Abstract
Carbon monoxide (CO) and hydrogen sulfide (H2S) used to be thought of simply as lethal and (for H2S) smelly gaseous molecules; now they are known to have important signaling functions in the gastrointestinal tract. CO and H2S, which are produced in the gastrointestinal tract by different enzymes, regulate smooth muscle membrane potential and tone, transmit signals from enteric nerves and can regulate the immune system. The pathways that produce nitric oxide (NO) H2S and CO interact—each can inhibit and potentiate the level and activity of the other. However, there are significant differences between these molecules, such as in half-lives; CO is more stable and therefore able to have effects distal to the site of production, whereas NO and H2S are short lived and act only close to sites of production. We review their signaling functions in the luminal gastrointestinal tract and discuss how their pathways interact. We also describe other physiologic functions of CO and H2S and how they might be used as therapeutic agents.
Keywords: Signal transduction, gastrointestinal motility, gases, neurotransmission, smooth muscle
The discovery that mammalian cells synthesize nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) caused a paradigm shift that led to a large amount of research over the past 20 years into the roles of these molecules in human physiology and disease. Early studies focused on NO, which was soon found to be a signaling molecule that regulates a large number of biologic processes, including blood flow, neurotransmission, immune reactions, and smooth muscle contraction. NO is an inhibitory neurotransmitter in the human small intestine.1, 2 CO has also emerged as an important signaling molecule. Under physiologic conditions, endogenously produced CO functions as a neurochemical signaling molecule in the brain,3–6 as a messenger molecule in the gastrointestinal tract,7–13 and as a paracrine messenger molecule that causes hyperpolarization of circular smooth muscle cells.7, 8, 13 CO also has anti-inflammatory and anti-apoptotic effects.14 For hundreds of years, H2S was thought of as only a toxic gas that smelled like rotten eggs, but it is now known to be an important signaling molecule in bacteria, plants, and animals including mammals.15, 16 H2S is ubiquitous in the mammalian body and has been reported to serve as a messenger molecule in the central and peripheral nervous, immune, endocrine, reproductive, and gastrointestinal systems.17, 18,19, 20
There are fundamental differences between the mechanism of action of CO, NO, and H2S. At physiological pH, H2S exists in whole blood for approximately 15 sec in fish, 51 sec in cows, 130 sec in rats, and 76 sec in pigs.21 Unlike H2S and NO, CO is stable, is not a radical, and does not alternate between different oxidative species. NO is highly reactive, existing as a radical (NO•), as a nitroxyl anion (NO−), and as a nitrosonium cation (NO+). H2S and HS− are each detected in animal cells.
The biological stability of CO means that, unlike NO and H2S, CO is able to have effects that are distant from the site of production. Most of the action of CO is exerted through the binding of CO to Fe2+ and other metals present in various proteins, including heme proteins, that may be far from the site of production or from the lungs where CO is inhaled. CO was thought to cross cell membranes by dissolving into and diffusing across the lipid membrane. There is now evidence that ion channels, particularly aquaporins, can transfer CO across lipid membranes.22–24 Preliminary studies have linked levels of heme oxygenase with those of aquaporin.25 NO and H2S have been assumed to enter and leave cells via free diffusion through lipid membranes. However, in recent experiments aquaporin 1 (AQP1) channels were found to facilitate NO efflux from endothelial cells into aortic vascular smooth muscle cells.22, 26 Rhesus proteins facilitate diffusion of CO2 and NH3 in xenopus oocytes.27 AQP1 and rhesus protein channels are also called gas channels.27
CO
Most biologically relevant CO is produced by the action of heme oxygenase (gene symbol HMOX) catabolizing heme into CO, biliverdin and free iron.28 While each product of heme breakdown has separate effects on cellular function, our focus in this review is solely on CO which appears to be the predominant mediator of the effects of HO induction.29
Three mammalian isoforms of HMOX have been described, although only HMOX1 and HMOX2 have been shown to be biologically active. HMOX3 may be a pseudogene in some species. HMOX1 and HMOX2 have separate and different functions which are reflected in how CO is generated as a result of the action of these isoforms. Heme oxygenase 1 is usually expressed at very low levels in the luminal gastrointestinal tract but can be markedly induced to high levels within hours by a large variety of molecules including cellular stressors.30–34 For example, in the stomach muscle wall, reactive oxygen species will markedly induce expression of heme oxygenase 1 in macrophages and in the mucosa.35 Heme oxygenase 1 is therefore referred to as the inducible form of heme oxygenase. The net result is that CO can be produced on demand by the actions of heme oxygenase 1. In contrast, heme oxygenase 2 is referred to as the constitutive form of heme oxygenase. It is constitutively expressed in the luminal gastrointestinal tract, in enteric nerves and interstitial cells of Cajal (ICC);36, 37 its levels are relatively stable and few inducers have been found. A major exception comes from glucocorticoids which act by binding to the promoter of HMOX2.38 Others include Ca2+ influx, activation of protein kinase C (PKC), CK239, 40 and tyrosine kinases.41 The net result is that cells that express heme oxygenase 2 stably produce CO, although when CO functions as a messenger molecule, pulsatile release is also possible. Although heme synthesis is usually associated with hematopoietic cells, heme synthesis can and does take place in most mammalian cells, which enables local production of CO wherever heme oxygenase 1 is induced or heme oxygenase is constitutively present. The exact local concentration of CO in different organs is not known. The best estimates come from the brain, where extracellular CO can be measured in cerebrospinal fluid.42 Concentrations of up to 1 µM have been reported, indicating that intracellular concentrations under stimulation could be higher.
