Abstract
Metabolism of nicotine to inactive cotinine by hepatic enzyme CYP2A6 is the principal pathway by which active nicotine is removed from circulation. We therefore hypothesized that inhibition of mouse CYP2A5, the orthologue of human CYP2A6, by methoxsalen (8-methoxypsoralen) should block nicotine metabolism and decrease its subsequent systemic clearance. Consequently, methoxsalen will alter dependence-related behaviors of nicotine in the mouse. In the first part of the study, a conditioned place preference (CPP) test was used in animals to explore the appetitive reward-like properties of nicotine. In the second part of the study, mice chronically exposed to nicotine were challenged with mecamylamine, a nicotinic antagonist, and tested for physical (somatic and hyperalgesia) and affective (anxiety-related behaviors) precipitated withdrawal signs. The nicotine plasma levels were also measured with or without methoxsalen pretreatment.
Methoxsalen (15 and 30 mg/kg, intraperitoneally) pretreatment 15 min before nicotine (0.1 mg/kg, subcutaneously) induced a significant enhancement of nicotine-induced preference in mice (p<0.05). This potentiation was significantly accompanied by an increase in nicotine plasma levels (p<0.05). However, there was a lack of enhancement of nicotine in the CPP test after the highest dose of the CYP-2A5 inhibitor. Similarly to the CPP results, repeated administration of methoxsalen with nicotine increased the intensity of mecamylamine-precipitated withdrawal signs on test day. The potentiation of withdrawal intensity by methoxsalen was accompanied by significant increase in nicotine plasma levels in these mice (p<0.05). Finally, methoxsalen enhanced the ability of nicotine to reverse withdrawal signs in mice undergoing spontaneous withdrawal after chronic nicotine infusion. Spontaneous withdrawal from chronic nicotine administration (36 mg/kg/day in minipumps for 7 days) significantly increased the number of somatic signs and induced hyperalgesia after mini pumps removal (p<0.05). Interestingly, methoxsalen (30 mg/kg, intraperitoneally) pretreatment significantly enhanced the reversal of these withdrawal signs by a very low dose of nicotine (0.05 mg/kg, subcutaneously) (p<0.05).
In conclusion, inhibition of nicotine metabolism by methoxsalen apparently enhances nicotine’s bioavailability and alters the behavioral effects of nicotine in the mouse. Combining CYP2A6 inhibitors with low dose nicotine replacement therapies may have a beneficial role in smoking cessation because it will decrease the drug elimination rate and maintain plasma and brain nicotine levels.
Keywords: nicotine, dependence, methoxsalen, mice, metabolism
1. Introduction
Smoking raises the risk of cardiovascular, pulmonary diseases and cancer. Nicotine is the main active ingredient in tobacco smoke that leads to and maintains tobacco addiction (Benowitz, 2008). Metabolism of nicotine to inactive cotinine by human microsomal cytochrome P450 CYP2A6 enzyme is the principal pathway by which active nicotine is removed from circulation in humans due to the major role that this pathway plays in nicotine clearance (Messina et al., 1997; Murphy et al., 2005; Benowitz et al., 2009). Interindividual variation in hepatic CYP2A6 activity influences the rate of nicotine metabolism and resulting smoking behaviors. Individuals with genetic deficiencies in CYP2A6-mediated nicotine metabolism have a lower risk for being a current smoker and if dependent, will smoke fewer cigarettes (Benowitz et al., 2002; Schoedel et al., 2004; Ray et al., 2009). Additionally, the inhibition of hepatic CYP-mediated nicotine metabolism leads to slower elimination of nicotine in smokers (Sellers et al., 2000; 2003) as well as increase in nicotine’s pharmacological actions in animals (Damaj et al., 2007; AlSharari et al., 2014). CYP2A6 inhibition works indirectly to slow the clearance of nicotine from the body, hence individuals respond by smoking less. Indeed, the selective CYP2A6 inhibitor methoxsalen was shown to inhibit the first-pass metabolism of orally administered nicotine and this nicotine-methoxsalen combination reduced smoking rates in smokers (Sellers et al., 2000). Animal studies in mice showed also that nicotine behavioral and pharmacological effects are affected by alteration in nicotine metabolism. For example, the amount of nicotine oral intake correlated positively with hepatic microsomal CYP2A5 protein levels in mice (Siu et al., 2006). In addition, methoxsalen inhibited CYP2A5-mediated nicotine metabolism in vivo and in vitro after subcutaneous and oral administration of nicotine in the mouse (Damaj et al., 2007; Alsharari et al., 2014). More recently, a study using the CYP2A(4/5) null mouse, showed an increases in the responses to nicotine’s acute pharmacological and rewarding effects along with a significant decrease in nicotine clearance in these mice (Li et al., 2013).
