Abstract
Scientists at the National Institute of Allergy and Infectious Diseases Integrated Research Facility at Fort Detrick, Frederick, Maryland, coordinate and facilitate preclinical research on infectious diseases to develop medical countermeasures for high‐consequence pathogens. This facility is unique in that it is the only maximum containment laboratory in the world where conventional and molecular medical imaging equipments are incorporated into the design of the facility. This capability provides investigators with unique tools to dissect disease pathogenesis, evaluate the ability of animal models to recapitulate human disease, and test candidate countermeasures. Importantly, advanced molecular imaging has the potential to provide alternative endpoints to lethality. Using these alternative endpoints, investigators can reduce the number of animals used in experiments and evaluate countermeasures in sublethal models. With the incorporation of medical imaging modalities, a clinical laboratory modeled after those existing in hospitals, and a highly trained veterinary medicine team, IRF‐Frederick is uniquely suited to advance our understanding of emerging infectious diseases and to facilitate the development of medical countermeasures and clinical care paradigms previously considered impossible.
Keywords: BSL‐4, countermeasures, medical imaging, viral hemorrhagic fever
We present an overview of the unique infrastructure and capabilities of the Integrated Research Facility in the investigation of high‐consequence pathogens.

The NIAID Integrated Research Facility at Fort Detrick
The Integrated Research Facility (IRF) encompasses 144 000 ft2 (13378 m2) that supports scientific research for biosafety level (BSL)‐2, BSL‐3, and BSL‐4 pathogens. The facility has 32 000 ft2 (2972 m2) of laboratory space including an 11 000 ft2 (1022 m2) BSL/animal BSL‐4 (ABSL‐4) laboratory and a 21 000 ft2 (1951 m2) BSL‐2 laboratory space. The IRF‐Frederick has six core laboratory groups that support scientific studies undertaken within the facility. These include: (1) Clinical Core, (2) Anatomic Pathology, (3) Cell Culture, (4) Medical Imaging, (5) Comparative Medicine, and (6) Aerobiology. Each of these core groups contributes to an integrated evaluation of disease progression in experimentally infected animals.
Before this facility was conceived, a wealth of phenotypic and empirical data had been accumulated to characterize these infections. However, many recently developed clinical tools had not yet been applied to the study of high‐consequence pathogens. Thus, results from many previous animal challenge studies conveyed only an imprecise understanding of the mechanisms of pathogenesis of emerging pathogens. To facilitate medical countermeasure (MCM) development, animal models must closely recapitulate the human disease. Thus, the IRF was designed to incorporate not only standard features seen in most BSL‐4 laboratories but also state‐of‐the‐art clinical equipment including medical imaging capabilities. The expectation is that increased understanding of the disease processes associated with high‐consequence pathogens will facilitate the development of MCM and management options.
IRF facilitation of emerging infectious disease and biodefense research
The primary mission of the IRF is to foster collaborations with a broad spectrum of the EID and BD Research community. The relationship between the IRF and each collaborating organization will be based on the scope of the research and its relevance to the unique capabilities of the IRF. IRF collaborator's will fall into two categories: funded research with the IRF stipulated as a resource and nonstipulated IRF research. This distinction is important as it affects how each collaborators research and contractual steps are planned. Based on the funding mechanisms of each collaborator, a negotiated agreement will be established such as a Project Grant (RO1), Memorandum of Understanding (MOU), or Inter Agency Agreement (IAA). This collaboration agreement ensures that each party's roles and responsibilities have been clearly outlined.
