Abstract
Genetic analysis of protein function requires a rapid means of inactivating the gene under study. Typically this exploits temperature sensitive mutations, or promoter shut-off techniques. We report the adaptation to Schizosaccharomyces pombe of the Anchor Away technique, originally designed in budding yeast (Haruki et al., 2008a). This method relies on a rapamycin-mediated interaction between the FRB and FKBP12 binding domains, to relocalize nuclear proteins of interest to the cytoplasm. We demonstrate a rapid nuclear depletion of abundant proteins as proof-of-principle.
Introduction
Analysis of gene function in yeasts typically requires an effective way to inactivate the protein of interest. The classic way to do this relies on temperature sensitive mutations (Bonatti et al., 1972; Hartwell, 1967). These protein products have attenuated activity at higher or lower temperature. However, temperature-sensitive mutants can be hard to isolate, slow to inactivate, and residual activity can give ambiguous results. A modification of this method uses a temperature-sensitive degron that leads to ubiquitylation and protein turnover by the proteasome in response to temperature (e.g, (Lindner et al., 2002)). An auxin-inducible degron system was developed to deplete protein level without introducing temperature stress (Kanke et al., 2011). However, not all proteins have proven amenable to these modifications.
Another option is to control gene expression. The challenge is identifying a promoter that can be rapidly repressed. In fission yeast, the nmt1+ promoter (Maundrell, 1990) and its derivatives (Basi et al., 1993) are frequently used. However, the lag in induction and leakiness upon repression have made these promoters useful primarily for already unstable proteins (Forsburg, 1993). The urg1+ promoter is capable of fast induction (>2 hours) in the presence of uracil and a 10-fold reduction of protein level was achieved 360 min after uracil removal (Watson et al., 2011). Genes regulated by a tetracycline-regulated promoter show even shorter induction time (~30 min) and reach maximum level around 4 hours (Zilio et al., 2012), but repression has not been characterized. Alternatively, DAmP (decreased abundance by mRNA perturbation) alleles were used to generate hypomorphic alleles by reducing the transcription level (Schuldiner et al., 2005). Again, although the mRNAs are subjected to fast turnovers, the proteins may still linger in the cells.
A third strategy is to sequester the protein. The hormone-binding domain (HBD) of the estrogen receptor attracts the Hsp90 complex. Proteins tagged with HBD form a complex with Hsp90, therefore are inactivated by steric hindrance (Boe et al., 2008). Upon addition of beta-estradiol, the HBD ligand, fusion proteins are released from the complex and activated. However, many HBD-fused nuclear proteins do not respond to beta-estradiol (Pai et al., 2012).
The anchor away (AA) system was developed in Saccharomyces cerevisiae to target nuclear proteins (Haruki et al., 2008a). This system exploits the rapamycin-driven interaction that occurs between two binding domains: the FRB domain, found in the TOR1/Tor1 protein, and the FKBP12 domain, found in the FRP1/Fkh1 protein (Otsubo and Yamamato, 2008; Weisman, 2004). The system places the FKBP12 domain on a highly expressed cytoplasmic protein (anchor), and the FRB domain on a target protein of interest. In the presence of rapamycin, the target protein is depleted from the nucleus and anchored in the cytoplasm, allowing analysis of its depletion phenotype. We engineered the anchor-away (AA) system in the fission yeast Schizosaccharomyces pombe to target nuclear proteins to the cytoplasm. To eliminate competition from the endogenous TOR system, we used an allele tor1-SE that specifically blocks rapamycin binding to Tor1, and a strain deleted for fkh1+ (FKBP12) (Weisman et al., 2001). We applied this approach to two abundant nuclear proteins, Rad11 and Mcm4. Our results suggest that while the AA system will be a useful tool to study protein function, it has some limitations for use in fission yeast.
Materials and methods
Strains, growth conditions and basic manipulations
Strains are listed in Table 1. FY7128 was constructed by integrating NruI linearized pLD141 into the leu1-32 locus in FY6653. Media and manipulations were performed as described in (Forsburg and Rhind, 2006).
Table 1.
Strains
| Strain | Genotype | Source |
|---|---|---|
| FY11 | h- ade6-M210 | Our stock |
| FY1107 | h- Δrad3::ura4+ ura4-D18 leu1-32 ade6-M216 | Our stock |
| FY6609 | h- fkh1::ura+ ura4 -D18 leu1-32 ade6-M210 | From TA96 (Weisman et al., 2001) |
| FY6641 | h+ tor1SE:KanMX ura4 -D18 leu1-32 | From TA1463 (Laor et al., 2014) |
| FY6792 | h+ tor1SE:KanMX leu1-32::[nmt1-rpl13-2FKBP12-leu1+] ura4-D18 | This study |
| FY6962 | h- rad11-FRB-GFP::kan leu1-32::[nmt1-rpl13-2FKBP12-leu1+] tor1SE:KanMX fkh1::ura+ his3-D1 ura4 -D18 leu1-32 | This study |
| FY6992 | h+ mcm4-FRB-GFP::kan leu1-32::[nmt1-rpl13-2FKBP12- leu1+] tor1SE:KanMX fkh1::ura+ ura4-D18 leu1-32 ade6- M210 | This study |
| FY7128 | h- tor1SE:KanMX fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12- leu1+] ura4 -D18 leu1-32 ade6-M210 | This study |
| FY7139 | h+ tor1SE:KanMX fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12- leu1+] ura4 -D18 leu1-32 ade6-M210 | This study |
| FY7135 | h- fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12-leu1+] ura4 -D18 leu1-32 ade6-M210 | This study |
| FY7189 | h+ rad11-FRB-GFP::kan leu1-32::[nmt1-rpl13-2FKBP12-leu1+] his3-D1 ura4-D18 leu1-32 ade6-M210 | This study |
| FY7222 | h+ rad11-FRB-GFP::nat tor1SE:KanMX fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12-leu1+] ura4 -D18 leu1-32 ade6- M210 | This study |
| FY7190 | h+ mcm4-FRB-GFP::kan leu1-32::[nmt1-rpl13-2FKBP12-leu1+] his3-D1 ura4-D18 leu1-32 ade6-M210 | This study |
| FY7188 | h+ mcb1-FRB-GFP::kan leu1-32::[nmt1-rpl13-2FKBP12-leu1+] his3-D1 ura4-D18 leu1-32 ade6-M210 | This study |
| FY7196 | h- mcb1-FRB::Nat tor1SE:KanMX fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12-leu1+] ura4 -D18 leu1-32 ade6-M210 | This study |
| FY7197 | h+ mcb1-FRB::Nat tor1SE:KanMX fkh1::ura+ leu1-32::[nmt1-rpl13-2FKBP12-leu1+] ura4 -D18 leu1-32 ade6-M210 | This study |
Rapamycin (Sigma, R0395) was dissolved in DMSO. Final concentration is 2.5μg/mL. Strains with the Rpl13-2FKBP12 anchor were maintained in EMM–LEU+thiamine (15μM = 5μg/mL). Experiments (serial dilution assay and microscopy) were done in YES media. Electroporation was used to transform cells (Forsburg and Rhind, 2006).