CO is often characterized as an anti-inflammatory, anti-proliferative, and anti-apoptotic molecule,14 but this view is too simplistic. Given that HMOX1 is rapidly inducible, CO can have different effects on the same cell type, based physiological and pathophysiological states. For example, inhalation of very low levels of CO (100 ppm) in by non-obese diabetic (NOD) mice has no effect on gastric emptying or cellular oxidative stress levels. Inhalation of CO by diabetic NOD mice reduces oxidative stress without changing gastric emptying, whereas inhalation of CO in diabetic NOD mice with delayed gastric emptying reduces oxidative stress and normalizes gastric emptying.29 These diverse effects are likely due to the actions of heme oxygenase 1/CO on the macrophage-ICC-enteric nerve-smooth muscle syncytium. There is little heme oxygenase 1 in non diabetic NOD mice and high levels of heme oxygenase 1 in M2 macrophages in diabetic NOD mice. CO converts the cellular profile of M1 non heme oxygenase 1 expressing macrophages to M2 macrophages.43
A Smooth Muscle Hyperpolarizing Factor
One of the main mechanisms of action of CO is to activate guanylyl cyclase resulting in production of cGMP.44, 45 cGMP activates several types of K+ channels leading to hyperpolarization.46, 47 CO also can directly activate K+ channels.48 All animal species studied so far have a smooth muscle membrane potential gradient across the circular muscle layer. In the stomach and small bowel, smooth muscle closer to the myenteric plexus region is hyperpolarized compared to circular smooth muscle cells closer to the submucosa.49–51 The gradient varies from species to species but is in the order of 10 mV. In the colon, the same gradient is present but in the opposite direction, that is the region of circular muscle that is more hyperpolarized is closest to the submucosa and the region most depolarized closest to the myenteric plexus.7 This membrane potential gradient is highly CO-dependent and appears to be due to CO produced from heme oxygenase 2 constitutively expressed in myenteric ICC from the stomach and small intestine and from heme oxygenase 2 constitutively expressed in submucosal ganglion neurons from the colon.10, 11, 52 The transwall gradient enables the circular muscle layer to produce weak contractions that involve only a portion of the circular muscle layer, strong propulsive contractions that involve the entire circular muscle layer to gradations in strength between these two extremes.53 The transwall gradient may be considered to function as a biological rheostat regulating how much of the circular muscle layer contacts with each electrical slow wave.11
As a Neurotransmitter
There is debate over whether NO is a neurotransmitter in the gastrointestinal tract. For a molecule to be called a neurotransmitter, it must be synthesized and present presynaptically, released from the synapse in response to specific signals (usually following Ca2+-dependent depolarization), and interact with post-synaptic receptors. If NO and CO are to be considered neurotransmitters, these criteria need to be relaxed. How NO is stored and released has not been fully worked out and the receptor for NO is intracellular. Even with relaxed criteria, it is still unclear if CO is a neurotransmitter. The strongest evidence comes from studies of the internal anal sphincter. HO2 is constitutively expressed in the internal anal sphincter54 and neuronal stimulation results in activation of HO2 via a PKC-CK2 dependent pathway.39 Non-adrenergic, non-cholinergic neurotransmission is markedly decreased in the upper gastrointestinal tract of Hmox2 knockout mice, but can be restored by addition of exogenous CO.55 NO produced by neuronal nitric oxide synthase 1 (NOS1) is an important inhibitory neurotransmission in several species.55 The full actions of NO appear to require CO. Although non-adrenergic, noncholinergic neurotransmission is reduced in Hmox2 knockout mice, it is also greatly decreased in Nos1 knockout mice and completely lost from Hmox2/Nos1 double-knockout mice.55 These findings, along with those from studies outside of the gastrointestinal tract, indicate that CO and NO function together in neurons. Until proven otherwise, it is best to refer to CO as a messenger molecule.
Mechanisms
The best known target of CO is soluble guanylyl cyclase. CO binds to guanylyl cyclase, resulting in increased levels of cGMP. The amount of endogenous cGMP generated through this mechanism is controversial because the potency of soluble guanylyl cyclase activation by CO is several fold lower than that of NO. The argument has been made that when NO is present, only a small amount of cGMP is produced via CO interaction with guanylyl cyclase. However, there is also evidence that endogenous substances, such as YC1, increase the sensitivity of soluble guanylate cyclase to CO.56 YC1 greatly enhances binding of CO to heterodimeric soluble guanylate cyclase (Kd ~1 µM) likely by binding near the heme domain, inducing a heme pocket conformation with a high affinity for CO.
CO also modulates ion channels. One example is the activation of the large conductance calcium-activated potassium channel (BK channel).57 CO may bind directly to the alpha subunit of BK resulting in activation of the channel and leading to membrane hyperpolarization. This mechanism is proposed for the vasodilatory effects of CO.58 Other mechanisms of action of CO include binding to other ion channels such as the L-type Ca2+ channel, redox regulation and oxygen transport, signaling molecule synthesis including of NO, prostaglandins and cytokines, activation of second messenger cascades including MAPK and Phosphatidylinositol 3 kinase, and activation of transcription factors (HIF1α, ACOT7, and NPAS2).59–61
A Modulator of Immune Function
CO has many effects on the adaptive immune system, such as inhibiting mast cell activation through polymorphonuclear cells, inhibiting activation and proliferation of T effector cells, and inhibiting basophil histamine release.62 CO also inhibits migration of polymorphonuclear cells and downregulates inflammatory pathways mediated by activated macrophages and dendritic cells.62 These actions of CO are thought to be central to how CO reduces ischemia reperfusion injury and post operative ileus and modulates the immune response to infection. Release of CO from macrophages is thought to be the main mechanism for protection against gastroparesis in diabetes.
Heme oxygenase 1 is expressed by Kupffer cells, but little heme oxygenase 1 is expressed in hepatocytes under normal circumstances. Inducers of heme oxygenase 1 result in robust upregulation of heme oxygenase 1 in both cell types. Deficiency of heme oxygenase 1 results in a hepatic phenotype including iron overload and hepatitis.63 In contrast, overexpression of heme oxygenase 1 protects against ethanol-induced injury, ischemia and reperfusion injury, and rejection of liver transplants by reducing production of cytokines, infiltration of CD4+ and CD8+ cells, and increased numbers of T regulatory cells.64
In Gastrointestinal Diseases and Therapy
Heme oxygenase 1 is highly inducible and protects against inflammation. CO and biliverdin are thought to mediate this protective effect of heme oxygenase 1; with most evidence for the role of CO. In animal models, CO reverses delayed gastric emptying associated with diabetes, reduces post-operative ileus, increases survival of grafts, increases survival from sepsis. CO also reduces intestinal inflammation in animal models of human inflammatory bowel disease model.59, 62 The data from human studies is severely limited. The best studied disorders are diabetic gastroparesis and post-operative ileus. Post-operative ileus animal models have shown that post-operative ileus is characterized by release of inflammatory mediators from activated macrophages. Early studies showed that a 24 hour exposure to 250 ppm of inhaled CO reduced the expression of inflammatory mediators and normalized muscle function.65 A subsequent study found that exposure of rats to even a very low dose of CO (75 ppm) for 3 hours before surgery (or pigs to 250 ppm for 3 hours) increased gastrointestinal transit and contractility, producing average carboxyhemoglobin levels of 5.8%—significantly lower than the upper limit set by the US Food and Drug Administration (FDA).66 In a mouse model of diabetic gastroparesis, loss of upregulation of heme oxygenase 1 by macrophages resulted in damage to ICC and nerves; upregulation of heme oxygenase 1 reversed the delay in gastric emptying and the cellular damage. These effects appear to be mediated by a decrease in reactive oxygen species and can be replicated with inhalation of CO.29 Upregulation of heme oxygenase 1 by type 2 alternatively activated macrophages is required for its protective effects. Loss of these macrophages, accompanied by activation of type 1 activated macrophages, results in release of injurious mediators that disrupt ICC and neural networks.67 CO therefore appears to have significant promise as a therapeutic agent.
