers (4.00 μV). The main effect of Proposer was also significant (F(1, 38) = 7.47, p = .009, ); the P3 was larger when the proposer was believed to be human being (4.86 μV) and smaller when the proposer was known to be the computer (4.00 μV). The main effect of Anxiety was not significant (F(1, 38) = 1.32, p = .257, ). The interaction of Proposer × Anxiety was significant (F(1, 38) = 5.84, p = .021, ); no P3 difference reached significance for the LTA group (F(1, 38) = 0.05, p = .82, ), but the P3 was larger in the human-proposer condition (4.65 μV) than in the computer-proposer condition (3.22 μV) for the HTA group (F(1, 38) = 13.25, p = .001, ). Neither the Proposer × Equality interaction (F(1, 38) = 2.75, p = .105, ) nor the Proposer × Equality × Anxiety interaction (F(1, 38) = 0.57, p = 0.45, ) was significant. No significant interaction between the experimental factors and Electrode was found (F values < 2.25, p values > .05). When adding depression as a covariate in the ANOVA test, the Proposer × Anxiety interaction remained significant (F(1, 37) = 5.07, p = .03, ).
Figure 5.
The effect of the Proposer factor on grand-average ERPs evoked by offers in the low-trait anxiety (LTA) group (a) and the high-trait anxiety (HTA) group (b) at mid-line recording sites Fz, Cz, and POz. The time point “0” indicates offer presentation onset. The gray shaded areas indicate the 400–600 ms time window for the calculation of the mean value. The scalp topographies of each condition are presented beneath. Note: The waveforms are presented to provide the midline scalp topographies for the P3 results. The electrode used in the ANOVA were CPz, Pz, POz, and Oz.
Discussion
Our results replicated the well-established behavioral pattern that participants’ acceptance rates decreased as levels of equality of UG offers decreased (Güth et al., 1982; Haselhuhn & Mellers, 2005). Also, the acceptance rates decreased in response to human-proposed offers compared to computer-proposed offers (see also Rilling & Sanfey, 2011; Sanfey et al., 2003). That is, both the equality factor and the social-interaction factor (i.e., the Proposer factor in the statistical analysis) manifested in the current study. Furthermore, the influence of anxiety on these two factors was evidenced on the behavioral performance and the ERPs, all of which remained significant after entering SDS scores as a covariate, thus ruling out the potential impact of depression on the current study.
The Impact of Anxiety on Social Rejection
Regarding the behavioral data, the interaction between the equal factor and the social-interaction factor were sensitive to anxiety levels, such that the tendency of rejecting human-proposed unequal offers compared to computer-proposed unequal offers appeared to be stronger in the HTA group than in the LTA group. The ERP results may help with understanding this phenomenon, since the impact of anxiety on the equality and social-interaction factors was detected on the FRN and the P3, respectively.
Anxiety and Electrophysiological Responses to Proposals
The FRN amplitude was larger in response to unequal offers compared with equal offers in the HTA group, while the equality factor was not significant in the LTA group. Seeing that the classical FRN component differentiates between “favorable” and “unfavorable” outcomes (Hajcak, Moser, Holroyd, & Simons, 2006; Nieuwenhuis, Slagter, von Geusau, Heslenfeld, & Holroyd, 2005), our FRN results indicate that high-anxious participants were more prone to evaluate inequitable outcomes as unfavorable than were their low-anxious counterparts. Interestingly, the FRN following negative outcomes was smaller among high-anxious participants than among low-anxious ones in single-player gambling tasks (Gu, Ge et al., 2010; Gu, Huang et al., 2010), indicating that the influence of anxiety on outcome evaluation show context-dependent patterns. High-anxious participants’ smaller FRN amplitudes in single-player scenarios were interpreted as evidence of their lower confidence of earning reward when outcome valence is probabilistic; in other words, negative outcomes match high-anxious people's pessimistic outcome expectations and thus elicit weaker neural responses (Gu, Huang et al., 2010). In contrast, in multiple-player scenarios, high-anxious individuals may be more likely to expect equitable distributions from their partners and consequently, perceive stronger prediction errors than their low-anxious counterparts when the offers were inequitable (see also T. Wu et al., 2013).
