Abstract
Epidemiologic data suggest an association between depot medroxyprogesterone acetate (DMPA), a progesterone-based hormonal contraceptive, and increased risk of HIV acquisition and transmission. DMPA is highly effective and is among the most commonly used form of hormonal contraception in areas of high HIV prevalence. Thus, defining the biological mechanisms that contribute to the potential negative synergy between DMPA and HIV is key and may facilitate the identification of alternative contraceptive strategies. Proposed mechanisms include thinning or disruption of the cervicovaginal epithelial barrier, induction of mucosal inflammation, interference with innate and adaptive soluble and cellular immune responses, and/or alterations in the vaginal microbiome. DMPA may also indirectly increase the risk of HIV by promoting genital herpes or other sexually transmitted infections. However, there is a paucity of rigorous in vitro, animal model and clinical data to support these potential mechanisms highlighting the need for future research.
Keywords: Depo-Provera, female genital tract, HIV, hormonal contraception, mucosal immunity
Introduction
A critical emerging issue that may impede efforts to control and reverse the HIV pandemic is the findings from multiple epidemiological studies that link the use of depot medroxyprogesterone acetate (DMPA), a progesterone-based contraceptive used by an estimated 35 million women worldwide,1 with an increased risk of acquiring or transmitting HIV and other sexually transmitted infections (STI).2–4 One basis for these concerns is epidemiological analysis of data collected in the Partners in Prevention HSV/HIV Transmission Study, which was conducted in seven countries in Africa. In a secondary analysis from this study, women using DMPA were at significantly increased risk of acquiring and transmitting HIV compared with women not using hormonal contraception; however, the primary study was not designed to address the impact of HC on HIV risk.5 DMPA is widely used in areas endemic for HIV because of its contraceptive efficacy, convenience (one dose every 3 months), low cost, and privacy. The accumulating epidemiological data suggesting that it may increase HIV acquisition and transmission underscore the need to identify underlying biological mechanisms and to evaluate alternative hormonal contraceptive strategies.
The female genital tract, the primary site of HIV acquisition/transmission, provides several levels of protection against infection including the epithelial barrier of the vagina and cervix, immune cells, and a diverse array of molecules secreted by epithelial cells, immune cells, and microbiota. The soluble mediators of defense include secretory immunoglobulin A (sIgA), antimicrobial peptides (AMPs) such as a and b defensins, cytokines and chemokines, and bacterial products such as hydrogen peroxide, lactic acid, and bacterocins.6 These humoral and innate defense mechanisms work in concert to protect the woman (and the fetus during pregnancy) from infection. However, hormonal contraception (HC) may adversely impact these mucosal defenses in ways that increase the risk of HIV. The negative impact of HC may be amplified by evidence, suggesting that HC also increases the risk of genital herpes and possibly other STI, which independently facilitate HIV acquisition and transmission.7–9
Studies in non-human primates support the contention that reproductive hormones may impact HIV risk. For example, there was no difference in the plasma viral load in rhesus macaques treated with 30 mg Depo-Provera 30 days prior to intravenous challenge with simian immunodeficiency virus (SIV) compared with controls.10 However, a significant increase in SIV acquisition was observed in rhesus macaques that received intrascapular progesterone implants compared with placebo pellets (14 of 18 versus 1 of 10), and the progesterone-treated animals progressed more rapidly to simian acquired immunodeficiency syndrome (AIDS).11 Limitations of the non-human primate studies include small numbers of animals and differences in species, dosing, and study design. Moreover, few human studies have been specifically designed to test the impact of Depo-Provera (or other HC) on HIV risk, and adjusting for the variability of human behavior (e.g., sexual exposure, condom use) presents a significant challenge. Defining the biological mechanisms that link HC to an increased risk of HIV/STI may clarify this complex clinical issue and facilitate the selection of alternative contraceptive strategies. This review summarizes the potential mechanisms that may link HC with HIV risk and highlights the research gaps (Fig. 1).