LiOAc-SDS-based colony PCR was optimized for S. pombe from Looke et al. (2011). Single colony was resuspended in 100μL LiOAc-SDS buffer (200mM LiOAc, 1% SDS) in a 1.5mL eppendorf tube and incubated at 70°C. Three volumes (300μL) of 96% ethanol were added after 15 minutes. The sample was mixed by vortexing. The mixture was centrifuged at 15,000g for 3 minutes. The supernatant was removed by aspiration. After the pellet was completely air-dried, it was resuspended in 100μL TE and centrifuged at 15,000g for 3 minutes. The entire tube can be stored at 4°C for months without separating the supernatant (contains genomic DNA) and the pellet. The refrigerated sample was vortexed and centrifuged again before setting up the PCR experiments. One microliter of supernatant is sufficient for a 20μL PCR reaction.
Crosses were performed on SPAS plates (Forsburg and Rhind, 2006) . Mating type was determined by colony PCR using primers: MT1: AGAAGAGAGAGTAGTTGAAG, MP: ACGGTAGTCATCGGTCTTCC and MM: TACGTTCAGTAGACGTAGTG (Roguev et al., 2007).
Plasmids
Plasmids are listed in Table 2. Restriction maps are in Figure S1. pFA6a-FRB-KanMX6, pFA6a-FRB-GFP-KanMX6, and pFA6a-2xFKBP12-His3MX6 were purchased from Euroscarf (P30578, P30580, and P30583).
Table 2.
Plasmids
| Plasmid | Purpose | Source |
|---|---|---|
| pFA6a-FRB-KanMX6 | to tag gene of interest with FRB at endogenous locus; nourseothricin resistant | EUROSCARF: P30578 |
| pFA6a-FRB-GFP-KanMX6 | to tag gene of interest with FRB-GFP at endogenous locus; nourseothricin resistant one XhoI site between FRB and GFP (pFA6a-FRB-(XhoI)-GFP-kanMX6) |
EUROSCARF: P30580 |
| pFA6a-2xFKBP12-His3MX6 | to tag gene of interest with FRB-GFP at endogenous locus; histidine prototroph | EUROSCARF: P30583 |
| pLD103 | pFA6a-2xFKBP12-hph: to tag gene of interest with 2xFKBP12 at endogenous locus; hygromycin B resistant | This study |
| pLD115 | pFA6a-FRB-natMX6: to tag gene of interest with FRB at endogenous locus; nourseothricin resistant | This study |
| pLD116 | pFA6a-FRB-(XhoI)-GFP-natMX6: to tag gene of interest with FRB-GFP at endogenous locus; nourseothricin resistant | This study |
| pLD133 | Episomal expression vector that allows 2FKBP12 to be fused to C-terminus; gene of interest under full strength nmt1 promoter, LEU2 | This study |
| pLD134 | Episomal expression vector of rpl13-2FKBP12, under full strength nmt1 promoter, LEU2 | This study |
| pLD138 | leu1-32 integration plasmid that allows 2FKBP12 to be fused to C-terminus; gene of interest under full strength nmt1 promoter | This study |
| pLD141 | leu1-32 integration plasmid that expresses rpl13-2FKBP12 under full strength nmt1 promoter | This study |
pLD103 is a C-terminus 2FKBP12 tagging plasmid. It was made by replacing the His3MX6 cassette between BglII and PmeI from pFA6a-2xFKBP12-His3MX6 (Haruki et al., 2008a) (EUROSCARF: P30583) with the HphMX6 cassette from pFA6a-hph (Hentges et al., 2005). pLD133 is an episomal expression vector. It was made by replacing the C-terminus 3HA tagging fragment between NotI and SalI in pSGP72 with C-terminus 2FKBP12 tagging fragment amplified from pLD103. pLD134 was made by inserting rpl13+ ORF (lacking the stop codon) cloned from the S. pombe genomic DNA into pLD133 between XhoI and NotI sites. pLD134 is capable of expressing rpl13-2fkbp12 from the nmt1 promoter on an episomal plasmid. We also created a tagging integration vector, pLD138 by inserting the 2fkbp12-c flanked by nmt1 promoter (Pnmt1) and terminator (Tnmt1) released from pLD133 with PstI and SacI into pJK148. Finally, we made pLD141 by inserting a fragment of Pnmt1-rpl13+ released from pLD134 with PstI and NotI into pLD138. pLD115 was made by replacing the KanMX6 cassette between BglII and PmeI in pFA6A-FRB-KanMX6 (EUROSCARF: P30578) with NatMX6 from pFA6a-NatMX6 (Hentges et al., 2005). pLD116 was made by replacing the KanMX6 cassette between BglII and PmeI in pFA6A-FRB-GFP-KanMX6 (EUROSCARF: P30580) with NatMX6 from pFA6a-NatMX6 (Hentges et al., 2005).
pLD114 is an episome that tags a protein of interest at the C terminus with FRB-GFP and expresses it under the nmt1+ promoter. pLD133 provides the 2FKBP12 tag in a similar nmt1 episomal vector, and can be used to screen anchors.
Generating anchor away strains
Amplification of C-FRB or C-FRB-GFP module
pLD115 can be used as template to fuse FRB to the carboxy terminus of your favorite protein. pLD116 can be used as template to fuse FRB-GFP to the carboxy terminus of your favorite protein. Both plasmids are pFA6a derivatives. The PCR primers used from the templates are Forward Primer: 5’-(gene-specific sequence)- CGGATCCCCGGGTTAATTAA-3’ and Reverse Primer: 5’-(gene-specific sequence)-GAATTCGAGCTCGTTTAAAC-3’ (Bahler et al., 1998). The procedure of amplify the transformation module is described in (Bahler et al., 1998).