CYP2A6 inhibitors have been proposed as a novel approach for decreasing smoking directly or combination with nicotine replacement therapies (NRT) (Sellers et al., 2000; Denton et al., 2005; Buchhalter et al., 2008; Yamaguchi et al., 2013). Indeed, while NRTs generally have low abuse liability due to their slow absorption into the brain, their ability to promote smoking cessation is very modest. Furthermore, more rapid metabolism of nicotine was reported to result in lower nicotine blood levels from nicotine replacement products and poorer smoking cessation outcomes in Caucasians (Lerman et al., 2006) and African-American light smokers (Ho et al., 2009). These studies support the notion that slowing nicotine metabolism may serve as a therapeutic approach to enhance NRT efficacy in smoking cessation. We therefore hypothesized that inhibition of CYP2A6 will result in an increase in the duration of nicotine’s effect and consequently will enhance its efficacy as a substitute treatment for withdrawal. Specifically, CYP2A6 inhibition would cause an increase in apparent dose observed as an increase in nicotine plasma levels. This increase in plasma nicotine levels would result in a left-shifted dose-response curve (increased apparent potency) in the conditioned place preference test. Likewise the apparent increase in dose would result in enhanced nicotine withdrawal intensity.
Here we used methoxsalen (8-methoxypsoralen or MOP), a potent inhibitor of human CYP2A6 and mouse orthologous CYP2A5 (Zhang et al., 2001; Damaj et al., 2007), to study the impact of inhibiting nicotine metabolism on nicotine reward and withdrawal using well-established mouse models. Methoxsalen is relatively selective for the CYPs involved in nicotine metabolism, having little effect on other CYPs (Zhang et al., 2001).
2. Materials and Methods
2.1. Animals
Male adult ICR mice (20–25g) obtained from Harlan Laboratories (Indianapolis, IN) were used throughout the study. Animals were housed in an AALAC approved facility in groups of five and had free access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-Methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] and mecamylamine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Methoxsalen was purchased from Sigma Chemical Company (Milwaukee, WI). All drugs except for methoxsalen were dissolved in physiological saline (0.9% sodium chloride) and injected at a total volume of 1ml/100 g body weight unless noted otherwise. Methoxsalen was dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ) and 18 volumes distilled water] and administered intraperitoneally (i.p.). All doses are expressed as the free base of the drug. Mecamylamine and nicotine were injected subcutaneously (s.c.).
2.3. Plasma nicotine and cotinine levels measurement
To determine plasma nicotine and cotinine levels in the CPP test, blood samples were drawn by cardiac puncture at 20 min after nicotine administration (0.1 mg/kg, s.c.) in the last conditioning day of the CPP test. Animals were pretreated with i.p. vehicle or methoxsalen (15, 30 and 45 mg/kg i.p.) 15 min before nicotine administration. Each time point represents data from 6 individual mice.
For the withdrawal studies, on test day (5th day) of the administration protocol, blood samples were drawn 1 h after the last nicotine dose (8:00 am) in mice. Immediately afterwards the plasma samples were prepared by centrifugation at 3000 × g for 10 min and frozen at −20°C until analysis. To measure total nicotine and cotinine levels (free and glucuronides) the samples were incubated with β-glucuronidase at a final concentration of 5 mg/ml in 0.2 M acetate buffer, pH 5.0, at 37°C overnight. After incubation the sam ples were processed and analyzed for nicotine and metabolite levels by using high-performance liquid chromatography/tandem mass spectrometry (HPLC/MS/MS) analysis as previously described (AlSharari et al., 2014).