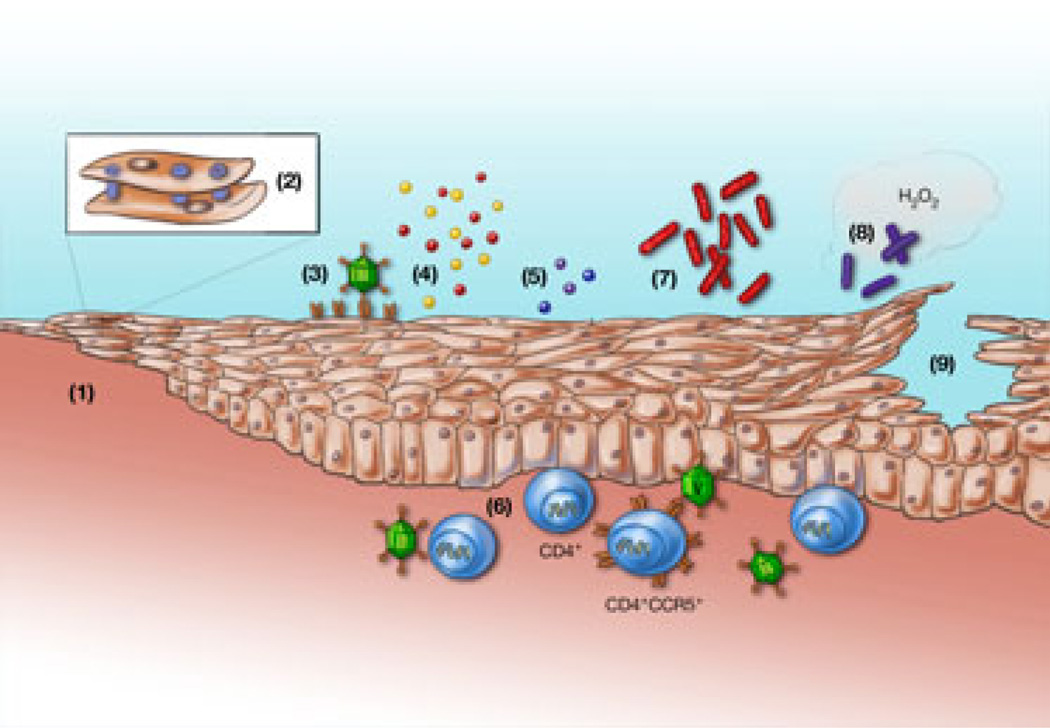
Fig. 1.
Biological mechanisms that may contribute to an increased risk of HIV acquisition and transmission among women using hormonal contraceptives such as DMPA. The numbers in the model shown above correspond to the following biological mechanisms: (1) thinning of the epithelial barrier, (2) disruption of intercellular junctional complexes; (3) upregulation of Trojan horse receptors that capture HIV particles such as heparan sulfate expressing syndecans; (4) increased secretion of inflammatory mediators that recruit or activate immune target cells and/or facilitate HIV replication; (5) decreased secretion of antimicrobial peptides (e.g., SLPI, defensins) that contribute to soluble host defense, (6) increased CCR5 expression on CD4+ cells rendering the cells more susceptible to HIV; (7) increased BV-associated bacteria; (8) decreased hydrogen peroxide producing lactobacilli; and (9) increased genital herpes shedding or clinical lesions with associated disruption of the epithelial barrier.
