Abstract
Schizophrenia is a disease typically associated with an adolescent onset. While there have been a considerable number of imaging studies investigating the transition to psychosis in prodromal patients, there are relatively few preclinical studies examining potential mechanisms that may contribute to adolescent onset. We have previously demonstrated, in the methylazoxymethanol acetate (MAM) rodent model of schizophrenia, that an enhanced activity within the ventral hippocampus may underlie the dopamine system hyperfunction, suggested to contribute to positive symptoms in patients. Here we demonstrate that the aberrant regulation of dopamine system function, in MAM-treated rats, is present prior to puberty. Furthermore, we now report that while the afferent regulation of ventral tegmental area dopamine neurons (from the hippocampus and pedunculopontine tegmental area) appears intact in pre-adolescent rats, the behavioral response to alterations in dopamine system function appears to be attenuated in pre-adolescent rats. Thus, we posit that the pathological alterations underlying psychosis may be present prior to symptom onset and that the ‘normal’ development of the post-synaptic side of the dopamine system may underlie the transition to psychosis.
Keywords: Preadolescent, Hippocampus, Schizophrenia, MAM rat, Parvalbumin
Introduction
A hallmark of schizophrenia is the adolescent onset of the disease (Hafner et al., 1992). The observation of early cognitive or negative deficits suggests that a level of pathology may be present prior to the transition to psychosis (Demjaha et al., 2012; Fusar-Poli P and et al., 2012). It is believed that aberrant dopamine system function is associated with the positive symptoms of schizophrenia based on several observations including: 1) psychomotor stimulants induce psychosis-like symptoms in the general population (Bell, 1965); 2) patients with schizophrenia demonstrate an increased sensitivity to psychomotor stimulants (Lieberman et al., 1987); 3) the observation of aberrant dopamine transmission (as determined by imaging studies) in schizophrenia patients (Abi-Dargham et al., 2000; Abi-Dargham et al., 2009); and 4) the clinical efficacy of dopamine D2-like receptor antagonists (Meltzer et al., 1989).
The cause of the aberrant dopamine system function in schizophrenia has not been conclusively demonstrated; however, we have previously suggested that the augmented dopamine neuron activity and hyper-responsivity to psychomotor stimulants observed in a rodent model of schizophrenia (namely methylazoxymethanol acetate (MAM)) are a consequence of aberrant activity within ventral regions of the hippocampus (Lodge and Grace, 2007). Thus, activation of the ventral hippocampus (vHipp) can increase dopamine neuron activity with corresponding increases in dopamine efflux observed in the nucleus accumbens (NAc) (Floresco et al., 2003). This regulation occurs via a projection to the NAc which, in turn, attenuates a tonic inhibitory drive to the ventral tegmental area (VTA) arising from the ventral pallidum (Floresco et al., 2003). MAM-treated rats display an increase in vHipp activity, an increased number of spontaneously active dopamine neurons, and an augmented locomotor response to amphetamine administration (Lodge and Grace, 2007). This enhanced dopamine system function has been attributed to increased vHipp activity since tetrodotoxin-inactivation of the vHipp completely normalizes both the aberrant dopamine neuron activity, as well as the behavioral hyper-responsivity to amphetamine (Lodge and Grace, 2007). Thus, we posit that aberrant vHipp activity may be the cause of the dopamine-dependent psychosis in schizophrenia. Indeed, this hypothesis is consistent with imaging studies demonstrating enhanced hippocampal activity at rest in schizophrenia patients, which is correlated with positive symptom severity (Heckers et al., 1998; Malaspina et al., 1999; Medoff et al., 2001; Lahti et al., 2006; Schobel et al., 2009).
Given that the positive symptoms of schizophrenia do not appear until adolescence or early adulthood, it is important to understand potential changes in the regulation of dopamine system function that occur across development. Consistent with the adolescent onset of the disease in humans, MAM-treated rats have been reported to display an augmented behavioral response to psychomotor stimulants only after puberty (Moore et al., 2006). Thus, the MAM-treated rat provides a model to examine potential neurophysiological alterations across development that may have relevance to the adolescent onset of psychosis. Here we examine the hippocampal regulation of dopamine system function and behavior across development and, determine whether the deficits in dopamine system function are present prior to puberty. Such information should provide insight relevant to our understanding of the mechanisms underlying the transition to psychosis.