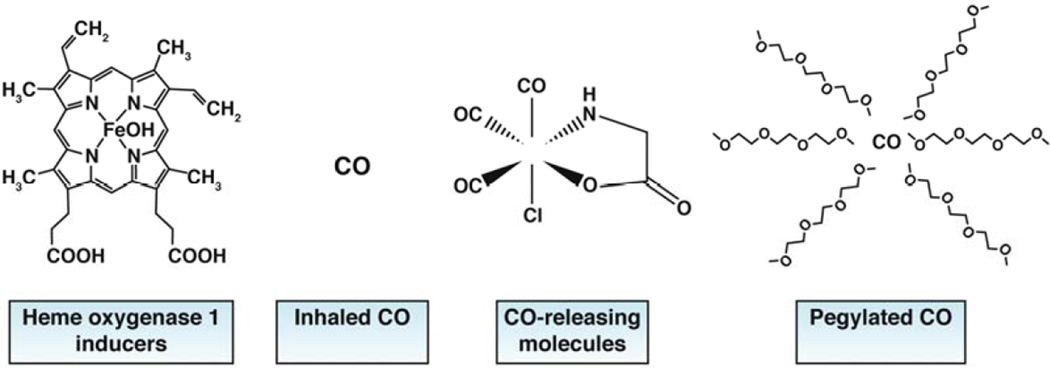
However, although several studies have shown that the amount of inhaled CO required to have a therapeutic effect is far below toxic levels, and despite the FDA statement that levels of carboxyhemoglobin below 12% are acceptable, there have been few clinical studies of inhaled CO. Reasons for this include the cumbersome equipment required to deliver precise, fixed amount of gas and the public perception of the toxicity of CO. There are currently only 2 active studies listed in clinical trials.gov testing the effects of inhaled CO. One study is investigating inhaled CO (range of 150 ppm for 3 hours once weekly to 150 ppm for 3 hours three times weekly) for treatment of pulmonary arterial hypertension. Another is assessing the ability of CO, inhaled 1 hour before and 1 hour after colon resection, to determine its utility to prevent or reduce post-operative ileus.
The real and perceived difficulties in administering inhaled CO have led to the development of transition metal compounds that covalently bind and deliver CO (CORMs). Initial compounds were lipid soluble, whereas the more recently developed are water soluble.68Although these compounds have shown efficacy in animal models of disease, including post-operative ileus, chronic colitis, necrotizing enterocolitis, and acute liver failure (see Table 1 of Gibbons et al.),59 none have tested in humans, because their safety for human use has not been resolved. More recently, products have been studied that use pegylated carboxyhemoglobin to deliver CO. One such product is being tested in a phase 1b study, in patients with sickle cell disease (NCT01848925). Safety analyses have shown good tolerability, despite the potential for binding and removal of NO.
H2S
H2S has been labeled as a gasotransmitter.69, 70 Feelisch and Olson71 stated that it is not accurate to label H2S, CO, or NO “gasotransmitters”, because “they do not move about and signal in the form of tiny gas puffs.” Instead, they are dissolved gases. The term gasotransmitter is a misnomer also because there is no definitive evidence that H2S functions as a transmitter in the classical meaning.18 Endogenous H2S has many regulatory functions throughout the gastrointestinal tract, but, there is no evidence that its production is regulated.18 Although exogenous H2S has several well-defined physiological effects, a receptor for H2S has not been identified.18 For these and other reasons outlined by Linden et al.,18 we refer to H2S as a signaling molecule or as a messenger molecule. The molecular entity that accounts for the biological effect is not known. At physiologic pH, nearly two thirds of H2S exists as hydrosulfide anion (HS−), a powerful nucleophile, rather than the acid (H2S).15 This is important because HS− is similar in size to Cl− and might be involved in Cl−-mediated processes.72 In this review, no distinction will be made, H2S can refer to HS− or H2S.
H2S is endogenously generated by the trans-sulfuration enzymes cystathionine β synthase (CBS) and cystathionine ɤ lyase (CTH).73 Both enzymes use L-cysteine as a substrate and depend on pyridoxal phosphate, NHDPH, and calcium and calmodulin.18, 73, 74 CBS and CTH have each been detected in the cytoplasm and the mitochondria.73, 75
Endogenous Production and Catabolism
H2S is synthesized by human cells, from head to foot. The rate of generation of H2S in the presence of non-physiologic concentrations of its substrate, L-cysteine (10 mM), has been reported to be 20 nM min−1 g protein−1 in brain tissue,76, 77 3.6 to 8.7 nM min−1 g tissue−1 in blood vessels,78 0.45 pmol/min/mg tissue in intact muscle layers of the mouse colon,18, 79 and 15.6 nmol/min/g tissue in rat colon muscle.80 In the absence of endogenous substrate, H2S production in the rat gastrointestinal tract was approximately 75 nmol/g/hr17 In the presence of 2 mM L-cysteine, the rate of production and release in the rat stomach, jejunum, and ileum was 750 nmol/g/hr, 590 nmol/g/hr, and 250 nmol/g/hr, respectively.17 According to these production rates, tissues that produce H2S appear to be exposed to endogenous concentrations close to the effective concentration range of exogenous H2S.
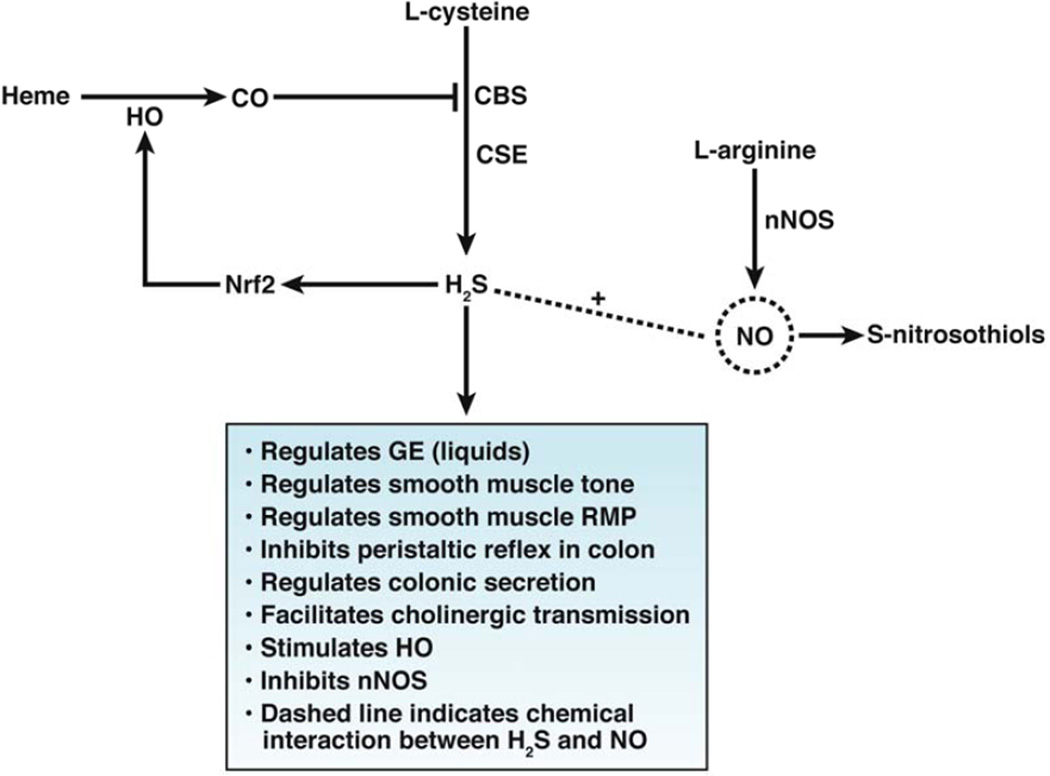
Perhaps the watershed discovery of the physiologic effects of endogenous H2S came when Abe and Kimura,76 who found that H2S functioned as an intracellular messenger during induction of long-term potentiation in the hippocampus. The discovery of the physiological role of H2S in the peripheral vasculature came when it was shown in mice that deletion of Cth, significantly decreased the endogenous level of H2S in the vasculature, and markedly altered vasorelaxation and resting blood pressure.81 Cth knockout mice were also found to have delayed gastric emptying of liquids, indicating a role for endogenous H2S in the regulation of gastrointestinal motility.82 In mice with wild-type Cth, endogenous H2S modulates gastric emptying of liquids through activation of KATP channels and TRPV1 receptors on gastric primary afferent nerves.82 Researchers recently reported a significant increase in colonic intraluminal pressure in conscious Cth knockout mice83 The physiological effects of endogenously generated H2S is summarized in Figure 2.