Potential mechanisms
Epithelial Barrier
The multilayered squamous epithelium lining the vagina and ectocervix, as well as tight junctions in the columnar epithelium of the endocervix, provides a physical barrier that may prevent HIV from reaching subepithelial immune cells. Depo-Provera may increase the risk of HIV by thinning epithelial barrier. Evidence for this emanates primarily from NHP models and may not accurately reflect events in the human genital tract. For example, NHP are more susceptible to SIV/SHIV in the second half of the menstrual cycle (when progesterone levels are high) and following treatment with MPA; this increase in susceptibility is associated with marked thinning of the vaginal epithelium.12 However, it is not clear that a similar phenomenon is observed in women. Mauck and colleagues found no significant decrease in vaginal epithelial thickness or increase in cervical ectopy (columnar epithelium in the vaginal portion of the cervix) in biopsies obtained 1 and 3 months following administration of DMPA when compared with baseline biopsies in 16 subjects.13 Another study also found no difference in vaginal epithelial thickness in women using DMPA compared with women on no HC.14 Small changes in the genital tract epithelium were observed after 6 months of DMPA use in a third study; these included a reduction from baseline in the mean number of epithelial cell layers (28.1 ± 0.7 to 25.9 ± 0.9; P = 0.05), thickness (1.02 ± 0.04 mm to 0.89 ± 0.05 mm; P = 0.005), and glycogen-positive thickness (0.81 ± 0. 04 mm to 0.66 ± 0.05; P = 0.005).15 However, the biological relevance of these changes is not clear.
Hormonal contraception may also impact HIV acquisition/transmission by disrupting the integrity of the epithelial barrier. A transmission electron microscopy (TEM) study of human tissue revealed the classic tripartite junctional complex of endocervical epithelial cells.16 Cells were joined apically by tight junctions, which prevent intercellular passage of molecules.16 Below the tight junctions, adherens junctions further reinforced the barrier by joining adjacent cells.16 Lastly, desmosomes were observed below the adherens junctions, fortifying the barrier between cells.16 Junctions were also observed in the lower two-thirds of the ectocervical epithelium, but not in the most apical region.16 Notably, because synthetic progesterone may reduce the risk of spontaneous preterm birth, studies have examined the impact of hormones on gene expression in myometrial tissue obtained during pregnancy and identified changes in expression of structural proteins including some of the junctional proteins.17 Similar changes have not yet been described in cervicovaginal epithelium of non-pregnant women.
The importance of epithelial barrier integrity is underscored by the need for HIV to cross the epithelium to reach submucosal T cells. Several studies indicate that epithelial and dendritic cells (DCs) may capture HIV and then transfer the virus to T cells through a Trojan horse-like mechanism. HIV gp120 may bind to heparan sulfate moieties on syndecan proteoglycans on epithelial cells and/or bind to C-type lectins such as DC-SIGN on DCs and subsequently be transferred to T cells.18–21 Heparan sulfate proteoglycans and DC-SIGN may stabilize HIV virions for days.18,21 Recent studies conducted in our laboratory indicate DMPA upregulates syndecan expression on genital tract epithelial cells to promote HIV capture and transfer in vitro, but clinical studies are needed to determine whether this contributes to an increase in HIV acquisition.
Soluble Immune Mediators
Genital tract epithelial and immune cells secrete an array of antimicrobial proteins, cytokines, and chemokines. Many of these proteins have direct inhibitory activity against HIV and other STI in vitro but may also facilitate HIV infection by recruiting or activating immune target cells or by enhancing viral replication through activation of the long terminal repeat (LTR) promoter. Changes in the expression of these soluble mediators have been observed throughout the menstrual cycle and during pregnancy, providing support for the hypothesis that reproductive hormones may increase HIV risk through modulation of soluble mucosal defense. For example, lower levels of immunoglobulins, β-defensins, and secretory leukocyte protease inhibitor (SLPI) were observed mid-cycle when estrogen levels were highest and decreased levels of IgA and IgG were observed in women treated with OCPs.22– 27 Similarly, the levels of polymeric immunoglobulin receptor (pIgR), which mediates IgA transport into the lumen, may be modulated by hormones.28 Levels of secretory component, the cleaved extracellular domain of pIgR, are highest during the secretory phase and lowest during menstruation.28 Genital tract tissue expression of the Fc neonatal receptor (FcRn), which mediates bidirectional transcytosis of IgG, may also be modulated by hormones.29,30