Materials and Methods
Animals
All experiments were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center, San Antonio. MAM treatments were performed as described previously (Moore et al., 2006). In brief, timed pregnant female Sprague Dawley rats were administered MAM (25 mg/kg, i.p.) or saline (1 ml/kg, i.p.) on GD17. Post-weaning, pre-adolescent rats were examined prior to puberty, (defined as postnatal day 42 based on previous observations of balano-preputial separation and increases in circulating androgens (Korenbrot et al., 1977)), whereas adult rats were over 8 weeks of age.
Extracellular Recordings and Drug Administration
Preadolescent MAM, saline and control rats were anaesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus. For acute administration of N-methyl-D-aspartic acid (NMDA) or vehicle, rats were implanted with 23 G injection cannula 2.0 mm dorsal to the vHipp (A/P −5.6, M/L +4.6, D/V −4.6 mm from bregma) and/or pedunculopontine tegmental nucleus (PPTg; A/P −7.4, M/L +1.4, D/V −4.0 mm from bregma). NMDA was infused (0.75 μg/0.5 μl) through a 30 G injection cannula protruding 2.0 mm past the end of the implanted guide cannula into either the vHipp, PPTg or both regions. Vehicle was infused into either region and data combined as reported previously (Lodge and Grace, 2006). Glass extracellular microelectrodes were lowered into the VTA (A/P −5.0, M/L +0.5 mm from bregma and −6.0 to −8.5 mm ventral of brain surface) and spontaneously active dopamine neurons were recorded while making 6–9 vertical passes throughout the VTA. Dopamine neurons were identified using previously established electrophysiological criteria (Grace and Bunney, 1983). Three parameters of activity were measured: (i) population activity (defined as the number of spontaneously active dopamine neurons recorded per electrode track), (ii) basal firing rate, and (iii) burst firing (Grace and Bunney, 1984). Statistics were performed on the average firing rate and burst firing obtained per rat.
Western Blot
MAM and saline treated rats were sacrificed on postnatal day (PD) 5, PD15, PD25, PD40 or as adults. The vHipp was dissected on ice and stored at −80 °C. The tissue was immersed in RNA Later ICE, equilibrated at −80 °C and stored at −20 °C. The tissue was then removed and homogenized (PARIS kit – Applied Biosystems). Protein concentrations were determined using the Bradford method before incubation with Laemmli Sample Buffer (10 min at 90 °C) and separated (20μg/lane) on “Any kD” Precast Gels. Proteins were transferred to nitrocellulose membranes and were incubated with either rabbit anti-parvalbumin 1:5000 (Abcam: AB11427) or mouse anti-GAPDH 1:1000 (Abcam: ab9484) followed by either HRP-anti-rabbit 1:10,000 or HRP-anti-mouse 1:5,000, respectively. Membranes were treated with a peroxidase substrate for enhanced chemiluminescence and exposed to film prior to scanning and quantification with ImageJ. Lanes that were obviously smeared were omitted from analysis.
Locomotor Activity
Preadolescent MAM and saline treated rats were anesthetized with sodium-pentobarbital (20 mg/kg) and implanted with bipolar, twisted, stainless steel electrodes (Plastics One) (A/P −5.6, M/L +4.6, D/V −4.6 mm from bregma), then fixed in place with four anchor screws and dental cement. One week after surgery, animals were examined for locomotor response to vHipp stimulation as described previously (Taepavarapruk et al., 2000). In brief, following a 30 minute baseline period, the vHipp was transiently activated (one stimulus train consisting of 200 pulses at 300 μA, 0.2 msec pulse width delivered at 20 Hz for 10 secs) and locomotor activity recorded. Next, rats received an injection of amphetamine (0.5 mg/kg i.p.) and activity recorded for 30 minutes followed by additional vHipp stimulation (parameters as above). A separate group of rats were examined for the locomotor response to increasing doses of amphetamine (0.5 mg/kg i.p. and 2.0 mg/kg i.p.).