The P3 component, which occurs immediately after the FRN temporally, showed an interaction between anxiety and the social-interaction factor. Specifically, the human-proposed offers elicited a larger P3 than the computer-proposed offers in the HTA group, but not in the LTA group. The P3 amplitude has been suggested to represent the motivational or affective significance of a stimulus (Gu et al., 2011; Martin & Potts, 2004; Yeung & Sanfey, 2004). Increased P3 amplitudes among anxious people has previously been reported in the studies using the oddball paradigm (Boudarene & Timsit-Berthier, 1997; Enoch, White, Harris, Rohrbaugh, & Goldman, 2001; Li, Hu, Liu, & Wu, 2011), indicating stronger motivations to detect novel or affectively salient information (Paulus, Feinstein, Simmons, & Stein, 2004). In the current study, an enhanced P3 among high-anxious participants indicates that these participants judged UG offers that reflect human players’ benevolent or selfish intentions to be more motivationally salient than the offers provided randomly by the computer. In contrast, low-anxious participants might be less concerned about the social context of an offer, indicated by an unchanged P3 between the human- and computer-proposer conditions for the group.
Further Directions and Concluding Remarks
Contrary to our behavioral results, Grecucci et al. (2012) reported that anxiety-disordered patients accepted significantly more unequal offers than the controls. To explain this discrepancy, it is important to note that the current study and that of Grecucci et al. (2012) recruited participants from a normal sample and from patients suffering from generalized anxiety disorder (GAD), respectively. Normal levels of anxiety and pathological anxiety are qualitatively distinct (Belzung & Griebel, 2001). It might be that high-anxious individuals in nonclinical populations and GAD patients are driven by different motivations during social decision-making. While high-anxious individuals tend to express their negative feelings towards unequal distributions, patients might be more worried about the potential interpersonal consequence of rejections, because GAD is strongly associated with low self-confidence and negative self-image (Butler, Cullington, Hibbert, Klimes, & Gelder, 1987; Masi et al., 2004). Further research that clarifies this unsolved issue would have significance to both clinical and non-clinical studies.
In sum, according to the ERP results, we suggest that high-anxious participants were more sensitive to the equality level of reward distribution than their low-anxious counterparts. Also, high-anxious participants were more sensitive to the underlying intention or motive of other people's actions. The interaction of these two factors on fairness consideration might explain high-anxious participants’ altered socioeconomic behavior. That is, they were more likely to reject human-proposed unequal offers in the UG, despite this being regarded as a suboptimal strategy from the perspective of the “economic man” (Camerer & Fehr, 2006). In real-life, hypersensitivity to unfairness may contribute to high-anxious people's social avoidance, because unfair treatment is sometimes inevitable in social interactions. Inspired by the emotional regulation research, we suggest that training people who suffer from anxiety symptoms to control their hyperactive responses would help improve their social performance (Hartley & Phelps, 2012).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31300847, 31300867), National Basic Research Program of China (973 Program, 2011CB711000), National Center for Research Resources (UL1RR033173), National Center for Advancing Translational Sciences (UL1TR000117), Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (Y2CQ013005), and National Institute of Drug Abuse (P50 DA005312) and National Institute of Aging (T32 AG000242) of the National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Suyong Yang for helping with data analysis.
Footnotes
Author Contributions
Conceived and designed the experiments: YL, TW, CF, RG. Performed the experiments: YL, TW, CF. Analyzed the data: YL, TW, DZ. Wrote the manuscript: YL, TW, LSB, RG. Contributed materials and analysis tools: YJL. Provided lab equipment for running the study: YJL.
Conflict of Interest
The authors declare no conflict of interest.