Figure 2.
Physiological effects of endogenously generated H2S
A specific catabolic pathway of signal transduction and termination is required to link the endogenous production of H2S with specific cells and protein targets. Few messenger molecules rely on passive diffusion for signal termination and inactivation. Enzymatic degradation and reuptake mechanisms by the releasing cell are used throughout the body. There is no evidence that any of these mechanisms terminate H2S signaling. The mitochondrial enzyme sulphide quinone reductase (SQR) contributes to catabolism in peripheral tissue, including the muscularis externa of mouse colon.79 The oxidation of H2S to thiosulphate and sulfate by SQR terminates H2S signaling.79, 84 Because of the low SQR threshold of 16 nM and half inhibition of the enzyme at 20 µM, the overall tissue concentrations of H2S are maintained at low levels, preventing inhibition of cytochrome C and thereby preventing H2S-induction of cytotoxicity. This catabolic pathway can be inhibited by stigmatellin, a mycobacteria-derived antibiotic.85 Stigmatellin significantly reduces H2S consumption in colonic musculature and potentiates fast nicotinic synaptic transmission in peripheral sympathetic ganglion.83 A second mechanism by which H2S-mediated signaling can be terminated is by binding of H2S to sulphane-sulfur pools and bound sulfate pools.86, 87 Ishigami et al.86 applied exogenous H2S to homogenates of mouse brain, liver, and heart and detected bound sulfur, rather than acid-labile sulfur.
The physiologic signal that leads to endogenous production and release of H2S is not known. H2S could be released immediately after its biogenesis, by enzymatic activity. Alternatively, it could be released from bound sulphane sulfur86, 87 and/or from acid labile sulfur pools located mainly in mitochondria pools.86 H2S is released from bound pools in neurons and astrocytes in mouse brain.86 The potential importance of release of bound H2S as a signaling mechanism in gastrointestinal tissue has not been determined. However, the release from acidlabile pools in the mitochondria occurs only when the pH falls below 5.5, which is unlikely because the mitochondrial pH is approximately 8.0.
Actions of Sodium hydrosulfide (NaHS)
NaHS is commonly used in in vivo and in vitro experiments as a source of H2S to study the possible physiologic functions of endogenous H2S. NaHS immediately dissociates and forms the hydrosulfide anion HS−, which then reacts with H+ to form H2S. The general construct is that NaHS mimics the physiologic environment of H2S-producing and/or targeted cells. As our emphasis is on the effect of H2S on gastrointestinal enteric system, we will discuss only briefly the potential role H2S has on mucosal function. For further information, the reader is referred to the following review articles.69, 73, 88–91
Effects on Smooth Muscle
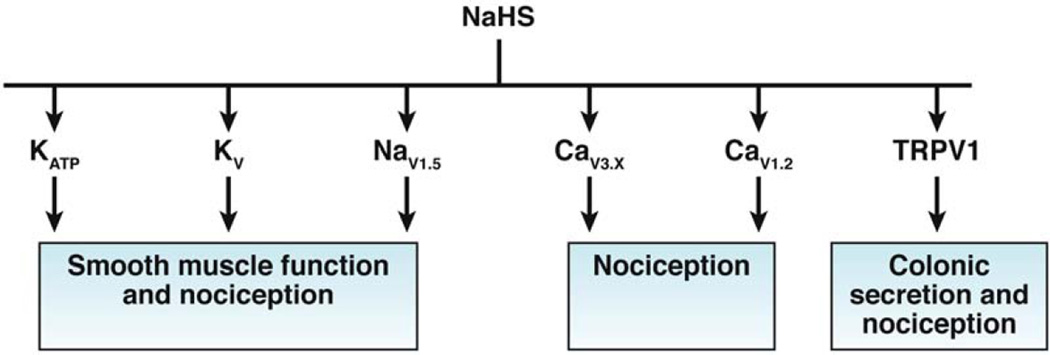
The effect of exogenous NaHS on motility is site specific and species dependent. In the mouse gastric fundus, NaHS relaxes the muscle—an effect that is not blocked by K-channel blockers.92 In muscle strips of the guinea pig antrum, high concentrations of NaHS (0.3 to 1.0 mM) suppress the amplitude of spontaneous contractions, by opening KATP channels. Low concentrations (0.1 to 0.3 mM) increase basal tension—an effect mediated by the inhibition of voltage-gated K-channels.93 Likewise in muscle strips of the stomach, low concentrations (<100 µM) of NaHS increase basal tension and the amplitude of contraction of muscle strips, and depolarize the resting membrane potential.94 This excitatory effect is mediated by inhibition of current carried through the potassium delayed rectifier channel. Inhibition of CBS, but not CTH, increases potassium current, indicating that release of endogenous H2S acts as an excitatory messenger molecule. H2S donors accelerate gastric emptying of liquids in conscious mice.82 These findings indicate that H2S relaxes antral smooth muscle and decreases antral-duodenal resistance, via activation of KATP channels, although the exact mechanisms of these processes are not known.82 The effect also involves TRPV1 receptors located on afferent nerves.82 In mouse stomach, endogenous H2S acts on KDR, KATP channels and TRPV1 receptors co-expressed on primary gastric afferents. The target sites for NaHS that have been identified are listed in Figure 3.
Figure 3.