Few studies have examined the impact of exogenous hormones on expression of these soluble mediators. Decreased expression of SLPI, an anti-inflammatory serine protease inhibitor with anti-HIV activity, was observed in endometrial biopsies obtained after DMPA treatment compared with biopsies obtained at baseline.31 Notably, SLPI may also be reduced in the setting of other conditions associated with an increased risk of HIV. For example, HSV-2 downregulates SLPI expression in vitro, and lower levels have been observed in genital tract secretions in women with bacterial vaginosis (BV).32,33 Expression of other antimicrobial peptides may also be hormonally regulated, although specific studies in women using DMPA have not been reported. Wira and colleagues found that estradiol significantly decreased the secretion of elafin (a serine protease inhibitor with anti-inflammatory properties similar to SLPI) and human beta defensin 2 (HBD2) from primary vaginal epithelial cells that were cultured overnight with exogenous hormone.34 In another study, endometrial biopsies were collected from 57 women; 24 received no contraception, 20 were using a combined oral contraceptive pill (OCP), and 13 were using a levonorgestrel intrauterine system.35 Both forms of HC were associated with decreased expression of HBD1, HBD2, and granulysin, but not SLPI.35
Changes in cytokines and chemokines have also been described in association with reproductive hormones. An increase in IL-8 and decrease in RANTES were observed in immortalized cervical cells treated in with MPA and TNF.36 The decrease in RANTES was blocked when an androgen receptor antagonist was added.36 RANTES, a ligand for CCR, inhibits HIV infection by competing for viral binding to the CCR5 coreceptor. Thus, lower levels of RANTES in the genital tract could reduce mucosal defense, but there is as yet no direct evidence linking genital tract RANTES levels with HIV risk. Translating in vitro findings to the clinical setting is complex. One concern is that the concentrations of exogenous hormones often used in vitro (and in animal models) may exceed levels that cells are exposed to clinically. The serum concentrations of MPA plateau at ~ 1 ng/mL following intramuscular injection of 150 mg of DMPA, although peak levels are likely much higher.37
Impact of Hormones on Immune Cell Populations
Hormonal contraception may facilitate HIV infection by increasing the number of HIV targets (activated T cells and macrophages/monocytes) within the genital tract and/or by interfering with cellular immune responses. For example, progesterone inhibited toll-like receptor 9 (TLR9)-induced interferon (IFN) production by human and mouse plasmacytoid dendritic cells (pDCs) and DMPA impaired TLR9- and virus-induced IFN production by pDCs in mice.38 Similar results were obtained in another study in which 10−7 m or higher concentrations of MPA inhibited the production of multiple cytokines and chemokines by activated T cells and reduced the production of IFNa and TNF by pDCs in response to TLR-7, TLR-8, and TLR-9 ligands in vitro.39
In that study, MPA also prevented the downregulation of HIV-1 coreceptors (CCR5 and CXCR4) on the surface of T cells after activation in vitro and increased HIV-1 replication in activated peripheral blood mononuclear cell cultures.39 The increased HIV replication could reflect the retention of coreceptors on the cell surface, activation of the LTR through inflammatory cytokines, and/or reduced production of antiviral factors. Increased expression of CCR5 in response to progesterone has been suggested in other studies. For example, one study that included pregnant women found that progesterone levels were significantly associated with CCR5 expression on PBMCs.40 A similar association was observed in a prospective clinical study of 15 women in which vaginal biopsies were obtained in the follicular and luteal phase of the menstrual cycle and approximately 12 weeks after a single dose of DMPA.41 A significant increase in the numbers of T cells, macrophages, and HLA-DR and CCR5-positive T cells were found in vaginal tissues following DMPA compared with the follicular and/or luteal phases of untreated cycles; no differences in immune cell populations were observed between the follicular and luteal phases of the cycle.41 Moreover, in another study of 32 healthy women, the number and cell surface density of CCR5 on peripheral blood T cells increased in women using OCPs.42 Increased CCR5 expression on endocervical CD4+T cells was also reported in healthy post-menopausal women.43 These data collectively suggest that increased expression of CCR5 may contribute to the epidemiological link between DMPA and HIV, but larger studies are needed to address the impact of HC on the number and function of mucosal and systemic immune cell populations.