Reference
- Alexopoulos J, Pfabigan DM, Lamm C, Bauer H, Fischmeister FPS. Do we care about the powerless third? An ERP study of the three-person ultimatum game. Frontiers in Human Neuroscience. 2012;6(59):1–9. doi: 10.3389/fnhum.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural Brain Research. 2001;125(1-2):141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Boksem MA, De Cremer D. Fairness concerns predict medial frontal negativity amplitude in ultimatum bargaining. Social Neuroscience. 2010;5(1):118–128. doi: 10.1080/17470910903202666. [DOI] [PubMed] [Google Scholar]
- Boudarene M, Timsit-Berthier M. Stress, anxiety and and event related potentials. Encephale. 1997;23(4):237–250. [PubMed] [Google Scholar]
- Butler G, Cullington A, Hibbert G, Klimes I, Gelder M. Anxiety management for persistent generalised anxiety. British Journal of Psychiatry. 1987;151(4):535–542. doi: 10.1192/bjp.151.4.535. [DOI] [PubMed] [Google Scholar]
- Camerer CF, Fehr E. When does “economic man” dominate social behavior? Science. 2006;311(5757):47–52. doi: 10.1126/science.1110600. [DOI] [PubMed] [Google Scholar]
- Camerer CF, Thaler RH. Ultimatums, dictators and manners. Journal of Economic Perspectives. 1995;9(2):209–219. [Google Scholar]
- Campanha C, Minati L, Fregni F, Boggio PS. Responding to unfair offers made by a friend: Neuroelectrical activity changes in the anterior medial prefrontal cortex. Journal of Neuroscience. 2011;31(43):15569–15574. doi: 10.1523/JNEUROSCI.1253-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM. Anxiety disorders: Why they persist and how to treat them. Behaviour Research and Therapy. 1999;37:S5–S27. doi: 10.1016/s0005-7967(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Clark JV, Arkowitz H. Social anxiety and self-evaluation of interpersonal performance. Psychological Reports. 1975;36(1):211–221. doi: 10.2466/pr0.1975.36.1.211. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educational and Psychological Measurement. 1973;33(1):107–112. [Google Scholar]
- Duronto PM, Nishida T, Nakayama S. Uncertainty, anxiety, and avoidance in communication with strangers. International Journal of Intercultural Relations. 2005;29(5):549–560. [Google Scholar]
- Enoch MA, White KV, Harris CR, Rohrbaugh JW, Goldman D. Alcohol use disorders and anxiety disorders: relation to the P300 event-related potential. Alcoholism-Clinical and Experimental Research. 2001;25(9):1293–1300. [PubMed] [Google Scholar]
- Feng C, Luo Y, Gu R, Broster LS, Shen X, Tian T, et al. The flexible fairness: equality, earned entitlement, and self-interest. Plos One. 2013;8(9):e73106. doi: 10.1371/journal.pone.0073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth W, Huck S, Müller W. The relevance of equal splits in Ultimatum Games. Games and Economic Behavior. 2001;37(1):161–169. [Google Scholar]
- Güth W, Schmittberger R, Schwarze B. An experimental-analysis of Ultimatum bargaining. Journal of Economic Behavior & Organization. 1982;3(4):367–388. [Google Scholar]
- Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depression and Anxiety. 2010;27(4):339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Grecucci A, Giorgetta C, Brambilla P, Zuanon S, Perini L, Balestrieri M, et al. Anxious ultimatums: How anxiety disorders affect socioeconomic behaviour. Cognition and Emotion. 2012;27(2):230–244. doi: 10.1080/02699931.2012.698982. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. P300 assessment of anxiety effects on processing novel stimuli. International Journal of Psychophysiology. 1994;17(3):205–217. doi: 10.1016/0167-8760(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Gu R, Ge Y, Jiang Y, Luo YJ. Anxiety and outcome evaluation: The good, the bad and the ambiguous. Biological Psychology. 2010;85(2):200–206. doi: 10.1016/j.biopsycho.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu R, Huang YX, Luo YJ. Anxiety and feedback negativity. Psychophysiology. 2010;47(5):961–967. doi: 10.1111/j.1469-8986.2010.00997.x. [DOI] [PubMed] [Google Scholar]
- Gu R, Lei Z, Broster L, Wu T, Jiang Y, Luo YJ. Beyond valence and magnitude: A flexible evaluative coding system in the brain. Neuropsychologia. 2011;49(14):3891–3897. doi: 10.1016/j.neuropsychologia.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, Simons RF. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biological Psychology. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Halko ML, Hlushchuk Y, Hari R, Schurmann M. Competing with peers: Mentalizing-related brain activity reflects what is at stake. Neuroimage. 2009;46(2):542–548. doi: 10.1016/j.neuroimage.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Harle KM, Sanfey AG. Incidental sadness biases social economic decisions in the ultimatum game. Emotion. 2007;7(4):876–881. doi: 10.1037/1528-3542.7.4.876. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Anxiety and decision-making. Biological Psychiatry. 2012;72(2):113–118. doi: 10.1016/j.biopsych.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselhuhn MP, Mellers BA. Emotions and cooperation in economic games. Cognitive Brain Research. 2005;23(1):24–33. doi: 10.1016/j.cogbrainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MGH, Holroyd CB, et al. Why humans deviate from rational choice. Psychophysiology. 