Quantitative PCR
Preadolescent and adult control rats were anesthetized with sodium pentobarbital and rapidly decapitated. The NAc and VTA were isolated by tissue punch and homogenized. RNA was precipitated and separated by filtration (Ambion - RNAqueous-4PCR). The concentration of RNA was determined by absorbance at 260nm and converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Ambion). Real time PCR was performed with FAM-labeled TaqMan primers targeting either the dopamine D1 receptor (Rn00432253_m1), D2 receptor (Rn00561126_m1), D3 receptor (Rn00567568_m1), D4 receptor (Rn00564071_m1), D5 receptor (Rn00562768_s1), dopamine transporter (DAT) (Rn00562224_m1), tyrosine hydroxylase (Rn00562500_m1), RGS 2 (Rn00584932_m1), RGS 9 (Rn00570117_m1), GIRK2 (Rn00755103_m1) or GAPDH (Rn01775763_g1). Detection of FAM labeled DNA was performed by a CFX384 Real-Time PCR Detection System (Bio-Rad). Data represented as ΔCt report the number of PCR cycles required for mRNA detection (compared to the control mRNA, GAPDH), while fold-changes are calculated by the 2−ΔΔCt method.
Histology
At the cessation of the electrophysiological or behavioral experiments, rats were decapitated, brains removed, and fixed for 24 hours (3.7% formaldehyde), cryoprotected (10% w/v sucrose in PBS) and sectioned (25 μm coronal sections) on a cryostat (Shandon/Leica). Sections containing either recording/stimulating electrode tracks or NMDA/vehicle injection sites were mounted onto gelatin-chrom alum coated slides and stained with neutral red (0.1%) and thionin acetate (0.01%) for histochemical verification with reference to a stereotaxic atlas (Paxinos and Watson, 1986).
Analysis
Electrophysiological analysis of dopamine neuron activity was performed using commercially available computer software (LabChart v7.1) and locomotor activity was collected with Activity Monitor (Med Associates). All statistics of firing rate and burst firing were performed on the average measures from each rat. PCR data were analyzed by commercially available software (BioRad CFX) while optical density of western blot images was quantified using ImageJ. All data are represented as the mean ± standard error of the mean (SEM).
Materials
Methylazoxymethanol acetate (MAM) was purchased from Midwest Research Institute (Kansas City, MO). Chloral hydrate, N-methyl-D-aspartate (NMDA), sodium pentobarbital, Dulbecco’s PBS and D-amphetamine sulfate were all purchased from Sigma (St Louis, MO). The RNAqueous-4PCR kit, RNA Later ICE, PARIS kit, High Capacity cDNA Reverse Transcription Kit as well as FAM-labeled TaqMan primers and Gene Expression Master Mix were purchased from Applied Biosystems (Carlsbad, CA). The anti-parvalbumin and anti-GAPDH antibodies were purchased from ABCAM while the HRP-labeled secondary antibodies were obtained from Invitrogen. Laemmli Sample Buffer and Mini-Protean gels were purchased from Biorad. All other chemicals and reagents were of either analytical or laboratory grade and purchased from various suppliers.