Alterations in the Vaginal Microbiome
Hydrogen peroxide producing lactobacilli (predominantly L. crispatus, L. jensenii and L. gasseri)44 contribute to host defense. Conversely, BV, which is characterized by overgrowth of commensal anaerobes and concomitant loss of lactobacilli,45–47 increases HIV acquisition,48 transmission to male partners,49 and genital HIV shedding.50 Healthy lactobacilli protect against infection by maintaining the vaginal pH (<4.7) and producing lactic acid, bacteriocins, and other small molecules. High concentrations of hydrogen peroxide producing lactobacilli are viricidal to HIV in vitro.6 In a study conducted among HIV-sero-negative sex workers in Mombasa, Kenya, absence of vaginal lactobacilli on culture was associated with an increased risk of acquiring HIV and other STI.51 Lactobacilli correlated negatively and Mycoplasma hominis (BV-associated bacteria) correlated positively with genital tract HIV RNA levels in an U.S. study, suggesting that BV influences genital tract viral shedding.52 Thus, if DMPA adversely impacts the vaginal microbiome, this may provide another mechanistic link contributing to the increased risk of HIV associated with DMPA.
However, the preponderance of studies does not support this mechanism. A systematic review of 36 studies found that combined oral contraception and DMPA reduced BV by 10–20% and 18–30%, respectively.53 These findings are further supported by a recent randomized controlled trial of young Australian women in which estrogen-containing combined OCPs were associated with a reduced risk of BV [AOR = 0.6; 0.4–0.9].54,55 Molecular vaginal microbiome studies confirm that high estrogen levels favor a vaginal microbiome composition dominated by lactobacilli, whereas the effects of progesterone are less clear.53
Indirect Effects: Increases in Genital Herpes in Association with HC
Epidemiological studies consistently demonstrate that HSV-2 infection is associated with increased rates of HIV acquisition, higher HIV plasma viral loads, and higher rates of transmission to HIV-naive partners including mother-to-child transmission.56–59 The negative synergy between HSV-2 and HIV has been attributed to alterations in local soluble and cellular host defense33,60 including the recruitment and persistence of immune cells that are targets for HIV infection to sites of genital ulcer disease.61 Thus, if HC or specifically DMPA increases the likelihood of HSV-2 infection and genital tract shedding, this may indirectly facilitate HIV infection. This notion is supported by a study of 550 women in Brazil and the Philippines, which found an association between HSV-2 sero-positivity and long-term OCP use (≥4 years).9 A subsequent longitudinal study of 330 HSV-2 sero-positive women found that HC (OCP and injectables) was an independent predictor of genital HSV-2 shedding (adjusted OR, 1.8; 95% CI, 1.1–2.8).8
Mouse models also suggest a role for reproductive hormones in promoting genital herpes infection. Mice are typically treated with DMPA prior to intravaginal HSV-2 infection because DMPA thins the epithelium and increases the vaginal expression of nectin-1, the primary receptor for HSV entry.62 However, no differences in nectin-1 expression were observed in biopsies from humans obtained at different phases of the menstrual cycle,62 although clinical studies of nectin-1 expression in the setting of HC have not been reported. Following DMPA treatment, mice have a prolonged diestrus phase (weeks) and are ~100-fold more susceptible to genital HSV compared with untreated or estradiol treated mice. DMPA treatment also had inhibitory effects on immune responses to HSV-2.63 Mice immunized with HSV-2 glycoprotein B and treated with Depo-Provera showed significant less local HSV-2-specific IgG and IgA in their vaginal washes.63