The IRF has established a process to support the setting of the research agenda through the identification and review of suitable projects. Extramural research collaborators with approved grants that include a signed letter of support from the Director, IRF, as well as Request for Applications (RFAs) that stipulate use of the IRF, have already undergone an extensive NIH approval process and therefore require no further review by the IRF Scientific Steering Committee. Research within this category goes directly to the IRF for prioritization, resource allocation, and operational planning. All other research projects require the Scientific Steering Committee review. This committee ensures fair and equitable reviews by NIH intramural division directors and external advisors. The committee makes its recommendations to the IRF Director based on qualifying research parameters:
Relevance to NIAID, DCR, and IRF missions
Requirement for unique capabilities of IRF
Ability to advance IRF capabilities for supporting cutting‐edge infectious disease research
Relevance to human disease
Use of complex biologic systems to answer questions regarding disease pathogenesis and strategies for intervention including antimicrobials, vaccines, and other countermeasures
Use of surrogate systems to test clinical hypotheses
Priority will be given to proposed projects that require medical imaging and involve category A viral agents requiring BSL‐4 containment (e.g. filoviruses, arenaviruses, henipaviruses). However, other projects will be considered based on the availability of resources. Work with these pathogens will commence immediately after CDC Select Agent approval which is anticipated in early 2014. Qualified investigators, including international colleagues, are welcome to participate at all levels, although in most cases they may wish to defer to IRF staff members with the specialized training required to work in a BSL‐4 laboratory.
The need for medical imaging of high‐consequence diseases
In vivo medical imaging is emerging as a powerful addition to conventional studies of viral pathogenesis and treatment. With the exception of X‐rays, application of medical imaging modalities focusing specifically on viral infection and diseases associated with BSL‐4 pathogens has thus far been impossible. The use of techniques such as single photon emission computed tomography (SPECT), positron emission tomography (PET), computed tomography (CT), and magnetic resonance imaging (MRI) will provide unique insights into disease pathogenesis including inflammation, altered systemic vascular function, coagulation, and tissue damage. With these technologies, investigators can chart the evolution of lesions in individual animals and link these findings with clinical manifestations, changes in laboratory values, and other indicators of disease severity. Investigators and veterinarians analyze generated data in real time and alter treatment protocols or clinical algorithms. These adjustments will not only improve the care and well‐being of the animal but will also begin to mimic normal care practices in clinical settings.
The wealth of data generated from experiments using medical imaging (MI) of experimentally infected animals should facilitate the scientific and regulatory review of MCM using 21 CFR Parts 314 and 601, more often referred to as the Food and Drug Administration Animal Rule (Food & Drug Administration & Department of Health & Human Services, 2002). Under this rule, regulators expect that the pathophysiologic emerging disease process in the animal model is reasonably well understood and recapitulates the human condition. Demonstration that the MCM mitigates this disease is also required. Usually, the Food and Drug Administration requires this demonstration in more than one animal species unless a model in a single species is demonstrated to be ‘sufficiently well characterized’ to predict therapeutic responses. In that case, a second species may not be needed. MI has the potential to refine animal models by more precisely defining how faithfully they recapitulate human disease and also demonstrating how candidate MCM are preventing or mitigating disease from high‐consequence pathogens.
Traditionally, reduction in ‘major morbidity or lethality’ has been viewed as the most acceptable or readily interpretable outcome from animal efficacy studies. This endpoint has presented significant challenges when the best available models are not uniformly lethal or when the disease severity does not match human disease from emerging pathogens. As a result, investigators are often faced with the daunting choices of using large numbers of animals, adapting the pathogen of interest to be more lethal in animal model(s), or developing models using genetically modified animals. By definition, adaptation changes the nature of the pathogen which raised concerns regarding relevance to the human pathogen as well as dual‐use regulatory concerns. While genetically altered animals may offer alternatives, the expression or deletion of the gene of interest usually fails to fully recapitulate the disease following infection. Generally, investigators are concerned that expression or deletion of a gene typically results in altered phenotypes that may impact the interpretation of results from these models. The availability of MI opens the potential to identify and use alternative outcomes in lieu of these other options.
MI modalities provide the opportunity to noninvasively and serially measure physiologic processes and disease development within a single subject over time. Thus, these modalities complement conventional animal studies that rely on serial euthanasia and necropsies to describe pathogenesis. Through MI, scientists at the IRF are already studying the development of consolidations in the lungs of nonhuman primates infected with Middle Eastern respiratory syndrome coronavirus (MERS‐CoV). With MI, scientists are able to chart the development and quantification of lung lesions (Fig. 1). This ability will be critical for assessing potential countermeasures in this very mild disease model. In addition, use of medical imaging also has the potential to reduce the number of animals needed during a study, complying with Russell and Burch's three Rs (refinement, reduction, and replacement of the use of animals in research) of animal research. For instance, the longitudinal approach may increase the statistical power needed to assess the efficacy of an investigational drug or treatment while decreasing total animal numbers needed for research (Rudin & Weissleder, 2003).
