Abstract
Non-adherence is common in adolescent and young adult kidney transplant recipients, leading to adverse graft outcomes. The aim of this study was to determine if adherence to immunosuppressant medications changes during transition from a pediatric to an adult program within the same transplant center. Adherence was assessed for a period of two years before and two years after the transfer. Sub-therapeutic trough levels of serum tacrolimus and level variability were used as measures of adherence. Twenty-five patients were transitioned between 1996 and 2011 at the median age of 22.3 [IQR 21.6 to 23.0] years. Young adults 21 to 25 years of age (n=26) and non-transitioned adolescents 17 to 21 years of age (currently followed in the program, n=24 and those that lost their grafts prior to the transfer, 22) formed the comparison groups. In the transitioned group, adherence prior to the transfer was not significantly different from the adherence after the transfer (p=0.53). The rate of non-adherence in the group of non-transitioned adolescents who lost their grafts (68%) was significantly higher than in the transitioned group (32%, p=0.01). In the group of young adults, adherence was not significantly different from the transitioned group (p=0.27). Thus, transition was not associated with differences in medication adherence in this single-center study. Large-scale studies are needed to evaluate the national data on medication adherence after transfer.
Keywords: adolescents, transition, medication, non-adherence, pediatric kidney transplantation
Introduction
Non-adherence to treatment regimens is prevalent among adolescents (1), is related to increased health care use (2) and constitutes a major cause of graft loss in kidney transplant recipients within this age category (3–5). Among all age groups, adolescents and young adults have the highest rate of kidney allograft loss beyond the first year post-transplant (6, 7). A recent, large-scale epidemiologic analysis failed to identify any subgroup of patients who were free from this significant risk of graft loss during adolescence and emerging adulthood (8). However, data on adherence and transition were not available in that registry.
Transition of adolescents to adult care has recently been recognized as a sensitive and multidimensional process that may lead to adverse outcomes (9–11). It has been proposed that transition of pediatric and adolescent transplant recipients to the adult services may be in part responsible for the high rate of graft loss in this vulnerable population (12).
Earlier reports support the concept of worsening graft survival (13) and medication adherence (14) following transfer of care from pediatric to adult transplant services. On the contrary, more recent investigations did not find a difference between rates of acute allograft rejection and graft loss before and after transfer (15–17). To clarify the possible effect of transfer on the well being of transplant patients, it may be worth exploring not only hard outcomes, such as graft loss, but also some intermediate outcomes, such as adherence. However, the relationship between transition and medication adherence in the US kidney transplant population has not been investigated.
In the current study, we examined an inner-city cohort of young kidney transplant recipients in order to determine if the transition from a pediatric to an adult program within the same transplant center was associated with a change of adherence to immunosuppressant medications.
Patients and methods
Patients
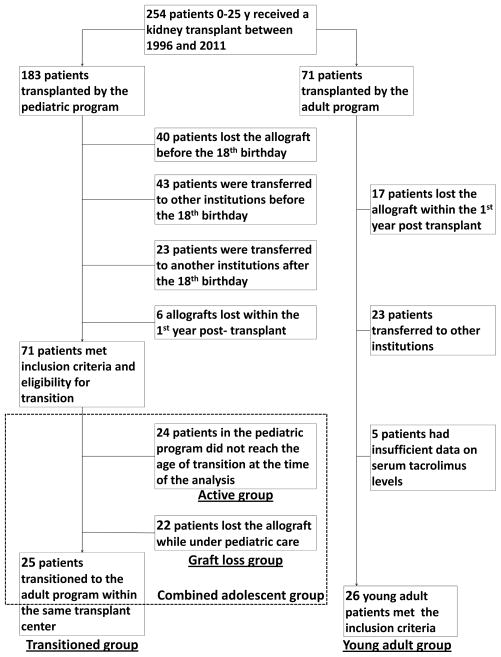
We used the institutional Online Transplant Tracking Record System (OTTR) to identify pediatric transplant recipients who were eligible for transition to the adult transplant program using the following inclusion criteria (Figure 1):
Figure 1.
Last kidney transplantation performed between 1996 and 2011.
Recipient’s age less than 21 years old at the time of the last transplantation.
Retention at Pediatric Montefiore Medical Center Transplant Program, with a functioning graft, until 18th birthday and for at least 1 year after the last transplantation.
We excluded patients who lost their grafts within the first post-transplant year, as the large percent of those losses may not be related to non-adherence but rather to medical and/or surgical post-transplant complications or recurrence of the primary disease.
In the cohort of patients eligible for transition, we then selected patients who were actually transferred (main or transitioned group). The rest of the patients, who either lost their grafts prior to the transfer, or are still currently active in the pediatric program, formed, respectively, the graft loss and active adolescent groups (Figure 1).
Pre-post comparison of adherence within the transitioned group may have an inherent selection bias because post-transfer outcomes are compared to the pre-transplant data only for the patients who were successful enough to reach the point of transfer with a functioning graft. In order to address this limitation, we compared adherence outcomes in the transitioned group after the transfer to pre-transition adherence in the combined adolescent group that included the active adolescent group, the graft loss adolescent group and the transitioned group (before the transfer).
In addition, we identified young adults who received their first kidney allografts in the adult transplant program in our institution using the following inclusion criteria:
First kidney transplantation performed between 1996 and 2011
Recipient age between 21 and 25 years at the time of transplantation
Retention at Montefiore Medical Center Transplant Program, with a functioning graft, for at least 1 year after the last transplantation.
These patients formed a comparison young adult group. Only patients with first transplants were included into this group in order to exclude patients who had previous transplants as adolescents and therefore were exposed to pediatric transplant program.
Immunologic risk was determined pre-transplant using the standard protocol based on the level of sensitization, primary diagnosis, rate of disease progression and previous transplants. Patients with high immunologic risk received induction immunosuppression with anti-thymocyte globulin. Patients with low immunologic risk received induction immunosuppression with interleukin 2 receptor antagonist (Basiliximab). Triple maintenance immunosuppression regimen (calcineurin inhibitor, mycophenolic acid, prednisone) was used for patients in all groups with target tacrolimus levels of 3–6 ng/mL beyond the first year post transplant.