2.4. Behavioral tests
2.4.1. Nicotine conditioned place preference (CPP) studies
Nicotine CPP was conducted using an unbiased design as previously described by Kota et al., (2007). In brief, separate groups of male ICR mice (n= 8 per group) were handled for three days prior to initiation of CPP testing. The CPP apparatus consisted of a three-chambered box with a white compartment, a black compartment, and a center grey compartment. The black and white compartments also had different floor textures to help the mice further differentiate between the two environments. On day 1, mice were placed in the grey center compartment for a 5 min habituation period, followed by a 15 min test period to determine baseline responses. A pre-preference score was recorded and used to randomly pair each mouse with either the black or white compartment. Drug-paired sides were randomized so that an even number of mice received drug on the black and white side. Over the next 3 days, mice were conditioned for 20 min twice a day with conditioning sessions no less than 5 hr apart. The saline group receiving saline on both sides of the boxes and drug groups receiving nicotine (0.1 mg/kg, s.c.) on one side of the box and saline on the opposite side. Animals in the drug group received drug each day. On test day (day 5), mice were exposed to the chambers, and day 1 procedure was repeated. In addition, mice were pretreated with vehicle (i.p.) or methoxsalen (15, 30 and 45 mg/kg i.p.) 15 min prior nicotine. Data were expressed as time spent on the drug-paired side post-conditioning minus time spent on the drug-paired side pre-conditioning. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
2.4.2. Studies of nicotine withdrawal
The effect of methoxsalen on nicotine withdrawal was tested using a modified precipitated nicotine withdrawal model as described in Jackson et al. (2008). We chose a low-dose nicotine exposure precipitated withdrawal protocol in order to test the hypothesis that nicotine withdrawal intensity is enhanced by methoxsalen treatment. Our experimental protocol consisted of administration of vehicle or methoxsalen (15 mg/kg, i.p.) 15 min before nicotine (2 mg/kg, s.c.), three times daily (8:00 am, 12:00 pm and 4:00 pm) for 4 days. On test day (5th day), mice received one last nicotine dose (8:00 am) and 1 h later they were given one injection of mecamylamine (2 mg/kg, s.c.). Withdrawal observations were made immediately after mecamylamine treatment. The mice were first evaluated for 5 min in the elevated plus maze test for anxiety-related behavior, followed by a 20 min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Hyperalgesia was evaluated using the hot plate (thermostat apparatus maintained at 52°C) immediately following the somatic sign observation period (The latency to jump or lick a hind paw served as the dependent measure). The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent result. The number of arm crosses in the plus maze test was also counted as a measure of locomotor activity. All testing was conducted blind to group assignment.
2.4.3. Nicotine withdrawal reversal studies
In this study, we used a high-dose nicotine exposure withdrawal protocol to investigate if methoxsalen will enhance nicotine-induced reduction of withdrawal signs. Mice were anesthetized with sodium pentobarbital (35 mg/kg i.p.) and implanted with Alzet osmotic mini pumps (Durect Corporation, Cupertino, CA) filled with (−)-nicotine or saline solution as described in Jackson et al. (2008). The concentration of nicotine was adjusted according to animal weight and mini pump flow rate. Mice chronically infused with saline or nicotine (36 mg/kg/day) for 7 days and mini pumps were removed on day 8. Mice were then monitored for somatic signs and assessed for hyperalgesia starting 30 min after receiving vehicle, nicotine (0.05 mg/kg s.c.) or methoxsalen (30 mg/kg, i.p.) as described above.
2.5. Statistical analysis
Statistical analysis of all behavioral studies was performed using one or two-way analysis of variance (ANOVA) with Student Newman Keuls test when appropriate. All differences were considered significant if at p < 0.05.
3. Results
3.1. Effects of methoxsalen on low dose of nicotine in the CPP test
Methoxsalen was reported to enhance and to extend nicotine-induced acute pharmacological effects, including antinociception and hypothermia in mice (Damaj et al., 2007; Alsharari et al., 2014). We therefore hypothesized that a lower nicotine dose would be required to produce reward-like effects after pretreatment with methoxsalen. Here, we used an unbiased CPP paradigm to explore our hypothesis. As shown in Fig. 1A, low dose of nicotine (0.1 mg/kg, s.c.) did not induce place preference in mice by itself compared to vehicle-treated group [F(1,14)=0.636, p=0.451]. Conversely, pretreatment with methoxsalen at 15 and 30 mg/kg, prior to nicotine produced a significant CPP in mice [F(2,17)=8.167, p=0.003]. However, at the highest dose of methoxsalen (45 mg/kg), nicotine preference in CPP test was no longer significant. No significant differences in locomotor activity of mice after different treatments were observed on the test day (Table 1; p>0.05).
Figure 1. Effects of methoxsalen on nicotine CPP in the mouse.