Ion channel targets for exogenous NaHS
With few exceptions, NaHS inhibits smooth muscle contraction and motility (in the small intestine and colon of mice, rats, and guinea pigs)80, 95, 96 and inhibits field-stimulated and acetylcholine-induced contractions.97 The direct inhibitory effect on smooth muscle is largely mediated through an action on multiple potassium channels, particularly apamin-sensitive small conductance and glibenclamide-sensitive KATP channels.88, 98 In contrast to the involvement of TRPV1 receptors in the mouse stomach, where H2S-donor molecules accelerates gastric emptying,82 the inhibition of the peristaltic reflex in the mouse ileum and colon is preserved in Trpv1 knockout mice.96 In the rat colon, the constitutive endogenous production of H2S by CTH maintains the membrane potential in circular smooth muscle cells.80 The CO-dependent transwall gradient of resting membrane potential that exists across the circular muscle layer in the mouse colon is modulated by the ongoing release of H2S.83 There is therefore sufficient data to support the hypothesis that ongoing, endogenously generated H2S in the circular smooth muscle layer maintains the resting membrane potential in the hyperpolarizing range.
Effects on Visceral Nociceptors
In the mouse, H2S acts as a pro-nociceptor via CaV3 channels99 whereas in the rat it acts as an anti-nociceptive molecule—an effect mediated by KATP channels.100 NaHS increases the frequency of action potentials in gut afferent neurons and in dorsal root ganglion neurons.101, 102 The effect is reduced by capsazepine, so TRPV1 has been implicated. NaHS and H2S donor molecules reduce pain-related behaviors in healthy rats and rats with colitis—an effect mediated by the opening of KATP channels.100 In mice, luminal release of H2S is nociceptive, involving CaV3 calcium channels.99 Intraluminal administration caused visceral pain-like behavior and abdominal hyperalgesia.99 The differences in the effects of NaHS on visceral nociceptors might be species related; in the rat, NaHS has anti-nociceptive effects, whereas in the mouse it has nociceptive effects. Development of chronic visceral hyperalgesia by 0.5% acetic acid increased expression of CBS in dorsal root ganglion neurons,102 and perfusion of dorsal root ganglion neurons with 25 µmol/L NaHS increased the number of action potentials102 in isolated colonic afferent neurons.102
Effects on Colonic Secretion
In the human and guinea pig colon, NaHS promotes secretion, acting through TRPV1 on colonic afferents, which causes the release of SP, which acts on tachykinin receptors (TACR1–3) to activate cholinergic secretomotor neurons.103 In the rat colon, NaHS increases secretion of chloride from the apical membrane of epithelial cells and secretion of potassium from the basolateral membrane.104 These secretory effects are mediated by nerves and via direct actions on calcium storage organelles in epithelial cells, through ryanodine receptors.104
Effects on ICC
ICC are another putative target for endogenous H2S. NaHS inhibits spontaneous intracellular Ca oscillations in cultured mouse ICC and inhibits pacemaker amplitude current and pacemaker frequency, and increases resting membrane currents in an outward direction—most likely through changes in oscillation of internal calcium.105 If endogenous H2S acts on ICC in mice, the source of H2S would have to be from surrounding cells, because CBS and CTH transcripts have not been detected in ICC.105
Mechanisms of H2S
H2S activates KATP channels; inhibits and activates CaV1.2 calcium channels in different tissues; and activates CaV3 calcium channels, TRPV1 and TRPA1 channels, and NaV1.5. For reviews, please see.18, 88, 90, 91 The mechanism by which H2S mediates these functions in gastrointestinal tissue is receiving increasing attention. One mechanism involves post-translational modification of protein cysteine residues (a process referred to as sulfhydration). The development of the modified biotin-switch technique106 and maleimide procedure107 has provided direct evidence that many proteins are sulfhydrated during basal and physiological contractions.107, 108 Sulfhydration, which adds an -SSH moiety to proteins, has been shown to increase activity,106 in contrast to NO-nitrosylation, which reduces activity.109 The EC50 of H2S required for S-sulfhydration in vascular tissue is within the range of levels detected in mouse tissues.106
In inflamed colons of rats, NaHS (100 µM) allosterically modulated KATP channels through sulfhydration of the SUR2B subunit,98 in contrast to the sulfhydration of the Kir6.1 subunit in vascular smooth muscle,106 resulting in activation of the channel. This activation alters motility under inflammatory conditions.98 These studies, which used tissues from inflamed colons, were performed using patch clamp recordings of isolated circular smooth muscle cells and from heterogeneously transfected cells.98 This was the first study to show that H2S had a specific effect on the SUR2B subunit. In a recent study investigating the potential role of H2S and KATP channels, placing rats in a water avoidance stress test, which increased colonic motility, was found to increase expression of the pore-forming Kir6.1 and SUR2B subunits.110 Although KATP channels appear to be regulated by H2S during mucosal secretion and in smooth muscle and visceral afferents, it is not clear whether sulfhydration is a form of cell signaling or a reversible process.
A number of questions need to be answered before we can understand the physiologic significance and importance of H2S as a messenger or signaling molecule in the enteric system. First and perhaps foremost is the molecular identity that confers biologic activity. Is H2S or HS− the ligand, or are both? Although a number of proteins are post-translationally modified by H2S and HS−, can these be considered to be receptors for these molecules? There is no evidence for the reversibility of the interactions between H2S or HS− and proteins. What is the effective concentration range for H2S and can this be supplied by NaHS? Although we have not reviewed the effects of inhibitors of CTH and CBS, all of which are non-specific, it is important to consider their pharmacologic effects in evaluating the physiological functions of endogenous H2S. There are relatively few studies of the phenotypic characterization and functional effects of targeted deletion of CTH and CBS on gastrointestinal motility and enteric function. Lastly, the relative physiological roles of H2S and CO in gastrointestinal motility require side-by-side comparisons of Cth vs Cbs and Hmox1 vs Hmox2 knockout mice, and integration of the findings with those from Nos1 knockout mice.