Data collection
We used the OTTR to obtain the following data about transplantation for the eligible patients: date of transplant, age at transplant, donor age and type of donation (living vs. deceased; standard vs. extended criteria), date of graft loss, immunologic risk and type of induction immunosuppression. We obtained patients’ demographics (gender, race/ethnicity, type of health insurance, socioeconomic status) using the “Clinical Looking Glass” (CLG), which is the institutional software that allows electronic data abstraction from medical records. We also used CLG to obtain clinical (blood pressure) and laboratory data (serum tacrolimus levels, serum creatinine), as well as information about clinic visits and inpatient sdmissions. We used patients’ medical records to verify the information about primary diagnosis and to identify the date of transfer.
Race/Ethnicity was categorized as non-Hispanic Black (Black), non-Hispanic White (White), Hispanic and other. Socioeconomic status (SES) was defined as a neighborhood score on the basis of factor analyses of data from census-block groups (18). Neighborhood scores for block groups can range from −11.3 to 14.4, with an increasing score signifying an increasing neighborhood socioeconomic advantage.
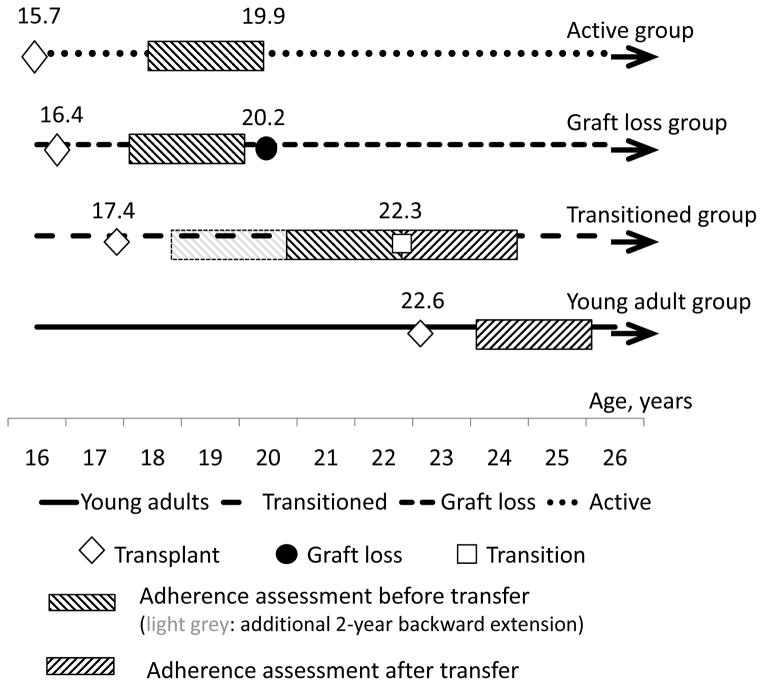
We used a time period of two years to abstract data on serum tacrolimus, blood pressure and serum creatinine: two years before and two years after the transfer for the transitioned group, two years preceding the date of graft loss for the adolescent group, and two years following the first post-transplant year for the young adult group (Figure 2). The last six months preceding the date of the graft loss (defined as a first dialysis treatment) were excluded from the analysis because a number of patients had changes in their immunosuppressant regimens during that time. When data were not available for the full 2 years, we used the longest available time period, but not less than 6 months and not less than 5 individual serum tacrolimus levels.
Figure 2.
Transfer definition and the transition process
For the purposes of this study, the date of transfer was defined as a first documented outpatient visit to the adult transplant clinic. In our kidney transplant program, the transition process is managed by a team that includes a pediatric transplant nephrologist, a pediatric transplant social worker and a pediatric transplant coordinator. Patients can be transferred as soon as they turn 18, however our common practice is to transfer transplant patients when they turn 21, with the goal to transfer before the 22nd birthday. We make an effort to avoid transferring patients who are not medically stable, which occasionally leads to retention of patients in the pediatric program beyond their 22nd birthday. At the last visit to the pediatric transplant clinic we make an appointment for the first visit to the adult transplant clinic, notify an accepting transplant nephrologist and complete a medical summary. Communication between adult and pediatric transplant teams continues during first adult transplant clinic visits on an as needed basis.
Measures of adherence
We used two measures of adherence to antirejection medications based on the serum trough tacrolimus levels that were checked on a routine basis as part of standard medical care:
Tacrolimus coefficient of variation (CV%), defined as standard deviation divided by mean and multiplied by 100% (19, 20) and
Percent of sub-therapeutic tacrolimus levels (“low levels”) defined as the number of serum tacrolimus levels less or equal to 2.5 ng/ml divided by the total number of serum tacrolimus determinations during the period of observation × 100.
We excluded from the analysis serum tacrolimus levels during inpatient admissions. When it was possible to identify a medical reason for low tacrolimus levels based on available records (e.g., diarrhea, viral infections), these levels were also excluded. We treated serum tacrolimus CV% as a continuous variable without categorization. Based on the percent of low tacrolimus levels we categorized patients as non-adherent if they had more than 10% of low levels during the period of observation, and as adherent otherwise.
For the graft survival analyses we used the beginning of observation as a starting point for the combined adolescent group, transplant date for the young adult group, and the date of transfer for the transitioned group.
Power considerations
An a priori power analysis for the Wilcoxon paired-rank sum test (21) comparing serum tacrolimus CV% before and after transfer revealed that with a sample size of 25 we would have at least 95% power to detect a difference in tacrolimus CV% equal to or greater than 15%.
A similar analysis for a Mann-Whitney U test (22) comparing tacrolimus CV% after transfer in the transitioned group to that of the group of young adults showed about 95% power to detect a difference at least 20% given the distribution of values we observed.