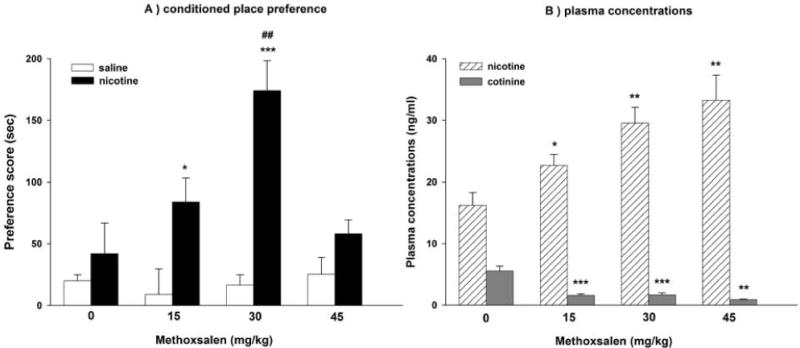
(A) The impact of increasing doses of methoxsalen on nicotine conditioned place preference (CPP). Male ICR mice were administered a low dose of nicotine (0.1 mg/kg, s.c.) 15 min after pretreatment with vehicle or methoxsalen (15, 30 and 45 mg/kg, i.p.) (B) Plasma concentrations of nicotine and cotinine in mice undergoing nicotine in combination with methoxsalen in the CPP test as described above. Data are presented as the mean ± SEM of 6–8 animals. *p<0.05, **p<0.01 and ***p<0.001 vs its control and ##p<0.01 vs vehicle + nicotine
Table 1.
Activity counts in the drug-paired or control compartment during assessment of nicotine (0.1 mg/kg, s.c.) or saline after pretreatment with different doses of methoxsalen (15, 30 and 45 mg/kg, i.p.) or vehicle in the conditioned place preference (CPP) test. Values represent the total activity counts in the drug-paired compartment on test day (drug free) for each group and are presented as the average activity count on test day. Data are expressed as mean ± S.E.M. of n=6–8 mice/group.
| Methoxsalen (mg/kg) |
Saline | Nicotine |
|---|---|---|
| 0 | 530 ± 69 | 512 ± 96 |
| 15 | 561 ± 83 | 524 ± 69 |
| 30 | 470 ± 105 | 488 ± 111 |
| 45 | 503 ± 89 | 513 ± 103 |
As predicted, the increase in nicotine preference by methoxsalen is consistent with the observed increase in nicotine plasma levels. Indeed, pretreatment with methoxsalen significantly increased nicotine’s plasma levels [F(3,18)=7.89, p<0.001] (Fig. 1B). At the highest dose of methoxsalen, nicotine plasma levels doubled, but preference scores were no longer significantly higher than control animals.
3.2. Effects of methoxsalen on nicotine withdrawal
In this part of the study, we examined whether greater nicotine bioavailability by methoxsalen treatment would enhance nicotine withdrawal, another important measure of nicotine dependence. Mice were treated with nicotine and methoxsalen (15 mg/kg, i.p.) or vehicle combination and then challenged with mecamylamine (2 mg/kg, s.c.) to precipitate withdrawal. Mice were tested for somatic withdrawal responses, anxiogenic-like effects in the elevated plus maze, and hyperalgesia in the hot plate test. As shown in Fig. 2, repeated administration of low dose nicotine did not precipitated somatic withdrawal signs [F(1,10)=1, p=0.341], but did induce a significant decrease in open arms time [F(1,10)=10.438, p=0.009], and paw withdrawal latency [F(1,9)=14.245, p=0.004] compared the vehicle group. The animals pretreated with methoxsalen exhibited significant somatic withdrawal signs [Fig. 2a; F(1,10)=17.962, p=0.008] and a further decrease in the average time spent in open arms [Fig. 2b; F(1,10)=79.697, p<0.001]. No significant differences in the total number of crossovers between arms in the plus-maze test between the different treatment groups were observed (Table 2; p>0.05).
Figure 2. Effects of methoxsalen on nicotine precipitated withdrawal in the mouse.