Hormonal contraception may also facilitate HSV infection by interfering with soluble mucosal defense. Genital tract secretions inhibit HSV infection in vitro, and this ‘endogenous activity’ correlates with the concentrations of human neutrophil peptides (HNP1–3), lactoferrin, inflammatory cytokines, and immunoglobulins (total IgA and IgG).64,65 The endogenous activity may prevent HSV from spreading from the more common external genitalia sites to the cervix, as suggested by the finding of increased anti-HSV activity in cervicovaginal lavage (CVL) collected at the time of an active external herpes lesion compared with HSV sero-negative women.65
One small study examined the impact of HC on the endogenous anti-HSV activity. CVL were obtained weekly from nine women normally cycling and from seven on HC (mostly OCPs). There was a modest decrease in the anti-HSV activity of CVL in women on HC (mean 57% during follicular or luteal phase compared with 36% in women on HC).66 However, there was substantial intra-and intersubject variability in the anti-HSV activity even among women using HC, suggesting that factors other than reproductive hormones contribute to the observed differences.66 These findings suggest that HC may facilitate HSV infection by modulating soluble mucosal defense, but larger prospective studies that compare different forms of contraception are needed. Similarly, the impact of HC on the anti-HIV activity of CVL, which has also been observed in numerous studies but is of unclear clinical significance, requires further investigation.67–69
Future directions
There is a paucity of data directly testing the mechanisms by which DMPA and other forms of HC might modulate the female genital tract mucosal immune environment to promote HIV infection. Hypothesis-generating in vitro studies are a critical first step, but results must be interpreted cautiously as differences in the phenotype of cell cultures and the hormonal concentrations applied may not reflect actual events in the female genital tract. Anderson and colleagues developed three immortalized, but not fully transformed, human epithelial genital tract cell lines expressing HPV type 16 E6 and E7 proteins: vaginal (VK2/E6E7), ectocervical (Ect1/E6E7), and endocervical (End1/E6E7) cells.70 These cells have normal karyotype and phenotypes70 and constitutively and/or inducibly express an array of immune mediators.71 Importantly, the Ect1/E6E7 and VK2/E6E7 cells express the progesterone, androgen, glucocorticoid, and estrogen receptor in the range of fmol/mL protein; however, in Ect1/E6E7 cells, only the glucocorticoid receptor is transcriptionally active with a steroid response element-driven reporter.36 The reproducibility of studies conducted with these cells is a clear advantage, but the findings must be confirmed with primary cell and tissue cultures. Animal models may also be hypothesis generating, but important limitations include the relatively high doses of exogenous hormones used in these studies and the differences in mucosal immune mediators and vaginal microbiome between species.
Results obtained from in vitro and animal model studies must be tested in human studies with sufficient genital tract sampling (including collections of secretions, swabs for microbiome analyses, and biopsies). Clinical studies should also include an assessment of how HC modulates the local and systemic innate and adaptive immune environment, which is critical for vaccine development. Identification of the mechanisms that contribute to the epidemiological link between DMPA and HIV risk will promote the advancement of safer alternative forms of HC. By defining the mechanisms, alternatives can be appropriately evaluated in vitro and in small clinical studies, as conducting large-scale clinical trials to test the impact of newer alternative HC modalities on HIV risk is a formidable task.
Acknowledgments
This work supported by U19 AI03461 and R01 AI065309 (B.C.H.), and by T32 A1007501 (K.M. and S.C.I.). We thank Margaret Nielsen for artwork.