Statistical analyses
All continuous variables were checked for normality. We used a Chi-square test for binary variables and a Kruskal-Wallis test for continuous variables that did not meet normality assumptions to compare patient characteristics between the 3 groups. A paired t-test was used to determine the statistical significance of the differences between CV% before and after transfer. The Mann-Whitney U-test was performed to compare the median frequencies of low tacrolimus levels and Chi-square test to compare the proportions of non-adherent patients. We conducted multivariable analyses to adjust the results for potential confounders using linear regression for continuous normally distributed dependent variables and logistic regression for binary dependent variables. Given the relatively small sample sizes of each group (all n <27), adjustment for potential confounding covariates was conducted individually for each covariate. IBM SPSS ©v.20 package was used for statistical analyses. Two-sided p-values <0.05 were considered statistically significant.
Results
Patient characteristics
The process of group selection is shown in Figure 1. After screening the database of 254 patients, we identified 25 patients that formed the transitioned group, 22 patients – the graft loss adolescent group, 24 patients – the active adolescent group, and 26 patients – the young adult group.
Table 1 displays demographic and disease characteristics of the 4 groups. There were no statistically significant differences in the gender or race/ethnicity between the groups (p=0.67). The median age at transplant was, as expected, significantly higher in the young adult group than in the adolescent groups. The median age at transplant was not significantly different between transitioned and adolescent groups (Figure 2, p=0.13). We had a greater number of young adult patients transplanted between 2006 and 2011 compared with the decade of 1996–2005. Between the transitioned and adolescent graft loss groups, we did not have significant differences in the distribution of patients by transplant eras. Active adolescent group, as expected, had higher proportion of patients transplanted within the recent years. Five patients in the graft loss adolescent group, four patients in the active adolescent group, and two patients in the transitioned group had more than one transplant. The young adult group did not have patients with more than one transplant by definition.
Table 1.
Patient characteristics
| Adolescent groups
|
Transitioned group (n=25) | Young adult group (n=26) | P-value | ||
|---|---|---|---|---|---|
| Active (n=24) | Graft loss (n=22) | ||||
| Male gender, n (%) | 14 (58%) | 14 (64%) | 15 (60%) | 14 (54%) | 0.87 |
| Race/ethnicity, n (%) | 0.06 | ||||
| Hispanic | 10 (42%) | 10 (45%) | 18 (72%) | 12 (46%) | |
| Black | 6 (25%) | 9 (41%) | 3 (12%) | 6 (23%) | |
| White | 2 (8%) | 3 (14%) | 2 (8%) | 7 (27%) | |
| other | 6 (25%) | 1 (4%) | 1 (4%) | 1 (4%) | |
| Median SES score*[IQR] | −3.0 [−7.0 to −1.0] | −4.0 [−6.5 to −1.0] | −4.6 [−6.3 to −1.0] | −2.7 [−6.2 to −1.2] | 0.95 |
| Commercial health insurance, n (%) | 2 (8%) | 4 (18%) | 2 (8%) | 5 (19%) | 0.56 |
| Primary diagnosis, n (%) | 0.61 | ||||
| FSGS | 4 (17%) | 9 (41%) | 7 (28%) | 6 (23%) | |
| other glomerulonephritidies | 6 (25%) | 3 (17%) | 3 (12%) | 6 (23%) | |
| CAKUT | 7 (29%) | 4 (18%) | 6 (24%) | 6 (23%) | |
| unknown | 1 (4%) | 4 (18%) | 4 (16%) | 6 (23%) | |
| other | 6 (25%) | 2 (9%) | 5 (20%) | 5 (19%) | |
| Median age at transfer, years [IQR] | 22.3 [21.8 to 23.2] | ||||
| Transplant era, n (%) | |||||
| 1996–2000 | 0 (0%) | 5 (16%) | 8 (32%) | 4 (15%) | 0.35 |
| 2001–2005 | 4 (17%) | 13 (59%) | 15 (60%) | 9 (35%) | 0.009 |
| 2006–2011 | 20 (83%) | 4 (18%) | 2 (8%) | 13 (50%) | <0.0001 |
| Median age at transplant, years [IQR] | 15.7 [11.8 to 17.9] | 16.4 [14.2 to 17.6] | 17.5 [15.6 to 19.1] | 22.8 [21.7 to 24.0] | <0.0001 |
| First transplants, n (%) | 20 (83%) | 17 (77%) | 23 (92%) | 26 (100%) | 0.08 |
| High immunologic risk#, n (%) | 16 (67%) | 7 (32%) | 8 (32%) | 9 (34%) | 0.23 |
| Living donation, n (%) | 3 (13%) | 7 (32%) | 6 (24%) | 16 (62%) | 0.01 |
| Median donor age, years [IQR] | 25.5 [20.1 to 31.0] | 32.4 [21.6 to 44.5] | 28.0 [22.3 to 40.0] | 39.7 [26.2 to 49.5] | 0.05 |
defined as a sum of census block z-scores for the income, education, and occupation
defined based on the level of sensitization, primary diagnosis, rate of disease progression and previous transplants
IQR – interquartile range
FSGS – focal segmental glomerulosclerosis
CAKUT – congenital abnormality of kidney and urinary tract
SES – socio-economic status
The median age at transfer in our center was 22.3 [IQR 21.6 to 23.0] years. No patients were transferred before their 21st birthday and 16 patients (64%) were transferred after their 22nd birthday. Transferring after the age of 22 was partly explained by our definition of the date of transfer as the first visit to the adult transplant clinic. Six patients (24%) were seen last time in the pediatric transplant clinic before their 22nd birthday but their first appointment with the adult provider occurred after they turned 22. Ten patients (40%) stayed in the pediatric program beyond their 22nd birthday, mostly because they were deemed to be not medically stable for the transfer at the time.
Patients in all three groups had comparable representation of glomerular and non-glomerular etiologies of end-stage renal disease, with somewhat higher prevalence of focal segmental glomerulosclerosis among the patients who lost their grafts.