Crosstalk
In the past few years, CO, H2S, and NO have been reported to interact (Figure 3). The actions of CO and H2S require consideration not only as molecules with specific targets but also as a network of messenger molecules that interact to produce diverse effects, through convergent signaling pathways, depending on the cellular state.62 A number of mechanisms have been identified. For example, CO inhibits the trans-sulfuration pathway.111 The heme prosthetic groups on the N-terminus of CBS can bind NO and CO. Since CBS binds CO approximately 200-fold more tightly than it does NO, CO has the potential to inhibit CBS activity and therefore the generation of H2S.112 CBS has been proposed to be a specific sensor for CO. The ligand of the 5th coordinate position of CBS is a thiolated anion. The binding of the thiolated anion to heme is weak and when CO binds to the heme moiety of CBS the thiolate anion ligand is displaced which results in a change in the enzymatic activity of CBS.113 The Ki for CO binding to CBS is approximately 5 µM, compared with approximately 320 µM for NO, indicating its higher specificity for CO.114 Binding of CO to CBS inhibits its activity. The physiological significance of this finding has been shown in the brain, where hypoxia inhibits heme oxygenase 1 and more H2S is produced, resulting in vasodilation countering hypoxia. CO also has been shown to inhibit NOS activity in vitro as well as directly stimulate NO formation.115, 116 CO derived from heme oxygenase 1 acts as a tonic regulator of NO-dependent vasodilation in the rat brain.117 H2S can regulate generation of NO,102, 118 facilitate release of NO in vascular tissue,119 and regulate generation of NO in the mouse colon.13 H2S also regulates the availability of NO by increasing its release from nitrosothiols.120 Heme oxygenase 1 and NOS each require NADPH as a cofactor, so substrate competition can limit enzymatic activity of one or both of these enzymes. NO and H2S compete for site recognition of cysteine residues for nitrosylation and sulfhydration, respectively. Sometimes, H2S and NO are both required for certain physiological actions.121
Finally, interaction can take place at the level of transcription. NaHS induces the nuclear localization of the transcription factor NRF2 (nuclear factor, erythroid 2-like 2) in hearts of rats during myocardial ischemia.122 NRF2, a nuclear basic leucine zipper transcription factor, controls expression of a number of genes that encode protective enzymes, including HOMX1 and thioredoxin1. Increased expression of these proteins is thought to limit tissue (cardiac) damage.
Conclusions
CO and H2S have been established as signaling molecules that have important physiologic roles in the gastrointestinal tract. CO and H2S signal through distinct pathways, but their functions overlap and each can influence the production and regulation of the other. To translate what is known about CO and H2S into therapeutic strategies, it is necessary to better understand how the enzymes that produce them are regulated, what are the most relevant biological functions of CO and H2S, and how we might accurately deliver the right concentration to a specific cell or tissue. Perhaps the key questions are ones that have yet to be identified.
Figure 1.
Delivery forms for CO
Acknowledgement
The authors thank Jan Applequist and Kristy Zodrow for preparing this article.
Grant Support: DK 57061, DK 52766, DK 17238, PO1 DK68055
Abbreviations
- CO
carbon monoxide
- H2S
hydrogen sulfide
- AQP
aquaporin channels
- CO2
carbon dioxide
- NH3
ammonia
- HOMX
heme oxygenase gene
- ICC
interstitial cells of Cajal
- PKC
protein kinase C
- NOD
non-obese diabetic
- cGMP
cyclic guanylyl cyclase
- CORM
CO releasing molecule
- CBS
cystathionine β synthase
- CTH
cystathionine ɤ lyase
- 3MST
3-mercaptopyravate sulfurtransferase
- SQR
sulphide quinone reductase
- NaHS
sodium hydrosulfide
- Nrf2
nuclear factor erythroid-related factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors disclose no conflicts.
Author Contributions: Joseph H. Szurszewski and Gianrico Farrugia were both involved in the manuscript analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and they both obtained funding.
References
- 1.Stark ME, Szurszewski JH. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- 2.Stark ME, Bauer AJ, Sarr MG, et al. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology. 1993;104:398–409. doi: 10.1016/0016-5085(93)90407-4. [DOI] [PubMed] [Google Scholar]
- 3.Ikegaya Y, Saito H, Matsuki N. Involvement of carbon monoxide in long-term potentiation in the dentate gyrus of anesthetized rats. Jpn J Pharmacol. 1994;64:225–227. doi: 10.1254/jjp.64.225. [DOI] [PubMed] [Google Scholar]
- 4.Nathanson JA, Scavone C, Scanlon C, et al. The cellular Na+ pump as a site of action for carbon monoxide and glutamate: a mechanism for long-term modulation of cellular activity. Neuron. 1995;14:781–794. doi: 10.1016/0896-6273(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 5.Verma A, Hirsch DJ, Glatt CE, et al. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo M, Small SA, Kandel ER, et al. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 7.Farrugia G, Lei S, Lin X, et al. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci U S A. 2003;100:8567–8570. doi: 10.1073/pnas.1431233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons SJ, Farrugia G. The role of carbon monoxide in the gastrointestinal tract. J Physiol. 2004;556:325–336. doi: 10.1113/jphysiol.2003.056556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanani M, Ermilov LG, Schmalz PF, et al. The three-dimensional structure of myenteric neurons in the guinea-pig ileum. J Auton Nerv Syst. 1998;71:1–9. doi: 10.1016/s0165-1838(98)00054-x. [DOI] [PubMed] [Google Scholar]
- 10.Sha L, Farrugia G, Harmsen WS, et al. Membrane potential gradient is carbon monoxide-dependent in mouse and human small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G438–G445. doi: 10.1152/ajpgi.00037.2007. [DOI] [PubMed] [Google Scholar]
- 11.Szurszewski JH, Farrugia G. Carbon monoxide is an endogenous hyperpolarizing factor in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 1):81–85. doi: 10.1111/j.1743-3150.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 12.Szursewski JH, Miller SM. Physiology of prevertebral ganglia. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Third ed. Volume 1. New York: Raven Press; 1994. pp. 795–877. [Google Scholar]
- 13.Sha L, Linden DR, Farrugia G, et al. Effect of endogenous hydrogen sulfide on the transwall gradient of the mouse colon circular smooth muscle. J Physiol. 2013 Dec 23; doi: 10.1113/jphysiol.2013.266841. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse D, Choi AM. Heme oxygenase-1: the "emerging molecule" has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 15.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid Redox Signal. 2011;15:379–391. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 16.Olson KR. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B. 2012;182:881–897. doi: 10.1007/s00360-012-0654-y. [DOI] [PubMed] [Google Scholar]
- 17.Martin GR, McKnight GW, Dicay MS, et al. Hydrogen sulphide synthesis in the rat and mouse gastrointestinal tract. Dig Liver Dis. 2010;42:103–109. doi: 10.1016/j.dld.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Linden DR, Levitt MD, Farrugia G, et al. Endogenous production of H2S in the gastrointestinal tract: still in search of a physiologic function. Antioxid Redox Signal. 2010;12:1135–1146. doi: 10.1089/ars.2009.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- 20.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- 21.Whitfield NL, Kreimier EL, Verdial FC, et al. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 22.Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension. 2006;48:157–164. doi: 10.1161/01.HYP.0000223652.29338.77. [DOI] [PubMed] [Google Scholar]