2011;48(4):507–514. doi: 10.1111/j.1469-8986.2010.01081.x. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Meynen T, Clark DM. Negative self-imagery in social anxiety contaminates social interactions. Memory. 2004;12(4):496–506. doi: 10.1080/09658210444000106. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: Electrophysiological responses to feedback stimuli. Brain Research. 2006;1105(1):93–101. doi: 10.1016/j.brainres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Larsen JT, Cohen JD. Context dependence of the event-related brain potential associated with reward and punishment. Psychophysiology. 2004;41(2):245–253. doi: 10.1111/j.1469-8986.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu Y, Liu T, Wu D. Dipole source analysis of auditory P300 response in depressive and anxiety disorders. Cognitive Neurodynamics. 2011;5(2):221–229. doi: 10.1007/s11571-011-9156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15(9):1519–1522. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- Masi G, Millepiedi S, Mucci M, Poli P, Bertini N, Milantoni L. Generalized anxiety disorder in referred children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(6):752–760. doi: 10.1097/01.chi.0000121065.29744.d3. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology-Science and Practice. 2002;9(1):85–90. [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJA, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005;21(11):3161–3168. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biological Psychiatry. 2004;55(12):1179–1187. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Biele G, Vavatzanidis N, Kazzer P, Heekeren HR. Temporal dynamics of prediction error processing during reward-based decision making. Neuroimage. 2010;53(1):221–232. doi: 10.1016/j.neuroimage.2010.05.052. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An Integrative Theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60(2):172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annual Review of Psychology. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Coles MGH. Electrophysiology of mind: Event-related brain potentials and cognition. Oxford University Press; New York, NY: 1996. [Google Scholar]
- Samochowiec J, Florack A. Intercultural contact under uncertainty: The impact of predictability and anxiety on the willingness to interact with a member from an unknown cultural group. International Journal of Intercultural Relations. 2010;34(5):507–515. [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shek DT. The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being. Journal of Clinical Psychology. 1993;49(3):349–358. doi: 10.1002/1097-4679(199305)49:3<349::aid-jclp2270490308>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Shu L. Self-rating depression scale and depression status inventory. Chinese Journal of Mental Health. 1993;7(Supplement):160–162. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Stavrakaki C, Vargo B. The Relationship of anxiety and depression - a review of the literature. British Journal of Psychiatry. 1986;149:7–16. doi: 10.1192/bjp.149.1.7. [DOI] [PubMed] [Google Scholar]
- van't Wout M, Chang LJ, Sanfey AG. The influence of emotion regulation on social interactive decision-making. Emotion. 2010;10(6):815–821. doi: 10.1037/a0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Veen FM, Sahibdin PP. Dissociation between medial frontal negativity and cardiac responses in the ultimatum game: Effects of offer size and fairness. Cognitive, Affective & Behavioral Neuroscience. 2011;11(4):516–525. doi: 10.3758/s13415-011-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Sheldon CT, Goering P. Psychiatric disorders and dissatisfaction with social relationships: Does type of relationship matter? Journal of Abnormal Psychology. 2000;109(4):803–808. doi: 10.1037//0021-843x.109.4.803. [DOI] [PubMed] [Google Scholar]
- Wray LD, Stone ER. The role of self-esteem and anxiety in decision making for self versus others in relationships. Journal of Behavioral Decision Making. 2005;18(2):125–144. [Google Scholar]
- Wu T, Luo Y, Broster LS, Gu R, Luo YJ. The impact of anxiety on social decision-making: Behavioral and electrodermal findings. Social Neuroscience. 2013;8(1):11–21. doi: 10.1080/17470919.2012.694372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Hu J, van Dijk E, Leliveld MC, Zhou X. Brain activity in fairness consideration during asset distribution: Does the initial ownership play a role? Plos One. 2012;7(6):e39627. doi: 10.1371/journal.pone.0039627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou XL. The P300 and reward valence, magnitude, and expectancy in outcome evaluation. Brain Research. 2009;1286:114–122. doi: 10.1016/j.brainres.2009.06.032. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24(28):6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, Saito H. Effect of conditioned fear stress on dopamine release in the rat prefrontal cortex. Neuroscience Letters. 1996;209(3):201–203. doi: 10.1016/0304-3940(96)12631-8. [DOI] [PubMed] [Google Scholar]
- Zung WWK, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic - further validation of SDS. Archives of General Psychiatry. 1965;13(6):508. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]