The percentage of living donations, as expected, was significantly higher in the group of young adults compared to the transitioned and adolescent groups (p = 0.02), likely due to the priorities of cadaveric organs for pediatric patients in the U.S. Donors were, as expected, older in the young adult group, although this was only borderline significant when compared with three other groups (p=0.05). Only standard criteria donations were received in the adolescent and transitioned groups. One extended criteria donation occurred in the young adult group. The socioeconomic status of patients in all cohorts was below the national average, which is typical for the Bronx. More than 80% of patients in all groups had state or federal health insurance (Medicaid or Medicare).
Adherence before and after transfer
In the transitioned group, medication adherence prior to the transfer (Table 2) was not significantly different from the adherence after transfer, based on both CV% and frequency of low serum tacrolimus levels. Out of 8 patients who were non-adherent after the transfer, three were also non-adherent prior to the transfer but 5 were adherent and developed non-adherence post-transfer. Among patients who were non-adherent prior to the transfer, the median percent of low tacrolimus levels was 22.5% [range 14.3% to 57.1%]. For the patients who were non-adherent after transfer, the median percent of low tacrolimus levels was 21.8% [range 13.7% to 47.5%]. In the regression models (table 3), there was no statistically significant relationship between the adherence before and after transfer, measured by either CV% or low tacrolimus levels. Adjustment for the series of individual covariates did not significantly change the model. The frequencies of drug monitoring, clinic visits and hospitalizations were not significantly different before (on the pediatric side) and after transfer (on the adult side) in the transitioned group (table 2).
Table 2.
Parameters of adherence and graft function during two-year periods of observation
| Adolescent group | Transitioned group | Young adult group (n=26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Graft loss (n=22) | P1 | Combined# (n=71) | P2 | before the transfer (n=25) | P3 | after the transfer (n=25) | P4 | ||
| Median serum tacrolimus CV% [IQR] | 46.7 [41.4 to 58.4] | 0.07 | 44.1 [29.9 to 50.4] | 0.32 | 42.9 [31.4 to 51.0] | 0.62 | 40.4 [29.9 to 50.4] | 0.98 | 38.5 [26.8 to 53.1] |
| Median frequency of serum tacrolimus monitoring, n per year [IQR] | 14.3 [11.2 to 18.2] | 0.004 | 7.7 [6.0 to 13.2] | 0.02 | 6.3 [5.0 to 8.1] | 0.61 | 5.5 [3.0 to 10.5] | 0.15 | 7.5 [4.4 to 8.0] |
| Non-adherent patients*, n (%) | 15 (68%) | 0.01 | 29(41%) | 0.43 | 8 (32%) | 1.0 | 8 (32%) | 1.0 | 9 (35%) |
| Median percent of low serum tacrolimus levels, [IQR] | 15.7 [0.0 to 23.3] | 0.04 | 6.6 [0.0 to 18.7] | 0.30 | 0.0 [0.0 to 18.6] | 0.59 | 0.0 [0.0 to 15.7] | 0.60 | 0.0 [0.0 to 11.5] |
| Median frequency of clinic visits, n per year [IQR] | 10.2 [6.9 to 13.8] | 0.003 | 6.2 [4.5 to 9.0] | 0.03 | 5.5 [3.8 to 6.7] | 0.75 | 4.7 [2.3 to 6.7] | 0.27 | 4.3 [2.9 to 8.0] |
| Median frequency of inpatient admissions, n per year [IQR] | 1.4 [0.5 to 3.0] | 0.06 | 0.5 [0.0 to 1.6] | 0.20 | 0.5 [0.0 to 1.0] | 0.95 | 0.25 [0.0 to 1.0] | 0.66 | 0.0 [0.0 to 1.0] |
| 4-year graft survival**, n (%) | 49(69%) | 0.07 | 22 (88%) | 0.72 | 22 (85%) | ||||
| Median serum creatinine [IQR] | 2.5 [1.6 to 3.5] | <0.0001 | 1.3 [1.13 to 2.0] | 0.71 | 1.2 [1.1 to 1.5] | 0.009 | 1.5 [1.1 to 2.3] | 0.58 | 1.4 [1.1 to 1.8] |
| Median systolic blood pressure [IQR] | 126 [121 to 136] | 0.17 | 123 [115 to 131] | 0.28 | 119 [113 to 130] | 0.11 | 124 [115 to 140] | 0.27 | 120 [110 to 130] |
| Median diastolic blood pressure [IQR] | 74 [68 to 82] | 0.92 | 73 [68 to 78] | 0.001 | 73 [69 to 78] | 0.048 | 79 [76 to 86] | 0.25 | 77 [70 to 82] |
| Median serum tacrolimus CV% [IQR] | 46.7 [41.4 to 58.4] | 0.07 | 44.1 [29.9 to 50.4] | 0.32 | 42.9 [31.4 to 51.0] | 0.62 | 40.4 [29.9 to 50.4] | 0.98 | 38.5 [26.8 to 53.1] |
combined adolescent group includes active adolescent group, graft loss adolescent group and transitioned group (before the transfer)
Based on the percent of low serum tacrolimus levels
From the beginning of observation for the combined adolescent group, from transplant date for the young adult group, from the date of transfer for the transitioned group
adolescent graft loss group vs. transitioned group before transfer
combined group vs. transitioned group after transfer
transitioned group before vs. after transfer
young adult group vs. transitioned group after transfer
Table 3.