References
- 1.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a Systematic Review. AIDS. 2013;27:787–794. doi: 10.1097/QAD.0b013e32835bb672. [DOI] [PubMed] [Google Scholar]
- 3.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 4.Butler AR, Smith JA, Polis CB, Gregson S, Stanton D, Hallett TB. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. AIDS. 2013;27:105–113. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klebanoff SJ, Coombs RW. Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med. 1991;174:289–292. doi: 10.1084/jem.174.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin HL, Mandaliya K, Ndinya-Achola JO, Bwayo JJ, Kreiss JK. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 8.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40:1422–1428. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 9.Smith JS, Herrero R, Munoz N, Eluf-Neto J, Ngelangel C, Bosch FX, Ashley RL. Prevalence and risk factors for herpes simplex virus type 2 infection among middle-age women in Brazil and the Philippines. Sex Transm Dis. 2001;28:187–194. doi: 10.1097/00007435-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sanders-Beer B, Babas T, Mansfield K, Golightly D, Kramer J, Bowlsbey A, Sites D, Nieves-Duran L, Lin S, Rippeon S, Donnelly G, Rhodes L, Spano YE. Depo-Provera does not alter disease progression in SIVmac-infected female Chinese rhesus macaques. AIDS Res Hum Retroviruses. 2010;26:433–443. doi: 10.1089/aid.2009.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 12.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 13.Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 14.Bahamondes L, Trevisan M, Andrade L, Marchi NM, Castro S, Diaz J, Faundes A. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception. 2000;62:23–27. doi: 10.1016/s0010-7824(00)00132-3. [DOI] [PubMed] [Google Scholar]
- 15.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96:431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 16.Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordeaux Y, Tattersall M, Charnock-Jones DS, Smith GC. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. J Clin Endocrinol Metab. 2010;95:E437–E447. doi: 10.1210/jc.2010-1541. [DOI] [PubMed] [Google Scholar]
- 18.Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 19.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondor I, Ugolini S, Sattentau QJ. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 22.Keller MJ, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, Cole AL, Cole AM, Profy AT, Wira CR, Hogarty K, Herold BC. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. Aids. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 23.King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 24.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol. 2003;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher GFB. Humoral immune factors in the female reproductive tract and their changes during the cycle. In: Dinsda D, Schumacher G, editors. Immunological Aspects of Infertility and Fertility Control. New York: Elsevier; 1980. pp. 93–141. [Google Scholar]
- 27.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan DA, Richardson GS, MacLaughlin DT, Wira CR. Variations in the levels of secretory component in human uterine fluid during the menstrual cycle. J Steroid Biochem. 1984;20:509–513. doi: 10.1016/0022-4731(84)90263-2. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li A, Felix JC, Yang W, Jain JK. Effect of mifepristone on the expression of endometrial secretory leukocyte protease inhibitor in new medroxyprogesterone acetate users. Fertil Steril. 2008;90:872–875. doi: 10.1016/j.fertnstert.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 32.Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, Hershow RC, Chen HY, Landay A. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007;14:1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fakioglu E, Wilson SS, Mesquita PM, Hazrati E, Cheshenko N, Blaho JA, Herold BC. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J Virol. 2008;82:9337–9344. doi: 10.1128/JVI.00603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate immunity in the vagina (part I): estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am J Reprod Immunol. 2013;69:463–474. doi: 10.1111/aji.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79:856–863. doi: 10.1016/s0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 36.Africander D, Louw R, Verhoog N, Noeth D, Hapgood JP. Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception. 2011;84:423–435. doi: 10.1016/j.contraception.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Mishell DR., Jr Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med. 1996;41:381–390. [PubMed] [Google Scholar]