Via direct transformation
Base strain (FY7128 or FY7129) was grown at 32°C in EMM-LEU+thiamine liquid to mid-log phase. Cells were harvested and electroporated with the transformation module. Transformed cells were plated on EMM-LEU+thiamine plates and incubated overnight at 32°C. The plates were replica plated on YES+G418+Nat plates and incubated at 32°C for 2-4 days (colonies usually appear in 2 days). Transformants were restreaked onto EMM-LEU+thiamine plates immediately after visible and incubated at 32°C for 3-4 days, then replica plated on YES+G418+Nat plates. The G418+Nat resistant transformants were tested by colony PCR and confirmed by sequencing. Proteins tagged with FRB-GFP were also confirmed by Western blotting.
Via genetic crosses
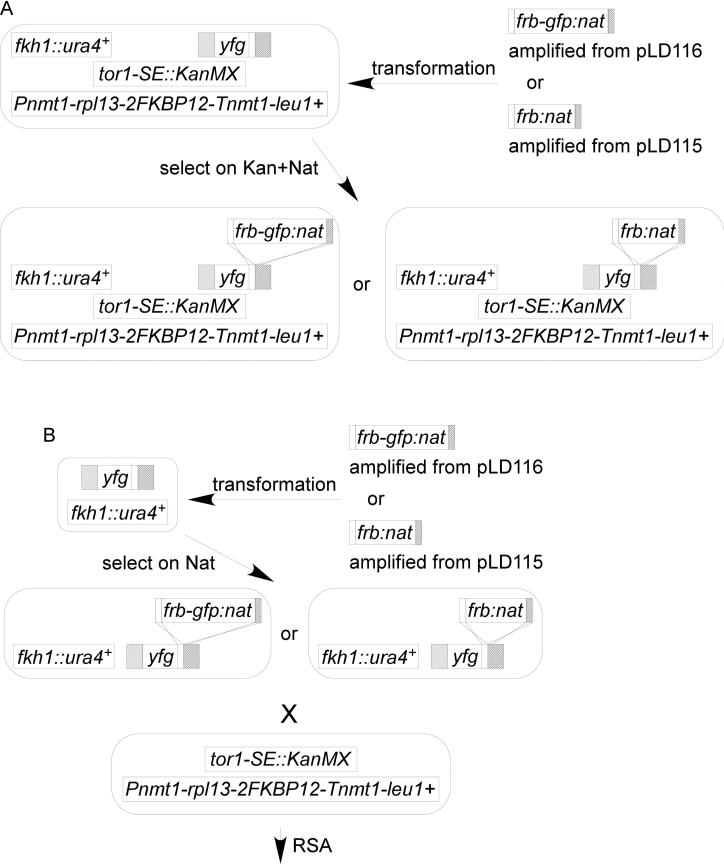
Δfkh1 (FY6609) was electroporated with the transformation module. Transformants were isolated as Nat resistant candidates and isolated using the same protocol as the via direct transformation section above. The correct transformant was crossed with tor1SE Pnmt1-rpl13-2FKBP12 strain (FY6792) on SPAS or ME. Random spore analysis (RSA) procedure was used to select the final assay strain. The spores were germinated and grow on EMM-LEU+thiamine plate then replica plated onto YES+G418+Nat and –URA plate.
Western blotting
The procedure was the same as in Ding and Forsburg (2011). Fifty micrograms of total protein were separated by SDS-PAGE in a 8% acrylamide gel. JL8 (Clontech; 1:1000) was used to detect GFP. PC10 (Delta Biolabs; 1:1000) was used to detect PCNA. Anti-mouse-HRP (Sigma; 1:2000) was used to recognize the primary antibodies. The signal was captured by the ChemiDoc system (Bio-Rad) under ChemHi Sensitivity setting. The exposure time was 999 seconds. The images were taken with Quantity One software (Bio-Rad).
Microscopy
A single colony was grown at 32°C in 3mL YES overnight to ~OD=0.8. One half milliliter of culture was mixed with 0.5mL YES+ 5μg/mL rapamycin (1μL 2000X stock) or 0.5mL YES+ 1μL DMSO in 15mL culture tubes. The culture tubes were shaken vigorously in a 32°C shaker during the time-course. One hundred microliters of culture was collected in 1.5mL eppedrof tube and centrifuged at 3000 rpm for 30 seconds. Ninety microliters of supernatant was removed and the pellet was resuspended in the rest of the media. Three microliters of the suspension was mounted on a 2% SPAS agar pad.
All images were taken on a DeltaVision core microscope (Applied Precision/GE) using a GFP/mCherry polychroic mirror and oil-immersion Olympus 60X lens with Numerical Aperture of 1.4. Eighteen z-sections at 0.3μm were taken to reconstruct the projected images in softWoRx 5.5. Cell length was measured using the distance tool in softWoRx 5.5 (Applied Precision/GE).
Digital image manipulation
All the plates and films were electronically scanned using a ScanJet IIcx scanner (Hewlett-Packard). Digitized pictures/photos were analyzed and contrast-enhanced by NIH ImageJ software and assembled in Canvas software (ACD Systems).
Results
Construction of the anchor-away strains
The TOR kinases are part of a conserved eukaryotic signaling system associated with stress response and metabolism (reviewed in Otsubo and Yamamato (2008)). S. pombe Tor1 and Tor2 each contain a conserved FRB (FKBP12-rapamycin binding) domain. Tor1 is not essential, but is required for proper response to stress, for amino acid uptake, and for mating (Kawai et al., 2001; Weisman and Choder, 2001; Weisman et al., 2005). Tor2 is essential for viability (Alvarez and Moreno, 2006; Matsuo et al., 2007; Uritani et al., 2006; Weisman and Choder, 2001; Weisman et al., 2007). Rapamycin binds to the FKBP12 domain in Fkh1, and results in formation of a stable complex with the FRB domain in TOR (reviewed in Otsubo and Yamamato (2008)). The Anchor-Away system exploits this interaction by fusing the FKBP12 domain to an abundant cytoplasmic protein and the FRB domain to a target nuclear protein (Haruki et al., 2008a). The cytoplasmic anchor traps the target outside of the nucleus.