- 23.Geyer RR, Musa-Aziz R, Enkavi G, et al. Movement of NH(3) through the human urea transporter B: a new gas channel. Am J Physiol Renal Physiol. 2013;304:F1447–F1457. doi: 10.1152/ajprenal.00609.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera M, Garvin JL. Aquaporins as gas channels. Pflugers Arch. 2011;462:623–630. doi: 10.1007/s00424-011-1002-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang QM, Yin XY, Duan ZJ, et al. Role of the heme oxygenase/carbon monoxide pathway in the pathogenesis and prevention of hepatic encephalopathy. Mol Med Rep. 2013;8:67–74. doi: 10.3892/mmr.2013.1472. [DOI] [PubMed] [Google Scholar]
- 26.Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. Am J Physiol Renal Physiol. 2007;292:F1443–F1451. doi: 10.1152/ajprenal.00353.2006. [DOI] [PubMed] [Google Scholar]
- 27.Boron WF. Sharpey-Schafer lecture: gas channels. Exp Physiol. 2010;95:1107–1130. doi: 10.1113/expphysiol.2010.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 29.Kashyap PC, Choi KM, Dutta N, et al. Carbon monoxide reverses diabetic gastroparesis in NOD mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G1013–G1019. doi: 10.1152/ajpgi.00069.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kacimi R, Chentoufi J, Honbo N, et al. Hypoxia differentially regulates stress proteins in cultured cardiomyocytes: role of the p38 stress-activated kinase signaling cascade, and relation to cytoprotection. Cardiovasc Res. 2000;46:139–150. doi: 10.1016/s0008-6363(00)00007-9. [DOI] [PubMed] [Google Scholar]
- 31.Chang AY, Chan JY, Cheng HL, et al. Hypoxia-inducible factor 1/heme oxygenase 1 cascade as upstream signals in the prolife role of heat shock protein 70 at rostral ventrolateral medulla during experimental brain stem death. Shock. 2009;32:651–658. doi: 10.1097/SHK.0b013e3181a71027. [DOI] [PubMed] [Google Scholar]
- 32.Naik JS, O'Donaughy TL, Walker BR. Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation. Am J Physiol Heart Circ Physiol. 2003;284:H838–H845. doi: 10.1152/ajpheart.00747.2002. [DOI] [PubMed] [Google Scholar]
- 33.Immenschuh S, Tan M, Ramadori G. Nitric oxide mediates the lipopolysaccharide dependent upregulation of the heme oxygenase-1 gene expression in cultured rat Kupffer cells. J Hepatol. 1999;30:61–69. doi: 10.1016/s0168-8278(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 34.Cooper KL, Liu KJ, Hudson LG. Enhanced ROS production and redox signaling with combined arsenite and UVA exposure: contribution of NADPH oxidase. Free Radic Biol Med. 2009;47:381–388. doi: 10.1016/j.freeradbiomed.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battish R, Cao GY, Lynn RB, et al. Heme oxygenase-2 distribution in anorectum: colocalization with neuronal nitric oxide synthase. Am J Physiol Gastrointest Liver Physiol. 2000;278:G148–G155. doi: 10.1152/ajpgi.2000.278.1.G148. [DOI] [PubMed] [Google Scholar]
- 37.Farrugia G, Szurszewski JH. Heme oxygenase, carbon monoxide, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:321–324. doi: 10.1002/(SICI)1097-0029(19991201)47:5<321::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Raju VS, McCoubrey WK, Jr, Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: characterization of a functional glucocorticoid response element. Biochim Biophys Acta. 1997;1351:89–104. doi: 10.1016/s0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- 39.Boehning D, Moon C, Sharma S, et al. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- 40.Dore S, Takahashi M, Ferris CD, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leffler CW, Balabanova L, Fedinec AL, et al. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H74–H80. doi: 10.1152/ajpheart.01081.2002. [DOI] [PubMed] [Google Scholar]
- 42.Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kashyap P, Farrugia G. Diabetic gastroparesis: what we have learned and had to unlearn in the past 5 years. Gut. 2010;59:1716–1726. doi: 10.1136/gut.2009.199703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996;16:835–842. doi: 10.1016/s0896-6273(00)80103-8. [DOI] [PubMed] [Google Scholar]
- 45.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 46.Farrugia G, Irons WA, Rae JL, et al. Activation of whole cell currents in isolated human jejunal circular smooth muscle cells by carbon monoxide. Am J Physiol. 1993;264:G1184–G1189. doi: 10.1152/ajpgi.1993.264.6.G1184. [DOI] [PubMed] [Google Scholar]
- 47.Rich A, Farrugia G, Rae JL. Carbon monoxide stimulates a potassium-selective current in rabbit corneal epithelial cells. Am J Physiol. 1994;267:C435–C442. doi: 10.1152/ajpcell.1994.267.2.C435. [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Cao K, Lu Y, et al. Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer AJ, Sanders KM. Gradient in excitation-contraction coupling in canine gastric antral circular muscle. J Physiol. 1985;369:283–294. doi: 10.1113/jphysiol.1985.sp015901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauer AJ, Sanders KM. Passive and active membrane properties of canine gastric antral circular muscles. Am J Physiol. 1986;251:C268–C273. doi: 10.1152/ajpcell.1986.251.2.C268. [DOI] [PubMed] [Google Scholar]
- 52.Sha L, Farrugia G, Linden DR, et al. The transwall gradient across the mouse colonic circular muscle layer is carbon monoxide dependent. FASEB J. 2010;24:3840–3849. doi: 10.1096/fj.10-156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, Christensen J, Jackson MJ, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. Second ed. Volume 1. New York: Raven Press; 1987. pp. 383–422. [Google Scholar]
- 54.Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993;265:G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- 55.Xue L, Farrugia G, Miller SM, et al. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci U S A. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 57.Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Z, Yue P, Lin DH, et al. Carbon monoxide stimulates Ca2+ -dependent big-conductance K channels in the cortical collecting duct. Am J Physiol Renal Physiol. 2013;304:F543–F552. doi: 10.1152/ajprenal.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gibbons SJ, Verhulst PJ, Bharucha A, et al. Review article: carbon monoxide in gastrointestinal physiology and its potential in therapeutics. Aliment Pharmacol Ther. 2013;38:689–702. doi: 10.1111/apt.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson WJ, Kemp PJ. Carbon monoxide: an emerging regulator of ion channels. J Physiol. 2011;589:3055–3062. doi: 10.1113/jphysiol.2011.206706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peers C. Ion channels as target effectors for carbon monoxide. Exp Physiol. 2011;96:836–839. doi: 10.1113/expphysiol.2011.059063. [DOI] [PubMed] [Google Scholar]
- 62.Wegiel B, Hanto DW, Otterbein LE. The social network of carbon monoxide in medicine. Trends Mol Med. 2013;19:3–11. doi: 10.1016/j.molmed.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Immenschuh S, Baumgart-Vogt E, Mueller S. Heme oxygenase-1 and iron in liver inflammation: a complex alliance. Curr Drug Targets. 2010;11:1541–1550. doi: 10.2174/1389450111009011541. [DOI] [PubMed] [Google Scholar]
- 64.Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- 65.Moore BA, Otterbein LE, Turler A, et al. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377–391. doi: 10.1053/gast.2003.50060. [DOI] [PubMed] [Google Scholar]
- 66.Moore BA, Overhaus M, Whitcomb J, et al. Brief inhalation of low-dose carbon monoxide protects rodents and swine from postoperative ileus. Crit Care Med. 2005;33:1317–1326. doi: 10.1097/01.ccm.0000166349.76514.40. [DOI] [PubMed] [Google Scholar]