Figure 1.
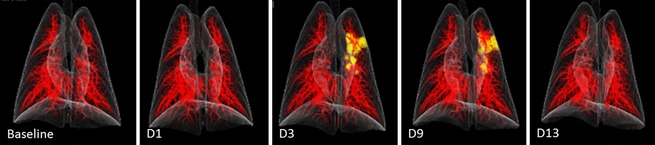
CT 3D volume‐rendered images of lungs of nonhuman primates acquired prior to intratracheal inoculation (baseline) with Middle Eastern respiratory syndrome coronavirus and on days 1, 3, 9, and 13 postinoculation. Images were segmented to highlight pulmonary vasculature (red) and areas of increased consolidation (yellow).
Current MI capabilities at the IRF
Advanced MI capabilities at the IRF include MRI, SPECT/CT, PET/CT, real‐time fluoroscopy and diagnostic C‐arm X‐ray, endoscopy, and ultrasound. All of these modalities bring unique capabilities to address a variety of questions related to infectious diseases such as the identification of disease lesions or pathology (e.g. areas of inflammation) and tracking of infected cells.
MRI uses a magnetic field in combination with pulses of radiowave energy to generate images of organs and structures inside the subject. MR images can be obtained with or without contrast agents. By exploiting endogenous sources of contrast, exogenous and potentially harmful contrast agents are not required. The wide variety of MRI techniques available provides flexibility in monitoring infectious disease processes in the BSL‐4 environment. For instance, a number of MRI techniques have been developed to assess vascular permeability, integrity of the blood–brain barrier, and edema formation. In addition to quantifying vascular permeability, MRI could potentially be used to track cell migration (Hoehn et al., 2007) during viral infections. MRI will therefore be a useful tool to follow the development of endothelial leakage during the progression of a viral hemorrhagic fever or to observe real‐time immune responses as visualized by macrophage migration postinoculation. In vivo magnetic resonance spectroscopy measures changes in relative amounts of certain metabolites in an infected tissue of interest during the course of infection or therapeutic intervention.
CT scans use X‐rays to visualize the internal structures of a subject. The images can be taken from a variety of angles to generate ‘digital or cross‐sectional slices’ or can be combined to generate three‐dimensional images. CT is suitable for morphological or functional imaging (e.g. multiphase perfusion scans) and provides attenuation correction. Combination of PET with CT is another technique to perform ‘functional imaging’.
For PET scans, a positron‐emitting radionuclide (tracer) or probe is administered to the subject. The subject is then imaged and the concentrations of the probe in tissues are measured. Images of probe uptake with PET are overlaid or co‐registered with the three‐dimensional images of the internal structures of the subject (CT). For example, if investigators are interested in identifying tissues or regions with increased metabolism as an indicator for inflammation or increased cellular replication, fluorodeoxyglucose (18F‐FDG), an analog of glucose, is administered to the subject. As cells take up this glucose analog, the probe accumulates, and the scintillation crystals on the detectors measure the released photons. When combined with CT and image analysis, investigators visualize and quantitate areas of inflammation in discrete anatomic locations. PET is presently being used at the IRF to establish the tissue distribution of T705 (an antiviral drug), the relationship between plasma and tissue levels of T705, and the effect of viral infection on these parameters, essential information required under the FDA Efficacy Rule. PET/CT has also been used at the IRF for studies of monkeypox (Dyall et al., 2011), a surrogate of smallpox, to show the development and progression of diseases both morphologically and physiologically. In addition to qualitative interpretation of the medical images, quantitative analysis of changes in morphology and function of animal anatomy allows for statistical analysis of findings for the animal models in vivo.
Similar to PET/CT, SPECT/CT combines a CT image with a radioactive probe. Traditionally, SPECT/CT has been most often used for cardiac studies. While often considered less sensitive than PET, SPECT has typically been used when there is a need to widen the observational period to look at changes in the probe distribution over time. Recent progress in iterative reconstruction techniques is improving the resolution of images generated by SPECT. However, PET remains the modality of choice when higher resolution is needed. With the availability of both modalities increases the choice of probes that can be radiolabeled and extends the range of studies that are possible in imaging diseases of emerging pathogens.