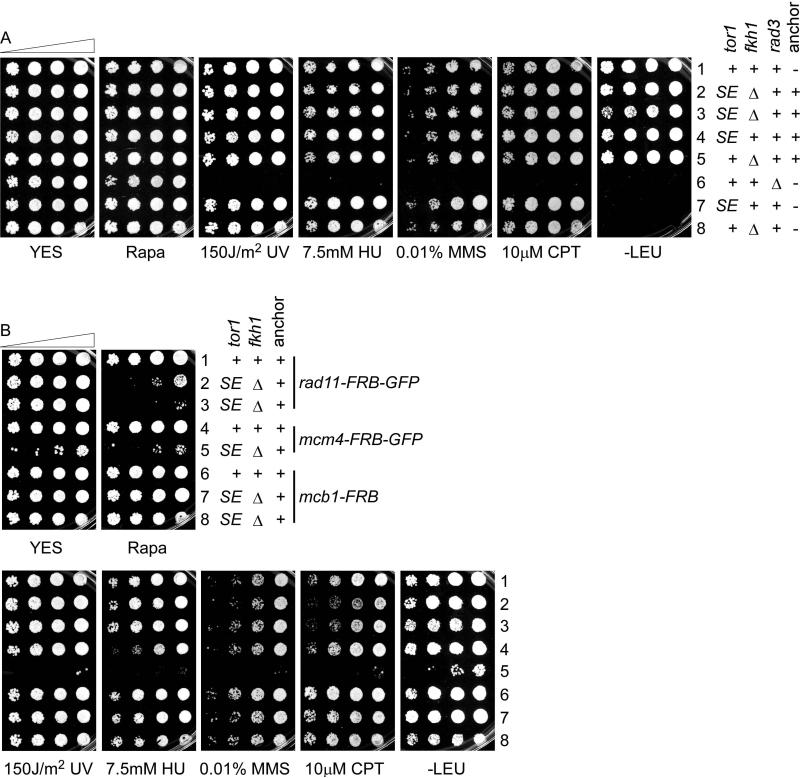
In contrast to S. cerevisiae, wild type S. pombe is not sensitive to rapamycin (Otsubo and Yamamato 2008; Figure 2A). However, to eliminate competition from the endogenous TOR signaling system, we used a strain with a deletion of fkh1+ (eliminating the native FKBP12 domain), and combined this with tor1-S1834E (tor1SE) (Laor et al., 2014; Weisman et al., 2005). Mutation of the conserved serine (S) residue in the FRB domain of Tor1 to glutamic acid (E) residue dramatically reduces the interaction with Fkh1 in the presence of rapamycin (Weisman et al., 2005). In analogy to its paralog in the S. cerevisiae anchor away system (Haruki et al., 2008b), and because the tor1SE fkh1Δ strain already had a mating phenotype, we left the essential tor2+ unchanged.
Figure 2. Drug sensitivity tests of indicated strains.
(A) FY11, FY7128, FY7139, FY6792, FY7135, FY1107, FY6641, FY6609. (B) FY7189, FY6962, FY7222, FY7190, FY6992, FY7188, FY7196, FY7197. 1:5 serial dilution on YES plates containing indicated drugs. Plates were scanned 2 days after incubated at 32°C.
Two abundant non-nuclear proteins ScPma1 (a plasma membrane proton pump/ATPase) and ScRpl13A (a cytoplasmic ribosomal 60S subunit protein) were used as anchors in S. cerevisiae (Haruki et al., 2008a). We therefore constructed anchors using their S. pombe orthologs, SpPma1 (pma1+: SPAC1071.10c) and SpRpl13 (rpl13+: SPAC664.05).
We generated a tagging plasmid, pLD103, from pFA6a-2xFKBP12-His3MX6 (EUROSCARF: P30583). The new tagging plasmid is marked by HphMX6 and used to tag potential anchors at the C-terminus. We tagged pma1+ at its native locus with two copies of FKBP12 (FY6554 and FY6555). However, the tagged strains had a slow growth and sterile phenotype (data not shown). Therefore, we focused on Rpl13, which was a better anchor in budding yeast (Haruki et al., 2008a). Unlike Sc RPL13, Sp rpl13+ is an essential gene (Hayles et al., 2013; Kim et al., 2010). We were not able to insert a C-terminal 2FKBP12 tag at its native locus. To solve this problem, we created pLD141, a leu1 integration plasmid that expresses rpl13-2FKBP12 from the nmt1 promoter. pLD141 was linearized with NruI and integrated at leu1-32 in a tor1SE strain (FY6641). This maintains the wild type rpl13+in addition to the integrated, tagged version. The resulting strains (FY6792 and FY6793) were unable to mate with h- wildtype cells on SPAS but mated successfully on ME. Interestingly, tor1-SE alone was mating competent (data not shown). This is consistent with a slightly decreased mating efficiency of tor1-SE (Weisman et al., 2005). S. pombe Tor1 is known to play a role in sexual development and nitrogen starvation responses (Kawai et al., 2001; Weisman and Choder, 2001; Weisman et al., 2007); this suggests some impairment of tor1-SE that is background dependent. Similarly, we observed that tor1-SE fkh1Δ in the absence of the anchor was also partially sterile on SPAS, although the mating efficiency was slightly better on ME (data not shown).
Despite the mating defects, none of the strains show any growth defects or sensitivity to DNA damaging agents (Figure 2A). To investigate whether the anchor away technique works in S. pombe, we conducted proof-of-principle experiments using three well-studied essential nuclear proteins: Rad11 (Rpa1; Parker et al., 1997), Mcm4 (Coxon et al., 1992), and Mcb1 (Ding and Forsburg, 2011; Li et al., 2011; Santosa et al., 2013). We tagged each gene with FRB-GFP domain using the tagging plasmid pFA6a-FRB-GFP-KanMX6 (EUROSCARF: P30580) in a wildtype strain. All three proteins were successfully tagged as assessed by Western blot (Figure S2). We constructed the assay strains as follows (Figure S3): we crossed each tagged candidate into a Δfkh1 strain (FY6609), then crossed it to tor1SE strain (FY6641). Since both our target genes and tor1SE were marked by Kan, we pulled tetrads and isolated nonparental ditypes (NPD) (we later made Nat marked pLD116 and pLD115 to avoid pulling tetrads). Finally, since tor1-SE fkh1Δ is partially sterile, we integrated the NruI-linearized pLD141 at the leu1-32 locus to provide the anchor.