Within and between-groups comparisons of medication adherence, adjusted for multiple covariates in regression models#
| Transitioned (before the transfer) group vs. adolescent group with graft loss§ | Transitioned (after the transfer) vs. combined adolescent groups§ | Transitioned group before vs. after the transfer$ | Transitioned (after the transfer) vs. young adult groups§ | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | OR (95% CI) | β (95% CI) | OR (95% CI) | β (95% CI) | OR (95% CI) | β (95% CI) | OR (95% CI) | |
| Unadjusted | −7.8 (−16.3 to 0.64) | 0.21 (0.06 to 0.70)* | 4.6 (−3.7 to 13.0) | 1.5 (0.6 to 3.8) | 0.28 (−0.14 to 0.70) | 1.56 (0.27 to 9.11) | −0.97 (−9.68 to 7.74) | 0.98 (0.29 to 3.33) |
| Era of transplant | −9.4 (−18.3 to −0.4)* | 0.19 (0.05 to 0.67)* | −4.5 (−9.9 to 1.0) | 0.7 (0.4 to 1.2) | 0.23 (−0.21 to 0.66) | 1.40 (0.23 to 8.46) | 2.86 (−8.76 to 14.49) | 1.18 (0.30 to 4.62) |
| Age at transplant | −6.9 (−15.6 to 1.8) | 0.25 (0.07 to 0.87)* | 0.9 (−0.2 to 2.1) | 1.0 (0.9 to 1.1) | 0.26 (−0.19 to 0.72) | 1.06 (0.15 to 7.50) | 0.53 (−12.33 to 13.40) | 3.02 (0.33 to 27.51) |
| Gender | −7.7 (−16.2 to 0.8) | 0.20 (0.06 to 0.70)* | −5.0 (−12.5 to 2.6) | 0.7 (0.3 to 1.6) | 0.27 (−0.20 to 0.75) | 1.59 (0.27 to 9.56) | −0.73 (−9.54 to 8.08) | 0.96 (0.28 to 3.28) |
| Race | −6.1 (−14.7 to 2.4) | 0.23 (0.07 to 0.80)* | 4.3 (−0.4 to 9.0) | 1.4 (0.8 to 2.4) | 0.22 (−0.30 to 0.75) | 1.52 (0.26 to 8.92) | −0.86 (−9.58 to 7.87) | 0.97 (0.29 to 3.29) |
| Primary diagnosis | −8.7 (−18.5 to 1.0) | 0.23 (0.06 to 0.86)* | 0.2 (−2.3 to 2.8) | 0.9 (0.7 to 1.3) | 0.24 (−0.24 to 0.73) | 0.90 (0.10 to 7.78) | 0.72 (−9.30 to 10.74) | 0.93 (0.23 to 3.76) |
| Type of donation | −7.8 (−16.4 to 0.8) | 0.21 (0.06 to 0.71)* | −0.4 (−9.1 to 8.4) | 0.7 (0.3 to 1.7) | 0.29 (−0.23 to 0.82) | 1.62 (0.27 to 9.70) | −3.15 (−12.48 to 6.17) | 0.88 (0.24 to 3.17) |
| Health insurance | −8.4 (−17.0 to 0.2)* | 0.20 (0.06 to 0.70)* | −4.9 (−17.0 to 7.3) | 1.0 (0.3 to 4.0) | 0.30 (−0.14 to 0.75) | 1.80 (0.29 to 11.16) | −0.53 (−9.56 to 8.49) | 0.88 (0.25 to 3.09) |
| SES | −8.2 (−16.7 to 0.4) | 0.21 (0.06 to 0.74)* | −0.9 (−2.2 to 0.4) | 0.9 (0.8 to 1.1) | 0.13 (−0.47 to 0.73) | 1.51 (0.25 to 8.97) | 1.35 (−8.04 to 10.74) | 1.36 (0.38 to 4.88) |
Beta coefficients are reported for tacrolimus coefficient of variation (CV%). Odds ratios (OR) are reported for non-adherence, defined as more than 10% of all measured tacrolimus levels being ≤ 2.5 ng/mL for the period of observation
Each coefficient (linear regression)/OR (logistic regression) is reported for the binary variable of being part of one vs. another group, adjusted for the single covariate of that row.
Each coefficient is reported for CV% before transfer adjusted for the single covariate of that row
P<0.05
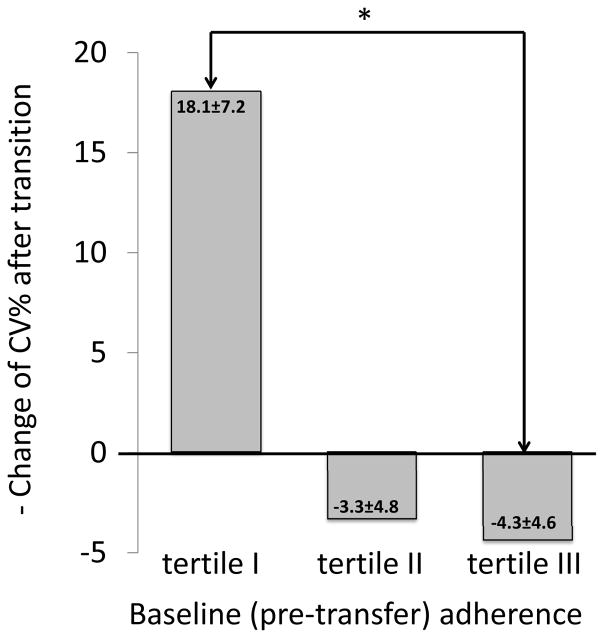
To examine the relationship between pre-transfer (baseline) adherence and change of adherence after transfer from the baseline, we divided the group of transitioned patients into tertiles based on the baseline adherence. Patients from the tertile I (those with the worst baseline adherence based on the highest CV%) had improvement of adherence after transfer, whereas adherence of patients from the tertile III did not significantly change post-transfer (Figure 3). The difference in adherence changes post-transfer was statistically significant between patients from tertiles I and III (p=0.02).
Figure 3.
The adolescent graft loss group had more than twice the percentage of non-adherent patients than the transitioned group before the transfer, based on the percent of serum tacrolimus levels ≤2.5 ng/ml (Table 2). This difference was still significant after individually adjusting for multiple covariates (Table 3). The median percentage of low tacrolimus levels was also significantly higher in the graft loss group compared with the transition group (table 2). The frequency of clinic visits, drug monitoring and inpatient admissions was, as expected, significantly higher in graft loss group than in the transitioned group (table 2). We observed a non-significant (p=0.07) trend towards a higher CV% in the graft loss group compared with the transitioned group before the transfer. The trend was still present after adjustments for covarites (Table 3).