Results
VTA Dopamine Neuron Activity
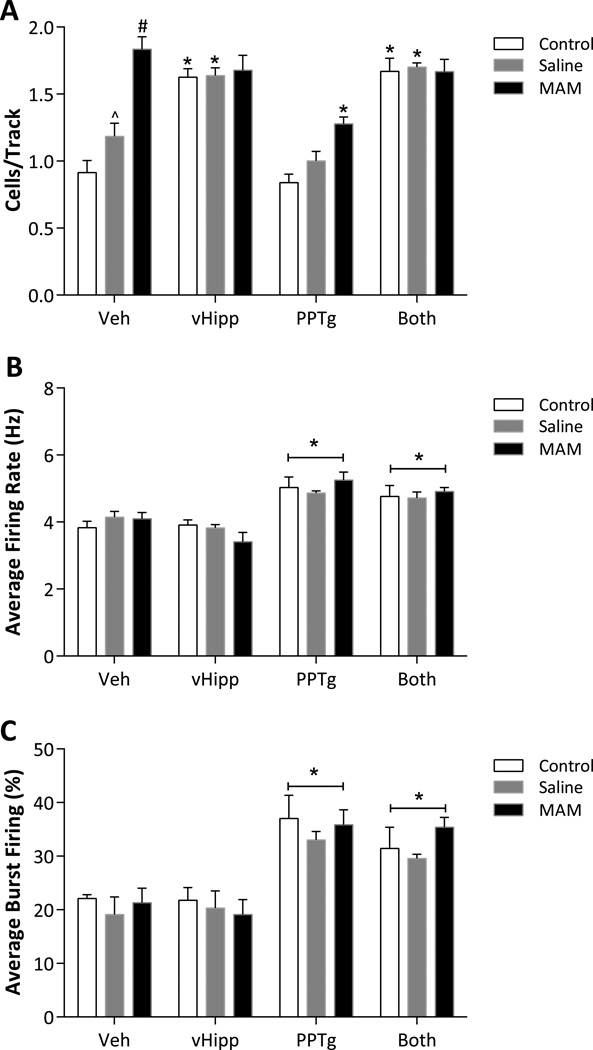
Dopamine neuron activity was examined by two-way ANOVA. The number of spontaneously active dopamine neurons per electrode track (i.e. population activity) demonstrated a significant main effect of ‘strain’ (F2,74 = 19.652, p < 0.05), ‘treatment’ (F3,74 = 43.601, p < 0.05) and a significant interaction between factors (F6,74 = 7.531, p < 0.05). Specifically, pre-pubertal untreated rats that received vehicle infusions (n = 9 rats, 74 neurons) exhibited an average of 0.91 ± 0.09 spontaneously active dopamine neurons per electrode track, with an average firing rate of 3.83 ± 0.19 Hz and 22.17 ± 0.7% of action potentials fired in a burst (Fig 1). Post-hoc analysis demonstrated that an infusion of NMDA into the vHipp (n = 8 rats, 104 neurons) selectively increased dopamine neuron population activity in control rats (1.63 ± 0.06 cells/track; 2-way ANOVA; Holm-Sidak; t = 6.857; p < 0.05; Fig 1A) while having no significant effect in MAM-treated rats (n = 6 rats, 99 neurons; 1.68 ± 0.11 cells/track; 2-way ANOVA; Holm-Sidak; t = 1.257; p > 0.05; Fig 1A) likely attributable due to the baseline increase in the number of spontaneously active dopamine neurons observed in vehicle-treated MAM rats (n = 6 rats, 90 neurons; 1.83 ± 0.09 cells/track; 2-way ANOVA; compared to both untreated (t = 8.161) and saline-treated (t = 5.257) rats; p < 0.05; Fig 1A). These data are similar to previous observations in adult rats demonstrating augmented dopamine neuron activity in MAM-treated rats (Lodge and Grace, 2007).
Figure 1.
Pre-adolescent untreated and saline treated rats display vHipp- and PPTg- induced increases in population activity and burst firing, respectively, of VTA dopamine neurons. In addition, pre-adolescent MAM-treated rats display a selective increase in the number of spontaneously active dopamine neurons observed per electrode track. While MAM-treated rats display PPTg-induced increases in burst firing and firing rate, similar to that observed in saline and untreated controls, activation of the vHipp does not further augment population activity. NMDA (0.75μg/0.5μl) was administered into either the vHipp, PPTg or both regions and compared to vehicle administration (Veh: 0.5μl Dulbecco’s PBS). Vehicle was infused into either the vHipp, PPTg or both regions and data combined as reported previously (Lodge and Grace, 2006). Three parameters of activity were recorded: population activity (A), average firing rate (B) and average percent burst firing (C). Data were analyzed by a 2-way ANOVA with a Holm Sidak post hoc as appropriate. *represents statistically significant difference from rats receiving vehicle infusions, ^ depicts statistically significant difference from untreated rats and # depicts statistically significant difference from both untreated and saline-treated rats.