Finally, the IRF has also incorporated diagnostic X‐ray equipment as well as ultrasound. With this equipment, the imaging team can obtain standard radiographic projection images and real‐time fluoroscopic series after the injection of iodinated contrast agent. Scientists and staff members are currently using ultrasound for the visualization of lesions as well as to guide various medical procedures (e.g. detection of fluid in the lungs, lymph node size, biopsies).
IRF design and infrastructure requirements
Incorporation and use of MI equipment in maximum containment present a unique set of challenges for architects, engineers, safety officials, scientists. If the imaging equipment is located inside high containment, subjecting the imaging device to decontamination procedures may increase the risk of damage (e.g. caustic etching of detectors, progressive degradation of device wiring insulation, corrosion of solder points). In general, BSL‐3 and BSL‐4 laboratory areas are decontaminated by exposure to paraformaldehyde gas followed by ammonium bicarbonate neutralization. Frequent maintenance of MI equipment is often necessary, and requiring service engineers to enter BSL‐4 areas is an untenable option. Two options were considered to permit safe insertion of the infected animals into the bore of the instrument without breaking the biocontainment barrier. One option is to place the animal in a closed containment tube that is then transported and positioned within the bore of the instrument. However, the logistics of maintaining ventilation and anesthesia of these animals while maintaining BSL‐4 conditions inside the tube seem insurmountable. The alternative approach is to reconfigure the equipment, leaving only the bare minimum of components within the BSL‐4 laboratory and developing an extension of the BSL‐4 laboratory that protrudes into the bore of each machine. These unique challenges were innovatively addressed (de Kok‐Mercado et al., 2011) by placing the bulk of the imaging equipment outside the BSL‐4 suite (Fig. 2). The high‐containment zone is extended into the bore of each imaging device with the use of transparent biocontainment tubes made of polycarbonate resin thermoplastic (Philips Bioshield™, North Ryde, Australia). This design facilitates routine maintenance of the delicate instruments by factory‐trained personnel without entering the BSL‐4 environment. With the animal inside the biocontainment tube, the veterinary care team has access to the animal during the procedures to ensure the health and well‐being of the animal. Photographs of the ‘hot’ and ‘cold’ sides of the SPECT imaging rooms are presented in Fig. 3.
Figure 2.
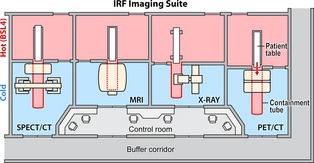
The imaging suite is separated into two sides: a hot side with pathogens present (pink rooms) and a cold side without pathogens (blue rooms). A biocontainment tube of polycarbonate resin thermoplastic extends from the hot side of the imaging suite into the bore of each imaging modality on the cold side.
Figure 3.
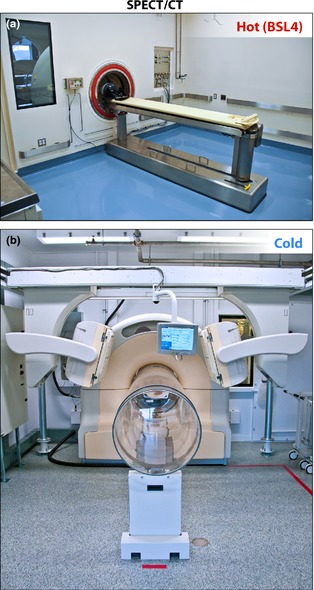
Representative biocontainment strategy for imaging. (a) The single photon emission computed tomography ‘hot side’ imaging room contains the subject table and the barrier wall that separates the hot side from the cold side (b). With the animal inside the biocontainment tube, the veterinary care team has access to the animal during the procedures to ensure the health and well‐being of the animal. Design of the subject table was simplified to facilitate decontamination and replacement of parts by scientists.