To simplify the system, we generated base strains (FY7139 and FY7128) that contain fkh1Δ tor1-SE and the integrated cytoplasmic anchor Rpl13-2FKBP12. These strains have neither growth defects nor drug sensitivity (Figure 2A), though they were partially sterile (data not shown). We converted the drug marker in the tagging plasmids from KanMX6 to NatMX6 (pLD115 and pLD116) to facilitate tagging the target protein with FRB or FRB-GFP, respectively. The assay strain of a gene of interest can be constructed simply by a direct transformation method (Figure 1A) or via a genetic cross (Figure 1B).
Figure 1. Outlines of anchor-away assay strain construction.
(A) Construction of an assay strain via direct transformation. (B) Construction of an assay strain via genetic crosses. See Materials and methods section for details.
Proof of concept
Rad11-FRB-GFP
RPA, replication protein A, is a nuclear protein complex composed of Rpa1, Rpa2, and Rpa3. The heterotrimeric complex coats the single-stranded DNA (ssDNA) and plays important roles in DNA replication, DNA repair, and homologous recombination (Wold, 1997). The accumulation of Rpa1/Rad11, the largest subunit, has been used as a measure of ssDNA level (Sabatinos et al., 2012). Since rad11+ is an essential gene, no full deletion can be made, but a temperature sensitive allele rad11A has been characterized (Parker et al., 1997), and a hypomorphic allele shows DNA damage sensitivity (Ono et al., 2003). We investigated whether the anchor-away system generates a reliable conditional rad11Δ with an appropriate phenotype. First, we followed the outline in Figure S3 to make an assay strain expressing rad11-FRB-GFP (FY6962). It has no growth defect or drug sensitivity, suggesting it is fully functional. As expected, viability was reduced in the presence of rapamycin. Importantly, expressing rad11-FRB-GFP in a wild type tor1+ fkh1+ strain with anchor (FY7189) was not sensitive to rapamycin (Figure 2B), indicating that the anchor-away system requires neutralization of the Tor1-Fkh1 interaction.
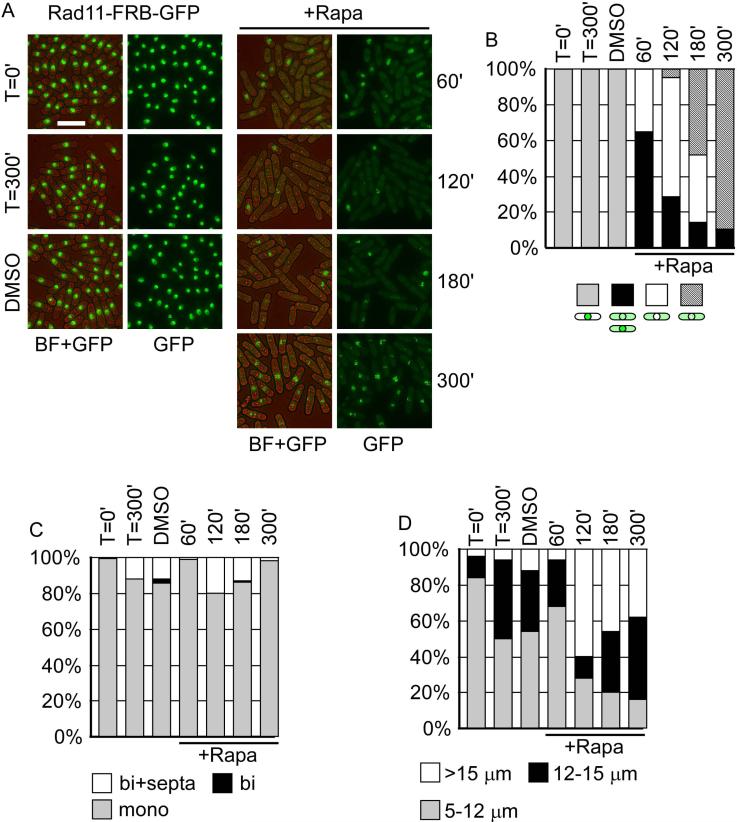
Next, we explored the dynamics of the nuclear depletion of Rad11 by visualizing the GFP signal every hour over a 5-hour time-course (Figure 3). Rad11-FRB-GFP localized in the nucleus in asynchronous cells at the beginning of the time-course (T=0). Following the addition of rapamycin, we observed translocation of Rad11-FRB-GFP from the nucleus to cytoplasm within an hour (Figure 3A). One hour after the treatment, 65% of the population exhibited GFP signal in the cytoplasm, and 35% showed a nuclear void (Figure 3B). Although binucleate cells were still observed, the population showed cell elongation, reminiscent of the cdc phenotype observed in rad11A mutants (Figure 3C, D; Parker et al. 1997). In two hours, the nuclear void population was predominant. There were about 5% of cells with punctate nuclear and cytoplasmic GFP signals, accompanied by a dramatic increase of cell length. Three hours into the treatment, 47% of cells had nuclear GFP puncta. By the end of the time-course, 89% of treated cells showed a punctate nuclear GFP signal, the nuclear GFP signal remained the same in untreated cells and mock cells. A large population of the treated cells were highly elongated (38% >15μm), compared to untreated (6% >15μm) and mock cells (12% >15μm).
Figure 3. Rad11-FRB-GFP is translocated from nucleus to cytoplasm in the presence of Rapamycin.
(A) Representative pictures of re-localization of Rad11-FRB-GFP. Left, no change in localization was observed in the absence of Rapamycin at 100 or 300 minute. DMSO is the solvent control. Right, Rad11-FRB-GFP was removed from the nucleus until approximately 300min after Rapamycin treatment. Bar = 15 μm. (B) Cells with different patterns of GFP signal were counted, n>50. At later time-points, speckles of Rad11, distinct from the original nuclear distribution, were observed. (C) Cells were counted and categorized as mononucleated, binucleated, and binucleated with septa, n>50. (D) Cell length was measured and categorized into 5-12 μm, 12-15 μm, and >15 μm, n=50.