- 38.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 39.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, Hel Z. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheffield JS, Wendel GD, McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16:20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 41.Chandra N, Thurman AR, Anderson S, Cunningham TD, Yousefieh N, Mauck C, Doncel GF. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29:592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prakash M, Kapembwa MS, Gotch F, Patterson S. Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol. 2002;54:117–131. doi: 10.1016/s0165-0378(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 43.Meditz AL, Moreau KL, MaWhinney S, Gozansky WS, Melander K, Kohrt WM, Wierman ME, Connick E. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–228. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hillier S. Normal vaginal flora. In: Holmes K, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, Cohen M, Watts D, editors. Sexually Transmitted Diseases. 4th edn. New York: McGraw-Hill; 2008. pp. 289–307. [Google Scholar]
- 45.Kumar N, Behera B, Sagiri SS, Pal K, Ray SS, Roy S. Bacterial vaginosis: etiology and modalities of treatment-A brief note. J Pharm Bioallied Sci. 2011;3:496–503. doi: 10.4103/0975-7406.90102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductiveage women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 48.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22:1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell C, Balkus JE, Fredricks D, Liu C, McKernan-Mullin J, Frenkel LM, Mwachari C, Luque A, Cohn SE, Cohen CR, Coombs R, Hitti J. Interaction between lactobacilli, bacterial vaginosis-associated bacteria, and HIV Type 1 RNA and DNA Genital shedding in U S. and Kenyan women. AIDS Res Hum Retroviruses. 2013;29:13–19. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell C, Hitti J, Paul K, Agnew K, Cohn SE, Luque AE, Coombs R. Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses. 2011;27:35–39. doi: 10.1089/aid.2010.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 52.Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, Spear GT. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 53.Van de Wijgert JH, Verwijs MC, Turner AN, Morrison CS. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013;27:2141–2153. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 54.Bradshaw CS, Walker J, Fairley CK, Chen MY, Tabrizi SN, Donovan B, Kaldor JM, McNamee K, Urban E, Walker S, Currie M, Birden H, Bowden F, Garland S, Pirotta M, Gurrin L, Hocking JS. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS ONE. 2013;8:e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bradshaw CS, Vodstrcil LA, Hocking JS, Law M, Pirotta M, Garland SM, De Guingand D, Morton AN, Fairley CK. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56:777–786. doi: 10.1093/cid/cis1030. [DOI] [PubMed] [Google Scholar]
- 56.Chen KT, Segú M, Lumey LH, Kuhn L, Carter RJ, Bulterys M, Abrams EJ. Group NYCPACTSP: Genital herpes simplex virus infection and perinatal transmission of human immunodeficiency virus. Obstet Gynecol. 2005;106:1341–1348. doi: 10.1097/01.AOG.0000185917.90004.7c. [DOI] [PubMed] [Google Scholar]
- 57.Drake AL, John-Stewart GC, Wald A, Mbori-Ngacha DA, Bosire R, Wamalwa DC, Lohman-Payne BL, Ashley-Morrow R, Corey L, Farquhar C. Herpes simplex virus type 2 and risk of intrapartum human immunodeficiency virus transmission. Obstet Gynecol. 2007;109:403–409. doi: 10.1097/01.AOG.0000251511.27725.5c. [DOI] [PubMed] [Google Scholar]
- 58.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 59.Corey L. Synergistic copathogens-HIV-1 and HSV-2. N Engl J Med. 2007;356:854–856. doi: 10.1056/NEJMe068302. [DOI] [PubMed] [Google Scholar]
- 60.Stefanidou M, Ramos I, Mas Casullo V, Trepanier JB, Rosenbaum S, Fernandez-Sesma A, Herold BC. Herpes simplex virus 2 (HSV-2) prevents dendritic cell maturation, induces apoptosis, and triggers release of proinflammatory cytokines: potential links to HSV-HIV synergy. J Virol. 2013;87:1443–1453. doi: 10.1128/JVI.01302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Linehan MM, Richman S, Krummenacher C, Eisenberg RJ, Cohen GH, Iwasaki A. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J Virol. 2004;78:2530–2536. doi: 10.1128/JVI.78.5.2530-2536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, Wallenstein S, Carlucci MJ, Tuyama AC, Lu W, Klotman ME, Lehrer RI, Herold BC. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 65.Keller MJ, Madan RP, Shust G, Carpenter CA, Torres NM, Cho S, Khine H, Huang ML, Corey L, Kim M, Herold BC. Changes in the soluble mucosal immune environment during genital herpes outbreaks. J Acquir Immune Defic Syndr. 2012;61:194–202. doi: 10.1097/QAI.0b013e31826867ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shust GF, Cho S, Kim M, Madan RP, Guzman EM, Pollack M, Epstein J, Cohen HW, Keller MJ, Herold BC. Female genital tract secretions inhibit herpes simplex virus infection: correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol. 2010;63:110–119. doi: 10.1111/j.1600-0897.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 68.Keller MJ, Madan RP, Torres NM, Fazzari MJ, Cho S, Kalyoussef S, Shust G, Mesquita PM, Louissaint N, Chen J, Cohen HW, Diament EC, Lee AC, Soto-Torres L, Hendrix CW, Herold BC. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS ONE. 2011;6:e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS ONE. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 71.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]