While significant differences in dopamine neuron population activity and the response to afferent activation were observed in pre-adolescent MAM-treated rats, changes in dopamine neuron firing rate and burst firing were not dramatically altered by MAM-exposure. Indeed, statistical analyses of average firing rate and average percent burst firing demonstrated main effects of ‘treatment’ (firing rate: F3,74 = 26.683, p < 0.05 ; burst firing: F3,74 = 23.888, p < 0.05) without significant effects of ‘strain’ (firing rate: F2,74 = 0.023, p > 0.05 ; burst firing: F2,74 = 1.130, p > 0.05) or interaction (firing rate: F6,74 = 1.077, p > 0.05 ; burst firing: F6,74 = 0.393, p > 0.05). Specifically, NMDA activation of PPTg or both regions (n = 7–8/group) resulted in significant increases in burst firing when compared to vehicle-treated rats, a response that was independent of strain (least square means for treatment; vehicle: 20.8 ± 1.6, PPTg: 35.29 ± 1.5; t = 6.486, p < 0.05, both regions: 32.1 ± 1.5; t = 5.072, p < 0.05: Fig 1C). A similar increase in firing rate was observed following PPTg activation (least square means for treatment; vehicle: 4.02 ± 0.13, PPTg: 5.05 ± 0.12; t = 5.914, p < 0.05, both regions: 4.80 ± 0.12; t = 4.467, p < 0.05: Fig 1C).
Similar responses to afferent activation were observed in rats following prenatal administration of saline (Fig 1). There were no significant differences between the untreated and saline-treated in any parameter of dopamine neuron activity, with the exception of a minor increase in dopamine neuron population activity in saline-treated rats (control 0.91 ± 0.09, saline-treated 1.19 ± 0.10 cells/track; 2-way ANOVA; Holm-Sidak; t = 2.403; p < 0.05; Fig 1A). Furthermore, saline-treated rats displayed similar responses to NMDA infusions into the vHipp and PPTg as untreated rats (Figure 1) and there were no significant differences between untreated and saline-treated rats following NMDA activation of afferent targets (n = 6–8/group).
Western Blot
A loss of parvalbumin expression has been observed in schizophrenia patients (Lewis et al., 2005; Konradi et al., 2011), and rodent models (Penschuck et al., 2006; Wang et al., 2008; Franois et al., 2009; Lodge et al., 2009; Jenkins et al., 2010; Amitai et al., 2012). Here, we demonstrate that deficits in vHipp parvalbumin occur before puberty in MAM-treated rats. Both saline-and MAM-treated rats display increases in vHipp parvalbumin across development (2-way ANOVA; F4,27(postnatal day) = 13.259; p < 0.05; Fig 2), with robust increases observed around postnatal day 25, consistent with previous observations (Solbach and Celio, 1991). The increase in parvalbumin was attenuated by ~50% in MAM-treated rats (2-way ANOVA; F1,27(strain) = 4.556; p < 0.05; Fig 2).
Figure 2.
MAM-treated rats display developmental changes in hippocampal parvalbumin expression. Representative western blots demonstrate the increase in parvalbumin expression, compared to GAPDH, across development (A). Quantitative analysis of the blots demonstrate that MAM-treated rats display similar levels of parvalbumin expression until postnatal day 25, where there is a clear decrease in expression that persists throughout adulthood (B). † denotes significant difference from saline; 2-way ANOVA.