To better match the ages of adolescent graft loss group and transitioned group prior to transfer, we extended the period of observation of the latter group to two more years backward. The data on adherence during this earlier period of time were available for the subgroup of 19 patients. The median tacrolimus CV% in this subgroup during years 2–4 prior to the transfer was 30.2 [IQR 25.1 to 62.0] %, the median percent of low tacrolimus levels was 0.0 [IQR 0.0 to 18.2] % and there were 7 (36.8%) non-adherent patients. The percent of non-adherent patients in the transitioned group during this earlier period of observation was still significantly lower than in the graft loss group (p<0.05), even after adjustment for covariates. Adherence during the extended earlier period of observation was not significantly different from the later adherence immediately preceding transfer in the transitioned group when measured by both CV% (p=0.16) and percent of low drug levels (p=0.75).
There were no significant differences between adherence measures (tacrolimus CV% and percent of low tacrolimus levels) between the combined adolescent group and transitioned group after the transfer in both univariate and multivariate analyses (Tables 2 and 3). The trend towards a poorer 4-years graft survival in the combined adolescent group (69%, vs. 88% in the transitioned group after the transfer) was not statistically significant (p=0.07).
In the group of young adults, adherence, measured both by serum tacrolimus CV% and percent of low tacrolimus levels, was not significantly different from the transitioned group after the transfer (Table 2). Adjustment for potential confounders did not significantly change the results (Table 3). The frequency of drug monitoring, clinic visits and hospitalizations in the young adult group was not significantly different from the transitioned group after the transfer (table 2).
Graft survival, graft function and blood pressure control
Three out of 25 transitioned patients (12%) lost their grafts within 4 years after transfer: at 0.64, 1.13 and 1.16 years post transfer respectively. All three patients who lost their grafts had more than 10% of low serum tacrolimus levels before the transfer and none of their pre-transition tacrolimus CV% were below the median for the transitioned group. All of them had documented non-adherence with medications and clinic visits while in the pediatric program, biopsy-proven episodes of rejection, and had poor graft function at the time of transfer. One of these patients died within 2 years after the graft loss, presumably due to a cardiovascular event, another patient remains on dialysis to date. The third patient was re-transplanted shortly after the graft loss at the age of 23 years old and now is more than 4 years after the second transplant with good graft function and no documented episodes of rejection. None of the transitioned patients died with a functioning graft within 4 years after the transfer.
The median serum creatinine after the transfer was significantly higher then before the transfer (table 2). The difference was still present when three patients who lost their grafts were excluded from the analysis (p=0.04). We observed a direct relationship between CV% before the transfer and serum creatinine after transfer (p=0.01) in univariate analysis; however, this association became not significant after adjustment for pre-transplant immunologic risk (data not shown).
In the graft loss adolescent group, the median graft life was 3.8 [IQR 1.8 to 5.8] years. We observed a strong inverse association between the age at transplant and graft life in this group (r=−0.85, p<0.0001), which was still significant after adjustment for immunologic risk and other covariates. Of the 22 patients, we were able to identify the cause of graft loss in 20 patients. Allograft rejection accounted for 80% of losses (16 patients). Eleven out of these 16 patients (69%) had documented evidence of non-adherence in their medical records. Three patients (15%) lost their allografts due to the recurrence of primary disease and one patient due to PTLD.
In the young adult group, 4 patients lost their grafts within the 4 years post transplant. In these 4 patients, the range of graft life was 1.97 to 3.77 years. One of the 4 patients had no low serum tacrolimus levels and had a CV% below the median for the young adult group. The other 3 patients all had more than 10% of low serum tacrolimus levels and their tacrolimus CV% were above the median for the group. All 4 patients lost their grafts due to biopsy-proven rejection, and three of them have documented evidence of non-adherence with medications in medical records (in one case it was related to the loss of health insurance).
We observed an increase of the diastolic blood pressure in the transitioned group after transfer, while systolic blood pressure did not significantly change (Table 2). Non-Hispanic black patients had higher systolic (p=0.03) and diastolic (p=0.05) blood pressure before (but not after) transfer, when compared with their white and Hispanic counterparts. Systolic (but not diastolic) blood pressure before the transfer significantly correlated with both systolic (r=0.69, p=0.007) and diastolic (r=0.61, p=0.02) blood pressure after transfer (data not shown). Systolic pre-transfer and diastolic post-transition blood pressure significantly correlated with the degree of creatinine increase post transfer (r=0.57, p=0.027 and r=0.53, p=0.04 respectively). We did not find significant differences between blood pressure values when comparing the transitioned group with the adolescent and the young adult groups respectively, except for the lower median diastolic blood pressure in the combined adolescent group (Table 2, p=0.001). We also did not find any significant association between blood pressure and adherence measures in the transitioned group.
Discussion
In this retrospective study, we observed that transition of pediatric kidney transplant recipients to the adult services within the same US inner-city tertiary medical center did not lead to any significant changes in adherence to immunosuppressant medications. The medication adherence of transitioned patients was also not significantly different from the adherence of young adults who received their first kidney transplants in the adult program and who as such were never exposed to the transplant transition process. In contrast, medication adherence was lower in the group of adolescent patients who were not transitioned to the adult transplant program due to graft losses while being still in the pediatric program. However, this difference disappeared when unsuccessful adolescents who lost their grafts prior to transfer were combined with those who were either currently active in the program or have been transitioned in the past.
Interestingly, in the subset of transitioned patients, whose adherence was the lowest in the pediatric program, it improved after the transfer. Improvement of outcomes following the transfer in selected transplant recipients, while observed in previous studies (16), has continued to receive little attention in the literature. Detailed characterization of this category of patients may provide new insights on the transition process and help to target the interventions more precisely. Possible reasons for adherence improvement post transfer may include psychosocial maturation; lower pill burden due to medical stabilization; and better fit of adult clinic settings for the subset of patients.