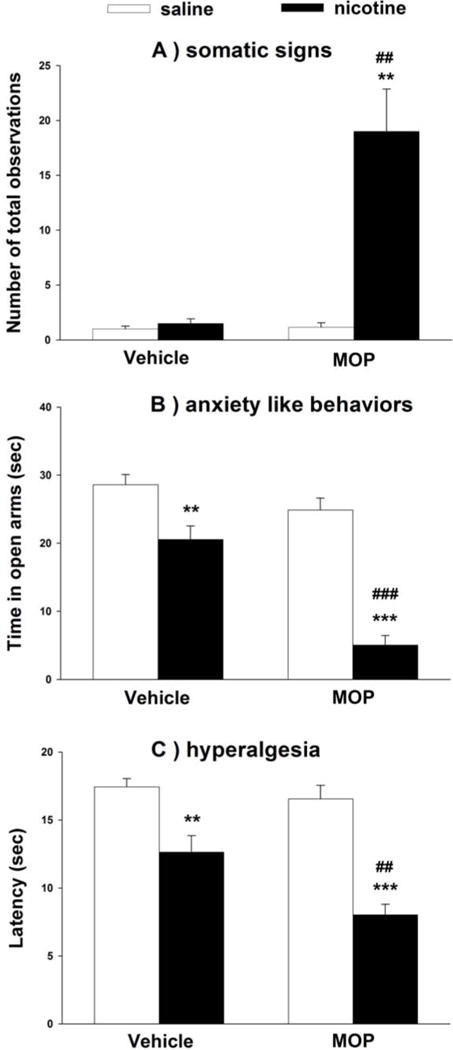
Nicotine was administered at 2 mg/kg (s.c.) 15 min after pretreatment with vehicle or methoxsalen (15 mg/kg, i.p.) three times daily for 4 days. On test day (5th day), mice received one last dose of nicotine and 1 h later they were injected with mecamylamine (2 mg/kg, s.c.). Ten min later, (A) somatic signs (B) anxiety-related behaviors and (C) hyperalgesia were measured in the mice. Data are presented as the mean ± SEM of 5–7 animals. **p<0.01, ***p<0.001 vs its control and ##p<0.01, ###p<0.001 vs vehicle + nicotine. (MOP = methoxsalen)
Table 2.
Total number of crossovers in the plus-maze test after precipitated nicotine withdrawal in mice. Nicotine was administered at 2 mg/kg (s.c.) 15 min after pretreatment with vehicle or methoxsalen (15 mg/kg, i.p.) three times daily for 4 days. On test day (5th day), mice received one last dose of nicotine and 1 h later they were injected with mecamylamine (2 mg/kg, s.c.). Ten min later, the total number of crossovers in the plus-maze test was measured in the different groups for 5 min. Data are presented as the mean ± SEM of 5–7 animals.
| Treatment Groups | Number Crossovers (Mean ± SEM) |
|---|---|
| Vehicle Control | 6.3 ± 0.5 |
| Vehicle / Nicotine / Mecamylamine | 5.5 ± 0.8 |
| Methoxsalen / Vehicle/ Mecamylamine | 6.0 ± 0.8 |
| Methoxsalen / Nicotine / Mecamylamine | 6.2 ± 0.6 |
Additionally, methoxsalen pretreatment displayed significant and increased hyperalgesic effects compared to vehicle group [Fig. 2c; F(1,10)=45.599; p<0.001]. The increase in nicotine withdrawal intensity in the group pretreated with methoxsalen was accompanied by an increase in nicotine plasma levels (Table 3). In summary, methoxsalen pretreatment of nicotine treated mice resulted in enhanced withdrawal intensity of all signs.
Table 3.
Plasma nicotine and cotinine levels in after treatment with methoxsalen or vehicle in chronically nicotine-treated mice. Mice were injected with vehicle or methoxsalen (15 mg/kg, i.p.) 15 min before nicotine (2 mg/kg, s.c.), three times daily for 4 days. On day 5, mice received one last nicotine dose and 1 h later, blood was collected and nicotine and cotinine plasma levels were measured. Data are expressed as mean ± S.E.M. of n=6 mice/group.
| Treatment | Nicotine Levels (ng/ml) |
Cotinine Levels (ng/ml) |
|---|---|---|
| Vehicle-treated group | 32 ± 7 | 144 ± 9 |
| Methoxsalen-treated group | 108 ± 8* | 38 ± 6* |
p<0.05 vs vehicle-treated group.