We also generated another assay strain of rad11-FRB-GFP (FY7222) using the base strain and following the protocol showed in Figure 1A. We found a similar response to rapamycin with FY7222 compared to FY6962 (Figure 2B, Figure S4), again, with evidence for nuclear puncta particularly by 5h. There was no significant difference in the effect observed using rich media (YES) compared to EMM (data not shown), so further experiments were performed in YES.
Mcm4-FRB-GFP
Mcm4 is a component of the heterohexameric MCM complex that is the primary replicative helicase (reviewed in Forsburg (2004)). An essential gene, mcm4+ has been studied using a number of conditional alleles (Kanke et al., 2011; Lindner et al., 2002; Nitani et al., 2008). We compared these phenotypes to an anchor away version of Mcm4. The anchor away strain of Mcm4-FRB-GFP exhibited a slow growth phenotype even in the absence of rapamycin, and was sensitive to a wide range of DNA damaging drugs (Figure 2B), suggesting that this protein function is attenuated in this background. Consistent with this, we observed substantial elongation of the cells in the starting strain.
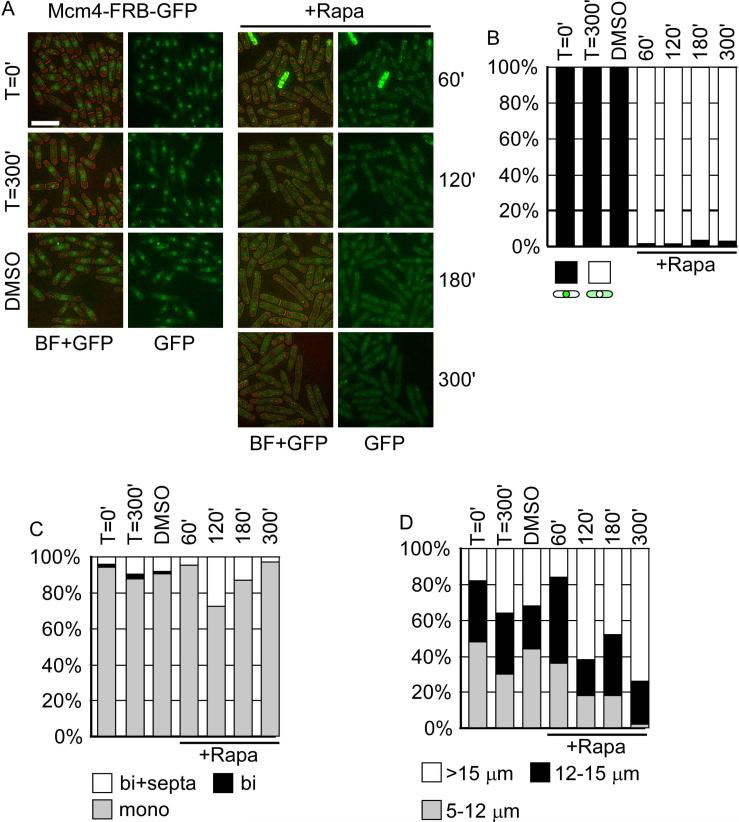
Mcm4 is an abundant nuclear protein (Kearsey et al., 2000). Prior to treatment, Mcm4-FRB-GFP localized in the nucleus, but the signal was not strong (Figure 4A). In the presence of rapamycin we observed rapid disappearance (~ 1 hour) from the nucleus. Unlike Rad11-FRBGFP, Mcm4-FRB-GFP remained in the cytoplasm at later time-points, and the cells ceased dividing (Figure 4B). The treated cells were dramatically elongated by the end of the time-course (Figure 4D).
Figure 4. Mcm4-FRB-GFP is translocated from nucleus to cytoplasm in the presence of Rapamycin.
(A) Representative pictures of re-localization of Mcm4-FRB-GFP. Left, no change in localization was observed in the absence of Rapamycin at 100 or 300 minute. DMSO is the solvent control. Right, Mcm4-FRB-GFP was removed from the nucleus until approximately 300min after Rapamycin treatment. Bar = 15 μm. (B) Cells with different patterns of GFP signal were counted, n>50. (C) Cells were counted and categorized as mononucleated, binucleated, and binucleated with septa, n>50. (D) Cell length was measured and categorized into 5-12 μm, 12-15 μm, and >15 μm, n=50.
Mcb1-FRB-GFP and Mcb1-FRB
Mcb1, an abundant essential protein, is localized in nucleus and cytoplasm (Ding and Forsburg, 2011; Li et al., 2011). We tagged the Mcb1 with FRB-GFP in a wildtype strain. Similar to Mcb1-GFP (Ding and Forsburg, 2011), we were not able to visualize it, although it is detectable by Western blot (Figure S2). We crossed the mcb1-FRB-GFP (FY6549) strain with Δfkh1 (FY6609), then crossed the resulting strain with FY6792 to make the assay strains of mcb1-FRB-GFP (FY7194 and FY7195). Neither assay strain is rapamycin sensitive (data not shown). To rule out the possibility that the GFP in FRB-GFP tag hindered rapamycin access, we constructed assay strains of mcb1-FRB (FY7196 and FY7197) followed the outline in Figure 1A using both base strains. Again, we found Mcb1 did not respond to rapamycin (Figure 2B).
Discussion
The anchor-away system depletes nuclear proteins by exploiting a rapamycin-dependent interaction between two protein domains, FKBP12 and FRB, derived from the TOR signaling network (Haruki et al., 2008a). We have adapted this system to fission yeast, with mixed results. We used a test strain tor1-SE fkh1Δ that eliminates Tor1-Fkh1 interactions in the presence of rapamycin, and expresses an ectopic copy of rpl13-2FKBP12 from the nmt1 promoter. However, this strain is sterile, so any genetic manipulations must be performed on its precursors.
We examined three abundant nuclear proteins required for DNA replication. We observed the most success with Rad11-FRB-GFP. The strain was sensitive to rapamycin only in the presence of tor1-SE fkh1Δ, but showed no other significant drug sensitivities. The protein was depleted rapidly from the nucleus accompanied by cell elongation upon treatment with rapamycin. However, at later time-points, discrete GFP puncta appeared in the nucleus. Since RPA foci are a metric for DNA damage and single stranded DNA (ssDNA) accumulation (Sabatinos et al., 2012), this suggests that the protein can re-enter into the nucleus and bind ssDNA. Importantly, we observed no effect on growth or sensitivity to DNA damaging agents in the absence of rapamycin.