The problem of the possible impact that transition may have on adherence has received limited attention. A small study of Annunziato, et al. (14) found worsening adherence to tacrolimus based on the standard deviations of serum levels in liver transplant recipients after their transfer, which occurred on average at 22 years of age. However, even before the transfer, adherence in the main group of patients was much lower than in comparison pediatric and adult groups. Therefore, an influence of age of the transitioned patients on their adherence could have been as important as transition itself in that study.
The majority of studies that addressed the issue of transition in the kidney transplant population has been focused on hard outcomes, such as graft loss and acute rejection episodes, and did not incorporate direct measures of adherence into their design. While an earlier study of Watson found a 40% rate of graft loss within 3 years following the transfer of twenty 18 year-old patients to the different transplant centers (13), a larger European study revealed that the rate of acute rejection was not significantly affected by the transition, except in an immigrant population (16). In our study, only 12% of transitioned patients lost their grafts within 4 years following the transfer. The difference between our study and the Watson study may be in part due to improvement of transition practices over the past two decades. Recent single center studies showed a dramatic improvement in graft survival following the implementation of a transition program (11, 23).
The age at transfer may be also an important factor determining the outcome. It is well documented that adolescents and emerging adults have the highest risk of graft loss (6), independent of any potential confounders (8). In their large-scale analysis, Van Arendonk et al. demonstrated that in the US patients with a functioning graft at age 17 years, 42.4% would be expected to lose the graft by age 24 (8). Based on the peak of the bell shaped curve of the graft loss distribution by age, it would be reasonable to expect that individuals who transition earlier would have worst outcomes after transfer than those transitioned later, even if transition itself had no impact on the graft loss rate. Indeed, despite the reports of relatively successful transition at the age of approximately 18 years of age (15–17), the analysis of graft failure rates among 440 kidney transplant recipients recorded in the UNOS database (1987–2007) who had been transferred from pediatric to adult care demonstrated that younger (before 21 years) age at transfer to adult care was associated with higher graft failure rates (24). The importance of age when transfer should occur was supported by the Canadian study of Samuel et al., in which transfer of 149 patients took place before or around the age of 18 years. This multicenter study showed a high risk of graft failure within the interval 0.5 years before to 2.5 years after the first adult transplant visit, which took place at a median age of 18.1 (IQR 18.0 to19.4) years (25).
In accordance with these data, the analysis of the adolescent graft loss cohort in our study showed a strong inverse correlation between the age at transplant and graft life. Dramatic differences in medication adherence and graft survival between adolescent and young adult groups in our study make it reasonable to hypothesize that one of the reasons for good outcomes after transfer might be a survival effect with respect to graft loss. It seems that only the most adherent patients were able to go through adolescence without losing their grafts and to make it to transfer at 22 years. At that point the majority of them had already passed the high-risk age interval and so the consistency in medication adherence and the low rate of graft loss after transfer would be not surprising. The low impact of transition on transplant outcomes in our study is further supported by the absence of differences in adherence and graft loss between transitioned and young adult groups.
Would it be reasonable to suggest a benefit of later transition based on these findings? In our opinion, the current data do not provide sufficient evidence to make this recommendation. While individuals transitioned later appear to have better outcomes, we do not know whether patients who lose their grafts between the ages 18 and 21 should have done better if transferred to adult programs at the age of 18. Based on our data, transferring patients at 18 would automatically shift 22 cases of graft loss from the adolescent group to the transitioned group, changing the percentage of graft failure from 12% to 30%. That result would be much closer to Watson’s data (40%) (13), and would be a more direct and appropriate comparison because of similarity of age groups. Therefore, a study comparing medication adherence and/or graft loss rate after transfer in a group of patients transitioned earlier, to the adherence/graft loss before transfer in a group of patients transitioned later, with matched ages in both groups, may provide the necessary evidence to support earlier vs. later transition.
This and other questions arising from our findings may be answered definitively only based on larger cohorts and ideally a randomized controlled trial of different ages at transfer. It would be warranted in the future to incorporate adherence monitoring into institutional and national transplant databases and to clearly document the transition process in the medical records and in the registries.
Our study has some obvious limitations. Because of the retrospective nature of the study, three patient groups had inherent differences and the 15-year period of study was quite long. The measures of adherence that we used, while objective, are not ideal. Single center data allowed us a modest sample size, which affected the power of our conclusions. Defining the date of transfer as a first documented visit to the adult transplant clinic we did not cover the patients who might be “lost” between the pediatric and the adult services. While we did not have a substantial number of such cases, the situation may be different in other programs. Nonetheless, availability of the exact dates of transfer and thorough collection of serum tacrolimus levels over the period of two years before and after transfer are some of the strengths of this study.
We would like to emphasize that our results by any means should not be regarded in cases of external transfer (transition from a pediatric program in one institution to an adult program in another institution). We transferred to other institutions almost the same number of patients as to our own adult center (Figure 1), however only the latter subgroup was included into the analysis due to absence of data for the patients transferred elsewhere. A study comparing the outcomes of internal vs. external transition is warranted.
In summary, transition to the adult services was not associated with differences in medication adherence in our study. When combining our findings with other recent publications (15–17), it is reasonable to suggest that the transition of pediatric kidney transplant recipients to the adult services within the same transplant center may not necessarily pose an additional risk for non-adherence in the current era of high awareness of problems associated with transition. Age-related risk of non-adherence appears to be a more significant factor that may substantially confound analyses of post-transfer outcomes. Therefore, it remains critical to develop targeted interventions to improve interventions in adolescence, the age group with the highest risk (26). Large-scale prospective studies are needed to elucidate national and international trends of medication adherence in relation to the transition from pediatric to adult transplant care in order to provide evidence for improving transition practices in kidney transplant programs.
Acknowledgments
This publication was supported in part by the CTSA Grant UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH. Authors appreciate the help of J. Lindower, K. Datta, P. Yeboa, and V. Boshuizen with data collection. Authors thank Dr. A. Spitzer for critical review of the manuscript.