3.3. Effects of methoxsalen on nicotine reversal of nicotine spontaneous withdrawal
Spontaneous withdrawal from chronic nicotine administration (36 mg/kg/day in minipumps for 7 days) significantly increased the number of somatic signs [Fig. 3A; F(1,10) = 61.801, p=<0.001] and decreased the time spent on the hot-plate [Fig. 3B; F(1,10)=43.479, p<0.001] after removing the mini pumps. The increase in somatic signs was partially reversed by pretreatment with nicotine at low dose (0.05 mg/kg) [Fig. 3A; F(1,8) = 20.56, p=0.002] which was totally reversed by the methoxsalen and nicotine combination [Fig. 3A; F(1,8) = 92.161, p<0.001] (Fig. 3A). In addition, nicotine at 0.05 mg/kg did not significantly reverse hyperalgesic withdrawal signs [Fig. 3B; F(1,7)=0.77, p=0.409] but methoxsalen pretreatment before nicotine administration totally reversed these withdrawal signs [Fig. 3B; F(1,7)=24.142, p= 0.002].
Figure 3. Effects of methoxsalen on nicotine reversal of spontaneous withdrawal in the mouse.
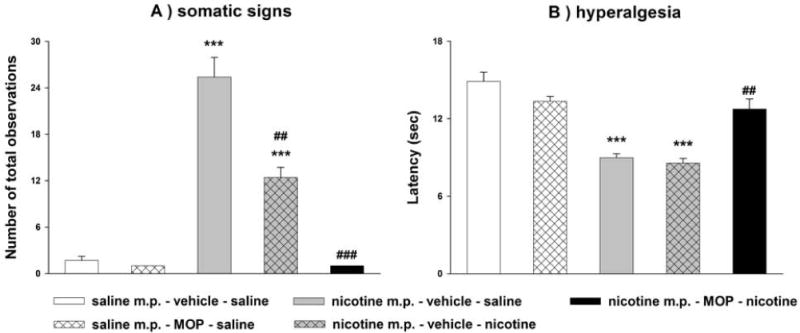
Male ICR mice were chronically infused with saline or nicotine (36 mg/kg/day) for 7 days. After mini pumps removal, mice received methoxsalen (30 mg/kg, i.p.), nicotine (0.05 mg/kg, s.c.) or their respective vehicles. Thirty min later, (A) somatic signs and (B) hyperalgesia were measured. Data are presented as the mean ± SEM of 5–7 animals. ***p<0.001 vs saline m.p. + vehicle + saline and ###p<0.001 vs nicotine m.p. + vehicle + saline in (A, B). (MOP = methoxsalen)
4. Discussion
The use of CYP2A5 Knock-out mice or inhibition of nicotine metabolism by methoxsalen, an inhibitor of CYP2A5, led to a prolongation of nicotine’s acute pharmacological effects as well as a decrease in nicotine elimination in mice (Damaj et al., 2007; Alsharari et al., 2014; Li et al., 2013). Our present results extend these previous findings, demonstrating that administration of methoxsalen enhances nicotine dependence-related behaviors in mice, by increasing and prolonging nicotine levels, providing a connection between nicotine metabolism and dependence liability of nicotine in animals.
In the present study we evaluated the impact of nicotine metabolism on two important aspects of nicotine dependence: reward and withdrawal. In the nicotine CPP, a test of conditioned reward, the low dose of nicotine (0.1 mg/kg) did not affect place preference in mice by itself. On the other hand, CYP2A5 inhibition by methoxsalen induced significant CPP in a dose-related manner. The increase in CPP by the low and middle doses of methoxsalen was correlated with an increase in plasma nicotine levels. However, inhibition of nicotine metabolism with the highest dose of methoxsalen (45 mg/kg) did not enhance nicotine preference. This lack of increase is probably due to a gradual development of the aversive properties at higher concentrations of nicotine and/or prolongation of nicotine levels and duration of effect in the animals. In other words, the highest dose of methoxsalen may have shifted nicotine’s effects to the descending part of the dose-response curve in the CPP test. The decrease in nicotine-induced CPP at the highest dose of methoxsalen pretreatment is interesting. Since at this dose of methoxsalen (45 mg/kg) there was no change in the locomotor activity of the animals in the test day of the CPP, the results do suggest a possible increase in nicotine aversive properties which may have contributed to the lack of enhancement in the CPP test after the highest dose of the CYP-2A5 inhibitor. This also may have some implications for the use of these CYP-2A5 inhibitors in smokers or nicotine users (e-cigarettes for example) as far as a possible increase of nicotine aversive properties and toxic effects.