Success was mixed with the Mcm4-FRB-GFP strain. In the absence of tor1-SE fkh1Δ, the strain was healthy. However, in the tor1-SE fkh1Δ background, even without rapamycin, the strain had a slow-growth phenotype and sensitivity to DNA damage. The cells were already elongated prior to rapamycin treatment. Following treatment, the GFP signal disappeared from the nucleus and the phenotype worsened. However, the growth defects without rapamycin make this of limited utility. It is not clear why the mcm4-FRB-GFP tor1-SE fkh1Δ rpl13-2FKBP12 strain shows evidence for damage without rapamycin treatment, when the individual components do not. These data suggest that Mcm4 function is attenuated. Cells lacking tor1 are also DNA damage sensitive (Schonbrun et al., 2009); the tor1-SE fkh1Δ mutant may be sufficiently attenuated to cause a synthetic phenotype. Previous studies have suggested that while tagged MCM proteins are functional, they may be prone to genetic interactions in combination with other mutations (Gómez et al., 2002).
We were unable to deplete the essential Mcb1 protein. In a previous study, we showed that Mcb1-GFP, despite being stable and abundant, could be detected immunologically but could not be observed by fluorescence (Ding and Forsburg, 2011). Similarly, we were unable to observe Mcb1-FRB-GFP and found no effect of rapamycin treatment on Mcb1-FRB-GFP or Mcb1-FRB. Together, these observations suggest that the C-terminal fusions on Mcb1 are inaccessible or otherwise structurally hidden. Therefore, not all proteins will be successfully tagged.
In addition to the bulky tag and relative lack of markers, the system may be affected by the mutations tor1-SE and fkh1Δ, which are required to see the depletion, but also cause sterility and potentially other defects. Importantly, Tor2 is still intact and contains an FRB domain and potentially also could contribute to the phenotypes. It may be possible to fine-tune the system by adjusting the tor alleles used, including use of a tor2-SE allele. Alternatively, it might be sufficient to use fkh1Δ and wild type tor1+. The effectiveness of the system may also be influenced by the anchor or target protein shuttling in and out of the nucleus. We conclude that this system may be of use on a case-by-case basis.
Supplementary Material
Figure S1. Plasmids Restriction maps of pLD103, pLD133, pLD134, pLD115, pLD116, pLD138, and pLD141. Not drawn to scale.
Figure S2. Expression of FRB-GFP tagged proteins Equal amount of whole cell lysates generated from asynchronous cultures were separated by SDS-PAGE and blotted for GFP. Lane 1: FY11, lane2: FY6962, lane3: FY6992, and line4: FY7194. PCNA is the loading control.
Figure S3. An outline of the initial way to make the assay strains (FY6962, FY6992, FY7194 and FY7195) using genetic crosses.
Figure S4. Representative pictures from a time-course experiment of the rad11-FRB-GFP in wildtype with anchor (A) FY7189, or assay strains (B) FY6962 and (C) FY7222.
Acknowledgements
This work was supported by NIH grants R01 GM059321 and R01 GM081418 to SLF, training support from NIH Cellular Biochemical, and Molecular Sciences Training Program at the USC Keck School of Medicine and USC-Dornsife College Doctoral fellowship to LD.
References
- Alvarez B, Moreno S. Fission yeast Tor2 promotes cell growth and represses cell differentiation. J Cell Sci. 2006;119:4475–4485. doi: 10.1242/jcs.03241. [DOI] [PubMed] [Google Scholar]
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, 3rd, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Boe CA, Garcia I, Pai CC, Sharom JR, Skjolberg HC, Boye E, Kearsey S, Macneill SA, Tyers MD, Grallert B. Rapid regulation of protein activity in fission yeast. BMC Cell Biol. 2008;9:23. doi: 10.1186/1471-2121-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonatti S, Simili M, Abbondandolo A. Isolation of temperature-sensitive mutants of Schizosaccharomyces pombe. J Bacteriol. 1972;109:484–491. doi: 10.1128/jb.109.2.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon A, Maundrell K, Kearsey SE. Fission yeast cdc21+ belongs to a family of proteins involved in an early step of chromosome replication. 1992;20:5571–5577. doi: 10.1093/nar/20.21.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Forsburg SL. Schizosaccharomyces pombe minichromosome maintenance-binding protein (MCM-BP) antagonizes MCM helicase. J Biol Chem. 2011;286:32918–32930. doi: 10.1074/jbc.M111.282541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev. 2004;68:109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg SL, Rhind N. Basic methods for fission yeast. Yeast. 2006;23:173–183. doi: 10.1002/yea.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez EB, Catlett MG, Forsburg SL. Different phenotypes in vivo are associated with ATPase motif mutations in Schizosaccharomyces pombe minichromosome maintenance proteins. Genetics. 2002;160:1305–1318. doi: 10.1093/genetics/160.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967;93:1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008a;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008b;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Hayles J, Wood V, Jeffery L, Hoe KL, Kim DU, Park HO, Salas-Pino S, Heichinger C, Nurse P. A genome-wide resource of cell cycle and cell shape genes of fission yeast. Open biology. 2013;3:130053. doi: 10.1098/rsob.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges P, Van Driessche B, Tafforeau L, Vandenhaute J, Carr AM. Three novel antibiotic marker cassettes for gene disruption and marker switching in Schizosaccharomyces pombe. Yeast. 2005;22:1013–1019. doi: 10.1002/yea.1291. [DOI] [PubMed] [Google Scholar]
- Kanke M, Nishimura K, Kanemaki M, Kakimoto T, Takahashi TS, Nakagawa T, Masukata H. Auxin-inducible protein depletion system in fission yeast. BMC Cell Biol. 2011;12:8. doi: 10.1186/1471-2121-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Nakashima A, Ueno M, Ushimaru T, Aiba K, Doi H, Uritani M. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr Genet. 2001;39:166–174. doi: 10.1007/s002940100198. [DOI] [PubMed] [Google Scholar]