Footnotes
Author contributions
Oleh M. Akchurin: Concept/design, Data collection, Statistics, Data analysis/interpretation, Drafting article
Michal M. Melamed: Concept/design, Data analysis/interpretation, Statistics, Critical revision of article
Becky L. Hashim: Data interpretation, Critical revision of article
Frederick J, Kaskel: Concept/design, Critical revision of article, Approval of article
Marcela Del Rio: Concept/design, Data collection, Critical revision of article, Approval of article
References
- 1.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatric transplantation. 2010;14:603–613. doi: 10.1111/j.1399-3046.2010.01299.x. [DOI] [PubMed] [Google Scholar]
- 2.McGrady ME, Hommel KA. Medication Adherence and Health Care Utilization in Pediatric Chronic Illness: A Systematic Review. Pediatrics. 2013;132:730–740. doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecka JM, Gjertson DW, Terasaki PI. Pediatric renal transplantation: a review of the UNOS data. United Network for Organ Sharing. Pediatric transplantation. 1997;1:55–64. [PubMed] [Google Scholar]
- 4.Michelon TF, Piovesan F, Pozza R, et al. Noncompliance as a cause of renal graft loss. Transplantation proceedings. 2002;34:2768–2770. doi: 10.1016/s0041-1345(02)03403-6. [DOI] [PubMed] [Google Scholar]
- 5.Morrissey PE, Reinert S, Yango A, Gautam A, Monaco A, Gohh R. Factors contributing to acute rejection in renal transplantation: the role of noncompliance. Transplantation proceedings. 2005;37:2044–2047. doi: 10.1016/j.transproceed.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA. Association between age and graft failure rates in young kidney transplant recipients. Transplantation. 2011;92:1237–1243. doi: 10.1097/TP.0b013e31823411d7. [DOI] [PubMed] [Google Scholar]
- 7.Magee JC, Bucuvalas JC, Farmer DG, Harmon WE, Hulbert-Shearon TE, Mendeloff EN. Pediatric transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4 (Suppl 9):54–71. doi: 10.1111/j.1600-6143.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Arendonk KJ, James NT, Boyarsky BJ, et al. Age at graft loss after pediatric kidney transplantation: exploring the high-risk age window. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1019–1026. doi: 10.2215/CJN.10311012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell LE, Sawyer SM. Transition of care to adult services for pediatric solid-organ transplant recipients. Pediatric clinics of North America. 2010;57:593–610. doi: 10.1016/j.pcl.2010.01.007. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Ferris ME, Mahan JD. Pediatric chronic kidney disease and the process of health care transition. Seminars in nephrology. 2009;29:435–444. doi: 10.1016/j.semnephrol.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi: 10.1136/bmj.e3718. [DOI] [PubMed] [Google Scholar]
- 12.Fredericks EM. Nonadherence and the transition to adulthood. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2009;15 (Suppl 2):S63–69. doi: 10.1002/lt.21892. [DOI] [PubMed] [Google Scholar]
- 13.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatric Nephrology. 2000;14:469–472. doi: 10.1007/s004670050794. [DOI] [PubMed] [Google Scholar]
- 14.Annunziato RA, Emre S, Shneider B, Barton C, Dugan CA, Shemesh E. Adherence and medical outcomes in pediatric liver transplant recipients who transition to adult services. Pediatric transplantation. 2007;11:608–614. doi: 10.1111/j.1399-3046.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Koshy SM, Hebert D, Lam K, Stukel TA, Guttmann A. Renal allograft loss during transition to adult healthcare services among pediatric renal transplant patients. Transplantation. 2009;87:1733–1736. doi: 10.1097/TP.0b013e3181a63ed9. [DOI] [PubMed] [Google Scholar]
- 16.van den Heuvel ME, van der Lee JH, Cornelissen EA, et al. Transition to the adult nephrologist does not induce acute renal transplant rejection. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:1662–1667. doi: 10.1093/ndt/gfp684. [DOI] [PubMed] [Google Scholar]
- 17.Kiberd JA, Acott P, Kiberd BA. Kidney transplant survival in pediatric and young adults. BMC nephrology. 2011;12:54. doi: 10.1186/1471-2369-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. The New England journal of medicine. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 19.Hsiau M, Fernandez HE, Gjertson D, Ettenger RB, Tsai EW. Monitoring Nonadherence and Acute Rejection With Variation in Blood Immunosuppressant Levels in Pediatric Renal Transplantation. Transplantation. 2011;92:918–922. doi: 10.1097/TP.0b013e31822dc34f. [DOI] [PubMed] [Google Scholar]
- 20.Prytula AA, Bouts AH, Mathot RA, et al. Intra-patient variability in tacrolimus trough concentrations and renal function decline in pediatric renal transplant recipients. Pediatric transplantation. 2012 doi: 10.1111/j.1399-3046.2012.01727.x. [DOI] [PubMed] [Google Scholar]
- 21.Zar JH. Biostatistical analysis. Englewood Cliffs, New Jersey: Prentice-Hall; 1984. [Google Scholar]
- 22.Machin D, Campbell M, Fayers P, Pinol A. Sample size tables for clinical studies. Malden, MA: Blackwell Science; 1997. [Google Scholar]
- 23.Prestidge C, Romann A, Djurdjev O, Matsuda-Abedini M. Utility and cost of a renal transplant transition clinic. Pediatr Nephrol. 2012;27:295–302. doi: 10.1007/s00467-011-1980-0. [DOI] [PubMed] [Google Scholar]
- 24.Foster BJ, Platt RW, Dahhou M, Zhang X, Bell LE, Hanley JA. The impact of age at transfer from pediatric to adult-oriented care on renal allograft survival. Pediatric transplantation. 2011;15:750–759. doi: 10.1111/j.1399-3046.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- 25.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, et al. Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation. 2011;91:1380–1385. doi: 10.1097/TP.0b013e31821b2f4b. [DOI] [PubMed] [Google Scholar]
- 26.Hashim BL, Vadnais M, Miller AL. Improving Adherence in Adolescent Chronic Kidney Disease: A Dialectical Behavior Therapy (DBT) Feasibility Trial. Clinical Practice in Pediatric Psychology. 2013 [Google Scholar]