To effectively use MI equipment for animal studies, the staff identified and procured a large assortment of specialized equipment and developed new protocols and practices for attempting to image animals safely under BSL‐4 containment. Perhaps the most appreciable challenge was the ability to maintain functions of the anesthetized animal (respiration rate, heart rate and rhythm, oxygen saturation, blood pressure, core temperature) during MI procedures while ensuring staff safety. To maintain these functions, advanced life support, including multiparameter patient monitors (e.g. electrocardiography), and forced air warming blankets were put into place. In addition, inhalation anesthesia equipment and mechanical ventilators for respiration‐compromised animals or for breathe holds required for imaging were used throughout the facility. All staff were trained in the use of the equipment and demonstrated proficiency before any experiments with live infectious agents were performed.
Safety features and protocols and communication protocols also have been instituted throughout the BSL‐4 facility. Importantly, all equipment, including encapsulating personal protective suits, were evaluated and determined to be MR compatible (contained no metal). Currently, staff is evaluating the suitability of telemetry implants for MI studies. Cameras and wireless communication systems for veterinary medicine personnel also have been developed and implemented for communication with imaging team outside of containment and for visualization of the operations and the animal in the bore of the containment tube. These protocols represent just a few examples of the myriad of procedures and practices that were developed de novo for this facility.
Development of IRF‐specific core teams
Execution of animal studies using MI in BSL‐4 are complex endeavors that require close coordination of core team activities. The Clinical Pathology Core provides technical support for investigators and collaborators for DNA/RNA high‐throughput extractions, validated quantitative real‐time polymerase chain reaction (qRT‐PCR) assays, DNA/protein microarray analysis, immunophenotyping/flow cytometry, cell sorting, cytokine profiling, and immunofluorescence analysis. Unique to the IRF is the incorporation of robotics into many of these processes including DNA/RNA extraction, viral plaque assays, microneutralization assays, plaque reduction neutralization tests and enzyme‐linked immunosorbent assays, sample registration via barcoding, high‐throughput clinical pathology procedures, and high‐throughput flow cytometry analysis. Clinical support is provided to the investigators throughout disease studies including animal prescreening. In addition, this group is also responsible for maintaining the central viral repository and inventory at the IRF‐Frederick.
The Anatomic Pathology Core (APC) performs necropsy, histopathology, and a wide variety of specialty pathology‐based disciplines, such as clinical pathology, electron microscopy, and immunopathology. The APC laboratories include two state‐of‐the‐art BSL‐4 necropsy suites and full‐service BSL‐2 histopathology and electron microscopy laboratories. Further, the Pathology Core laboratories offer investigators real‐time gross pathology images from the postmortem table and gross‐tissue trimming stations. The histopathology service prepares standard hematoxylin and eosin‐stained tissue sections and offers specialized histochemical stains, immunohistochemical staining using chromogenic and fluorescent dyes, and in situ hybridization. The APC staff analyzes manual and/or colorimetric quantitative digital images of immunohistochemically stained slides and performs cell‐type‐specific molecular quantitative analysis of nucleic acids and proteins. A full‐service transmission and scanning electron microscopy laboratory is available for ultrastructural studies, which include immunogold labeling of individual antibody epitopes in both in vitro cells and ex vivo tissue sections.
The Comparative Medicine staff at the IRF‐Frederick provide rapid intervention and medical of animals during a disease study. Veterinarians work closely with board‐certified veterinary pathologists to coordinate studies from the in‐life portion of the study. The vivarium is designed to provide a procedure/support room for each of the animal rooms to limit transport of animals and the possibility of cross‐contamination. Each of these spaces is large enough to contain down draft tables, anesthesia equipment, ventilators, ultrasound equipment, and endoscopy/bronchoscopy for direct visualization or collection of samples from the respiratory or gastrointestinal tract. Minor surgical procedures, such as peripheral lymph node biopsy, tracheostomy, and placement of central venous catheters, can be performed in the procedure rooms. Staff is skilled at the performance of many sampling methods including cystocentesis for sterile urine collection, bronchoalveolar lavage, bone marrow biopsy or aspiration, and cerebrospinal fluid collection.
The animal housing is designed to accommodate indwelling chronic catheterization for studies involving constant intravenous administration of therapeutics or to provide supportive care, such as intravenous fluids or medications for highly compromised animals on study. Equipment sterilization or sanitization between animals or study groups is accomplished by autoclaving, ethylene oxide gas, or by an automatic scope reprocessor for endoscopy equipment.