- Kearsey SE, Montgomery S, Labib K, Lindner K. Chromatin binding of the fission yeast replication factor mcm4 occurs during anaphase and requires ORC and cdc18. Embo J. 2000;19:1681–1690. doi: 10.1093/emboj/19.7.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, Yoo HS, Duhig T, Nam M, Palmer G, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nature biotechnology. 2010;28:617–623. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laor D, Cohen A, Pasmanik-Chor M, Oron-Karni V, Kupiec M, Weisman R. Isp7 Is a Novel Regulator of Amino Acid Uptake in the TOR Signaling Pathway. Mol Cell Biol. 2014;34:794–806. doi: 10.1128/MCB.01473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Schnick J, Hayles J, MacNeill SA. Purification and functional inactivation of the fission yeast MCM(MCM-BP) complex. FEBS Lett. 2011;585:3850–3855. doi: 10.1016/j.febslet.2011.10.033. [DOI] [PubMed] [Google Scholar]
- Lindner K, Gregan J, Montgomery S, Kearsey SE. Essential role of MCM proteins in premeiotic DNA replication. Mol Biol Cell. 2002;13:435–444. doi: 10.1091/mbc.01-11-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looke M, Kristjuhan K, Kristjuhan A. Extraction of genomic DNA from yeasts for PCR-based applications. BioTechniques. 2011;50:325–328. doi: 10.2144/000113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol. 2007;27:3154–3164. doi: 10.1128/MCB.01039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Nitani N, Yadani C, Yabuuchi H, Masukata H, Nakagawa T. Mcm4 C-terminal domain of MCM helicase prevents excessive formation of single-stranded DNA at stalled replication forks. Proc Natl Acad Sci U S A. 2008;105:12973–12978. doi: 10.1073/pnas.0805307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Tomita K, Matsuura A, Nakagawa T, Masukata H, Uritani M, Ushimaru T, Ueno M. A novel allele of fission yeast rad11 that causes defects in DNA repair and telomere length regulation. Nucleic acids research. 2003;31:7141–7149. doi: 10.1093/nar/gkg917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo Y, Yamamato M. TOR signaling in fission yeast. Crit Rev Biochem Mol Biol. 2008;43:277–283. doi: 10.1080/10409230802254911. [DOI] [PubMed] [Google Scholar]
- Pai CC, Schnick J, MacNeill SA, Kearsey SE. Conditional inactivation of replication proteins in fission yeast using hormone-binding domains. Methods. 2012;57:227–233. doi: 10.1016/j.ymeth.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Parker AE, Clyne RK, Carr AM, Kelly TJ. The Schizosaccharomyces pombe rad11+ gene encodes the large subunit of replication protein A. Molecular and cellular biology. 1997;17:2381–2390. doi: 10.1128/mcb.17.5.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev A, Wiren M, Weissman JS, Krogan NJ. High-throughput genetic interaction mapping in the fission yeast Schizosaccharomyces pombe. Nature methods. 2007;4:861–866. doi: 10.1038/nmeth1098. [DOI] [PubMed] [Google Scholar]
- Sabatinos SA, Green MD, Forsburg SL. Continued DNA synthesis in replication checkpoint mutants leads to fork collapse. Molecular and cellular biology. 2012;32:4986–4997. doi: 10.1128/MCB.01060-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa V, Martha S, Hirose N, Tanaka K. The fission yeast minichromosome maintenance (MCM)-binding protein (MCM-BP), Mcb1, regulates MCM function during prereplicative complex formation in DNA replication. J Biol Chem. 2013;288:6864–6880. doi: 10.1074/jbc.M112.432393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbrun M, Laor D, Lopez-Maury L, Bahler J, Kupiec M, Weisman R. TOR complex 2 controls gene silencing, telomere length maintenance, and survival under DNA-damaging conditions. Molecular and cellular biology. 2009;29:4584–4594. doi: 10.1128/MCB.01879-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner M, Collins SR, Thompson NJ, Denic V, Bhamidipati A, Punna T, Ihmels J, Andrews B, Boone C, Greenblatt JF, et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell. 2005;123:507–519. doi: 10.1016/j.cell.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Uritani M, Hidaka H, Hotta Y, Ueno M, Ushimaru T, Toda T. Fission yeast Tor2 links nitrogen signals to cell proliferation and acts downstream of the Rheb GTPase. Genes Cells. 2006;11:1367–1379. doi: 10.1111/j.1365-2443.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- Watson AT, Werler P, Carr AM. Regulation of gene expression at the fission yeast Schizosaccharomyces pombe urg1 locus. Gene. 2011;484:75–85. doi: 10.1016/j.gene.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Weisman R. The fission yeast TOR proteins and the rapamycin response: an unexpected tale. Curr Top Microbiol Immunol. 2004;279:85–95. doi: 10.1007/978-3-642-18930-2_6. [DOI] [PubMed] [Google Scholar]
- Weisman R, Choder M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J Biol Chem. 2001;276:7027–7032. doi: 10.1074/jbc.M010446200. [DOI] [PubMed] [Google Scholar]
- Weisman R, Finkelstein S, Choder M. Rapamycin blocks sexual development in fission yeast through inhibition of the cellular function of an FKBP12 homolog. J Biol Chem. 2001;276:24736–24742. doi: 10.1074/jbc.M102090200. [DOI] [PubMed] [Google Scholar]
- Weisman R, Roitburg I, Nahari T, Kupiec M. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics. 2005;169:539–550. doi: 10.1534/genetics.104.034983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman R, Roitburg I, Schonbrun M, Harari R, Kupiec M. Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics. 2007;175:1153–1162. doi: 10.1534/genetics.106.064170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- Zilio N, Wehrkamp-Richter S, Boddy MN. A new versatile system for rapid control of gene expression in the fission yeast Schizosaccharomyces pombe. Yeast. 2012;29:425–434. doi: 10.1002/yea.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Plasmids Restriction maps of pLD103, pLD133, pLD134, pLD115, pLD116, pLD138, and pLD141. Not drawn to scale.
Figure S2. Expression of FRB-GFP tagged proteins Equal amount of whole cell lysates generated from asynchronous cultures were separated by SDS-PAGE and blotted for GFP. Lane 1: FY11, lane2: FY6962, lane3: FY6992, and line4: FY7194. PCNA is the loading control.
Figure S3. An outline of the initial way to make the assay strains (FY6962, FY6992, FY7194 and FY7195) using genetic crosses.
Figure S4. Representative pictures from a time-course experiment of the rad11-FRB-GFP in wildtype with anchor (A) FY7189, or assay strains (B) FY6962 and (C) FY7222.