- 67.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399–2409. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 69.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 70.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 71.Feelisch M, Olson KR. Embracing sulfide and CO to understand nitric oxide biology. Nitric Oxide. 2013;35:2–4. doi: 10.1016/j.niox.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Olson KR. Hydrogen sulfide and oxygen sensing: implications in cardiorespiratory control. J Exp Biol. 2008;211:2727–2734. doi: 10.1242/jeb.010066. [DOI] [PubMed] [Google Scholar]
- 73.Kolluru GK, Shen X, Bir SC, et al. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide. 2013;35:5–20. doi: 10.1016/j.niox.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 75.Kabil O, Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal. 2014;20:770–782. doi: 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Warenycia MW, Goodwin LR, Benishin CG, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 78.Zhao W, Zhang J, Lu Y, et al. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linden DR, Furne J, Stoltz GJ, et al. Sulphide quinone reductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br J Pharmacol. 2012;165:2178–2190. doi: 10.1111/j.1476-5381.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gil V, Gallego D, Jimenez M. Effects of inhibitors of hydrogen sulphide synthesis on rat colonic motility. Br J Pharmacol. 2011;164:485–498. doi: 10.1111/j.1476-5381.2011.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Medeiros JV, Bezerra VH, Lucetti LT, et al. Role of KATP channels and TRPV1 receptors in hydrogen sulfide-enhanced gastric emptying of liquid in awake mice. Eur J Pharmacol. 2012;693:57–63. doi: 10.1016/j.ejphar.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Sha L, Linden DR, Farrugia G, et al. Hydrogen sulfide selectively potentiates central preganglionic fast nicotinic synaptic input in mouse superior mesenteric ganglion. J Neurosci. 2013;33:12638–12646. doi: 10.1523/JNEUROSCI.4429-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–378. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 85.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishigami M, Hiraki K, Umemura K, et al. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 87.Wallace JL, Vong L, McKnight W, et al. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 88.Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- 89.Jimenez M. Hydrogen sulfide as a signaling molecule in the enteric nervous system. Neurogastroenterol Motil. 2010;22:1149–1153. doi: 10.1111/j.1365-2982.2010.01600.x. [DOI] [PubMed] [Google Scholar]
- 90.Kimura H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid Redox Signal. 2013 May 25; doi: 10.1089/ars.2013.5309. 2013 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 92.Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Zhao P, Huang X, Wang ZY, et al. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223–228. doi: 10.1016/j.ejphar.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Han YF, Huang X, Guo X, et al. Evidence that endogenous hydrogen sulfide exerts an excitatory effect on gastric motility in mice. Eur J Pharmacol. 2011;673:85–95. doi: 10.1016/j.ejphar.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Nagao M, Linden DR, Duenes JA, et al. Mechanisms of action of the gasotransmitter hydrogen sulfide in modulating contractile activity of longitudinal muscle of rat ileum. J Gastrointest Surg. 2011;15:12–22. doi: 10.1007/s11605-010-1306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gallego D, Clave P, Donovan J, et al. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogastroenterol Motil. 2008;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 97.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gade AR, Kang M, Akbarali HI. Hydrogen sulfide as an allosteric modulator of ATP-sensitive potassium channels in colonic inflammation. Mol Pharmacol. 2013;83:294–306. doi: 10.1124/mol.112.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsunami M, Tarui T, Mitani K, et al. Luminal hydrogen sulfide plays a pronociceptive role in mouse colon. Gut. 2009;58:751–761. doi: 10.1136/gut.2007.144543. [DOI] [PubMed] [Google Scholar]
- 100.Distrutti E, Sediari L, Mencarelli A, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 101.Krueger D, Foerster M, Mueller K, et al. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol Motil. 2010;22:1224–1231. doi: 10.1111/j.1365-2982.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 102.Xu GY, Winston JH, Shenoy M, et al. The endogenous hydrogen sulfide producing enzyme cystathionine-beta synthase contributes to visceral hypersensitivity in a rat model of irritable bowel syndrome. Mol Pain. 2009;5:44. doi: 10.1186/1744-8069-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schicho R, Krueger D, Zeller F, et al. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 104.Hennig B, Diener M. Actions of hydrogen sulphide on ion transport across rat distal colon. Br J Pharmacol. 2009;158:1263–1275. doi: 10.1111/j.1476-5381.2009.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parajuli SP, Choi S, Lee J, et al. The inhibitory effects of hydrogen sulfide on pacemaker activity of interstitial cells of cajal from mouse small intestine. Korean J Physiol Pharmacol. 2010;14:83–89. doi: 10.4196/kjpp.2010.14.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S-sulfhydration. Sci Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sen N, Paul BD, Gadalla MM, et al. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paul BD, Snyder SH. H(2)S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 109.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, Luo H, Liang C, et al. Actions of hydrogen sulfide and ATP-sensitive potassium channels on colonic hypermotility in a rat model of chronic stress. PLoS One. 2013;8:e55853. doi: 10.1371/journal.pone.0055853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shintani T, Iwabuchi T, Soga T, et al. Cystathionine beta-synthase as a carbon monoxide-sensitive regulator of bile excretion. Hepatology. 2009;49:141–150. doi: 10.1002/hep.22604. [DOI] [PubMed] [Google Scholar]
- 112.Taoka S, Banerjee R. Characterization of NO binding to human cystathionine beta-synthase: possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 113.Omura T. Heme-thiolate proteins. Biochem Biophys Res Commun. 2005;338:404–409. doi: 10.1016/j.bbrc.2005.08.267. [DOI] [PubMed] [Google Scholar]
- 114.Kajimura M, Nakanishi T, Takenouchi T, et al. Gas biology: tiny molecules controlling metabolic systems. Respir Physiol Neurobiol. 2012;184:139–148. doi: 10.1016/j.resp.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 115.White KA, Marletta MA. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992;31:6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- 116.Dallas ML, Yang Z, Boyle JP, et al. Carbon monoxide induces cardiac arrhythmia via induction of the late Na+ current. Am J Respir Crit Care Med. 2012;186:648–656. doi: 10.1164/rccm.201204-0688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ishikawa M, Kajimura M, Adachi T, et al. Carbon monoxide from heme oxygenase-2 Is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–e114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- 118.Wallace JL, Dicay M, McKnight W, et al. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 119.Bhatia M, Wong FL, Fu D, et al. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 120.Ondrias K, Stasko A, Cacanyiova S, et al. H(2)S and HS(−) donor NaHS releases nitric oxide from nitrosothiols, metal nitrosyl complex, brain homogenate and murine L1210 leukaemia cells. Pflugers Arch. 2008;457:271–279. doi: 10.1007/s00424-008-0519-0. [DOI] [PubMed] [Google Scholar]
- 121.Coletta C, Papapetropoulos A, Erdelyi K, et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]