Similar findings of a nicotine dose-response shift in the CPP test were recently reported in the CYP2A(4/5) Knock-out mouse, where mice showed an increase in the sensitivity of nicotine-induced place preference (Li et al., 2013). The CYP2A(4/5) null mouse has a 90% decreased rate of nicotine clearance, so this is a well-described mouse model with slow nicotine metabolism (Wei et al., 2013). The results from the CYP2A(4/5) null mice, showed a direct proof of the impact of nicotine clearance on nicotine’s effects, more definitive evidence for the connection between nicotine metabolism and nicotine’s actions on the central nervous system (Li et al., 2013). The CYP2A(4/5) null mouse showed increases in the magnitude and persistence of responses to nicotine’s acute pharmacological effects such as hypothermia and antinociception. The authors suggested that their result were supporting the hypothesis that rates of nicotine clearance can affect the rewarding effects of nicotine. Our results are in agreement with this study.
One potential limitation of our CPP study is that we did not investigate the impact of CYP2A5/6 on nicotine reinforcement and reinstatement behaviors such as nicotine i.v. self-administration paradigm. While this approach has merit, human studies have also shown that nicotine, given subcutaneously or orally, with methoxsalen reduces VAS measures such as desire to smoke and also can reduce the amount smoked (Sellers et al., 2000; 2003). This suggests that whether the route of nicotine intake is through self-administration via smoking, or via investigator administrated nicotine, once nicotine is in the systemic system it acts to importantly alter nicotine-induced behaviors. This is consistent with the two most popular routes of nicotine replacement therapy, nicotine gum (oral delivery, self-administered) and nicotine patch (systemic delivery, non titratable). In addition, a previous study has already found that increased nicotine self-administration is associated with increased rates of nicotine metabolism between mouse strains (Sui et al., 2006). Together this suggests that human and mouse studies have provided evidence that the impact of variation in nicotine levels, when nicotine is delivered by numerous different methods, can be used to translate to human smoking behaviors.
Chronic nicotine administration in rodents induces the appearance of physical (somatic signs and hyperalgesia) and affective (anxiety-related behavior) nicotine withdrawal signs. Similar to our CPP results, pretreatment of nicotine-exposed mice with methoxsalen significantly enhanced the intensity of all three nicotine withdrawal signs measured in our study: somatic signs, anxiety-related behaviors and hyperalgesia. The potentiation of withdrawal intensity by methoxsalen was consistent with the accompanying significant increase in nicotine plasma levels in these mice. Collectively, our findings supports the notion that decreases in nicotine clearance (e.g., as a result of CYP2A5 inhibition) will not only affect nicotine’s acute pharmacological actions, but also modify dependence-related behaviors in rodents. Our observations are in line with those recently reported using the CYP2A5 Knock-out mouse (Li et al., 2013).
Importantly, methoxsalen enhanced the ability of nicotine to reverse withdrawal signs in mice undergoing spontaneous withdrawal after chronic nicotine infusion. These results suggest that inhibitors of nicotine metabolism may enhance the efficacy of substitute treatments such as NRT for smoking dependence. Indeed, slow nicotine inactivators were reported to be less likely to be adult smokers and smoked fewer cigarettes per day (Schoedel et al., 2004). In addition, nicotine metabolism rate predicts efficacy of transdermal nicotine for smoking cessation (Lerman et al., 2006). Therefore, studies assessing the impact of nicotine metabolism in the different aspects of nicotine-induced behaviors will enhance our understanding of dependence and possibly support the development of new strategies for smoking cessation therapies.
Overall, the current study was undertaken to determine whether in vivo CYP2A5 inhibition by methoxsalen would alter dependence-related behaviors in the mouse. Our findings show that inhibition of nicotine metabolism by methoxsalen enhances nicotine’s bioavailability and alters nicotine reward and withdrawal in mice. Together with the studies in humans, this suggests that the effects observed here are due to the enhancement, and prolongation, of nicotine plasma levels via inhibition of CYP2A5-mediated nicotine metabolism. Furthermore, our results suggest that combining CYP2A6 inhibitors with low dose nicotine replacement therapies may have a beneficial role in smoking cessation.
Highlights of the manuscript.
Nicotine is metabolized to cotinine by the liver P-450 CYP-2A5/6 in humans and mice.
Methoxsalen is an inhibitor of mouse CYP2A5, the orthologue of human CYP2A6.
Methoxsalen enhanced nicotine-induced conditioned reward and withdrawal in mice.
Methoxsalen enhanced the ability of nicotine to reverse withdrawal signs in mice.