The final core competency developed at the IRF is aerobiology. The IRF‐Frederick Aerobiology Core laboratory analyzes the impact of diverse, hazardous, biologic infectious aerosols (differing in regional deposition, inhaled dose) on disease presentation and disease progression. Aerosol experimentation involving high‐consequence pathogens is performed within Class III biosafety cabinets inside an animal BSL‐4 cabinet laboratory using the Biaera Technologies Automated Aerosol Management Platform, as described previously (Lackemeyer et al., 2014).
Conclusion
The US National Academy of Sciences Committee on ‘Animal Models for Assessing Countermeasures to Bioterrorism Agents’ recently evaluated the progress of animal model development and refinement for high‐consequence infectious diseases (Korch et al., 2011). In addition to the three Rs, the committee stressed the importance of using methods to develop animal models that will transfer to a culture of good laboratory practices for the development of MCM. In the past, many investigations relied on the similarities in the phenotypic presentation of disease in animals compared to humans, rather than similarities in the underlying molecular mechanisms of disease. By relying on phenotypic similarities and assumed absolute conservation of molecular processes in closely related animals of different species, investigators have raised doubts about the ability of existing animal models to accurately recapitulate human disease or predict efficacy of candidate therapeutics in humans (Seok et al., 2013). In addition, the scientific, funding, and regulatory communities realize that one animal model may not recapitulate all aspects of disease. Expectation of uniform or near uniform lethality from pathogen challenge is neither reasonable or in all cases appropriate. As a result, with the use of alternative endpoints besides lethality, investigators can evaluate MCM in nonlethal models rather than forcing a disease course more severe than the human condition.
The incorporation of medical imaging modalities, a clinical laboratory modeled after those existing in hospitals, and a highly trained veterinary medicine team make the IRF‐Frederick uniquely suited to address the recommendations of the National Academy of Sciences. The data generated from these experiments will not only advance our understanding of emerging diseases but will the advance development of countermeasures and clinical care paradigms previously considered impossible.
Acknowledgements
The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors. JHK performed this work as an employee of Tunnell Government Services, Inc.; MGL as an employee of Lovelace Respiratory Research Institute; and JK and LB as employees of Battelle Memorial Institute, all under Battelle Memorial Institute's prime contract with NIAID, Contract No. HHSN272200700016I. LK is an independent contractor for Advanced Health Education Center. MSC, PBJ, and LEH are employees of NIH.
This article describes the newly constructed IRF that will accommodate BSL4 animal work. Imaging capabilities are truly unique.
References
- de Kok‐Mercado F, Kutlak FM & Jahrling PB (2011) The NIAID Integrated Research Facility at Fort Detrick. Appl Biosaf 16: 58–66. [Google Scholar]
- Dyall J, Johnson RF, Chen DY et al (2011) Evaluation of monkeypox disease progression by molecular imaging. J Infect Dis 204: 1902–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration & Department of Health and Human Services (2002) New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Fed Regist 67: 37988–37998. [PubMed] [Google Scholar]
- Hoehn M, Wiedermann D, Justicia C, Ramos‐Cabrer P, Kruttwig K, Farr T & Himmelreich U (2007) Cell tracking using magnetic resonance imaging. J Physiol 584: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch GW, Niemi SM, Bergman NH et al (2011) Animal Models for Assessing Countermeasures to Bioterrorism Agents. Institute for Laboratory Animal Research, Division on Earth and Life Studies, The National Academies of Sciences, Washington, DC. [Google Scholar]
- Lackemeyer MG, Kok‐Mercado F, Wada J, Bollinger L, Kindrachuk J, Wahl‐Jensen V, Kuhn JH & Jahrling PB (2014) ABSL‐4 aerobiology biosafety and technology at the NIH/NIAID integrated research facility at Fort Detrick. Viruses 6: 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin M & Weissleder R (2003) Molecular imaging in drug discovery and development. Nat Rev Drug Discov 2: 123–131. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG et al (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. P Natl Acad Sci USA 110: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]