Acknowledgments
The authors greatly appreciate the technical assistance of Tie Han. This work was supported by National Institute on Drug Abuse grant # DA-05274 (MID). Deniz Bagdas would like to thank The Scientific and Technical Research Council of Turkey (TUBITAK) for her postdoctoral research scholarship (2219-2013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsharari SD, Siu EC, Tyndale RF, Damaj MI. Pharmacokinetic and pharmacodynamics studies of nicotine after oral administration in mice: effects of methoxsalen, a CYP2A5/6 inhibitor. Nicotine Tob Res. 2014;16:18–25. doi: 10.1093/ntr/ntt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Pérez-Stable EJ, Herrera B, Jacob P., 3rd Slower metabolism and reduced intake of nicotine from cigarette smoking in Chinese-Americans. J Natl Cancer Inst. 2002;94:108–115. doi: 10.1093/jnci/94.2.108. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin Pharmacol Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhalter AR, Fant RV, Henningfield JE. Novel pharmacological approaches for treating tobacco dependence and withdrawal: current status. Drugs. 2008;68:1067–1088. doi: 10.2165/00003495-200868080-00005. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Siu EC, Sellers EM, Tyndale RF, Martin BR. Inhibition of nicotine metabolism by methoxysalen: Pharmacokinetic and pharmacological studies in mice. J Pharmacol Exp Ther. 2007;320:250–257. doi: 10.1124/jpet.106.111237. [DOI] [PubMed] [Google Scholar]
- Denton TT, Zhang X, Cashman JR. 5-substituted, 6-substituted, and unsubstituted 3-heteroaromatic pyridine analogues of nicotine as selective inhibitors of cytochrome P-450 2A6. J Med Chem. 2005;48:224–239. doi: 10.1021/jm049696n. [DOI] [PubMed] [Google Scholar]
- Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther. 2008;325:302–312. doi: 10.1124/jpet.107.132977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, Benowitz N. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Li L, Jia K, Zhou X, McCallum SE, Hough LB, Ding X. Impact of nicotine metabolism on nicotine’s pharmacological effects and behavioral responses: insights from a Cyp2a(4/5)bgs-null mouse. J Pharmacol Exp Ther. 2013;347:746–754. doi: 10.1124/jpet.113.208256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282:1608–1614. http://jpet.aspetjournals.org/content/282/3/1608.long. [PubMed] [Google Scholar]
- Murphy SE, Raulinaitis V, Brown KM. Nicotine 5′-oxidation and methyl oxidation by P450 2A enzymes. Drug Metab Dispos. 2005;33:1166–1173. doi: 10.1124/dmd.105.004549. [DOI] [PubMed] [Google Scholar]
- Ray R, Tyndale RF, Lerman C. Nicotine Dependence Pharmacogenetics: Role of Genetic Variation in Nicotine Metabolizing Enzymes. J Neurogenet. 2009;23:1–10. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM, Kaplan HL, Tyndale RF. Inhibition of cytochrome P450 2A6 increases nicotine’s oral bioavailability and decreases smoking. Clin Pharmacol Ther. 2000;68:35–43. doi: 10.1067/mcp.2000.107651. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Ramamoorthy Y, Zeman MV, Djordjevic MV, Tyndale RF. The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo. Nicotine Tob Res. 2003;5:891–9. doi: 10.1080/14622200310001615231. [DOI] [PubMed] [Google Scholar]
- Siu EC, Wildenauer DB, Tyndale RF. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl) 2006;184:401–418. doi: 10.1007/s00213-006-0306-6. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Wei Y, Li L, Zhou X, Zhang QY, Dunbar A, Liu F, Kluetzman K, Yang W, Ding X. Generation and characterization of a novel Cyp2a(4/5)bgs-null mouse model. Drug Metab Dispos. 2013;41:132–140. doi: 10.1124/dmd.112.048736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Akimoto I, Motegi K, Yoshimura T, Wada K, Nishizono N, Oda K. Synthetic models related to methoxalen and menthofuran-cytochrome P450 (CYP) 2A6 interactions. benzofuran and coumarin derivatives as potent and selective inhibitors of CYP2A6. Chem Pharm Bull (Tokyo) 2013;61:997–1001. doi: 10.1248/cpb.c12-00872. http://dx.doi.org/10.1248/cpb.c12-00872. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kilicarslan T, Tyndale RF, Sellers EM. Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos. 2001;29:897–902. http://dmd.aspetjournals.org/content/29/6/897. [PubMed] [Google Scholar]