Locomotor Activity
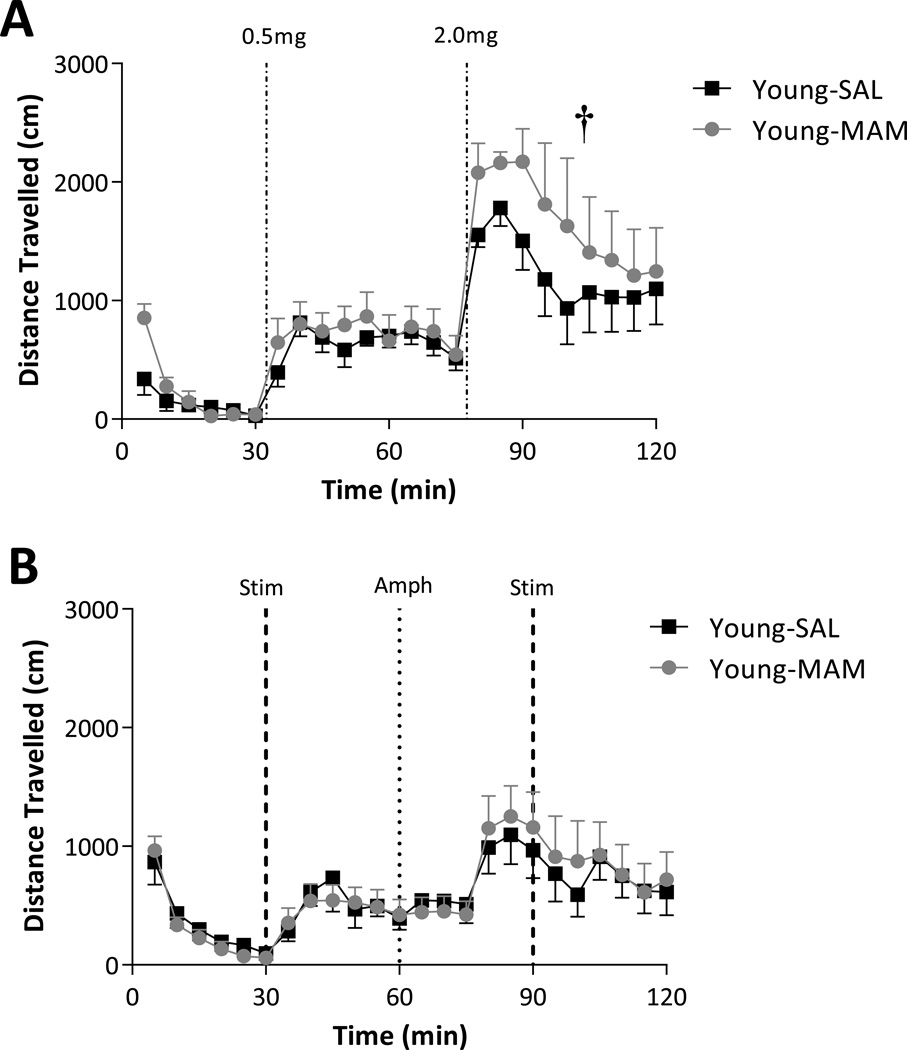
Consistent with previous studies (Moore et al., 2006), pre-adolescent MAM-treated rats did not display an augmented activity at baseline (2-way ANOVA of Basal; F1,224(strain) = 3.731, p > 0.05; Fig 3A) or following the low-dose of amphetamine (2-way ANOVA of 0.5 mg/kg dose; F1,224(Age) = 3.050, p > 0.05; Fig 3A). Interestingly, pre-adolescent MAM-treated rats did display an augmented locomotor response to amphetamine, but in contrast to that observed in adult rats, this was only observed with the higher dose of amphetamine (2-way ANOVA of 2.0 mg/kg dose; F1,224(strain) = 5.615; Holm-Sidak, t = 2.370, p < 0.05; Fig 3A).
Figure 3.
Pre-adolescent MAM-treated rats display a significantly greater locomotor response to high-dose (2.0 mg/kg), but not low-dose (0.5 mg/kg) amphetamine when compared to saline-treated rats (A). Both saline and MAM-treated rats display a weak locomotor response to vHipp stimulation in preadolescent rats with no significant differences between groups (B). † represents significant effect of MAM when compared to saline-treated rats; 2-way ANOVA.
Given that the vHipp regulation of dopamine neuron activity is dysfunctional in MAM rats, we examined the locomotor response to vHipp stimulation. Consistent with the data presented above, transient electrical stimulation of the vHipp failed to induce a greater response in juvenile MAM-treated rats (2-way ANOVA of Stim; F1,224(strain) = 0.054, p > 0.05; Fig 3B).
Quantitative RT-PCR
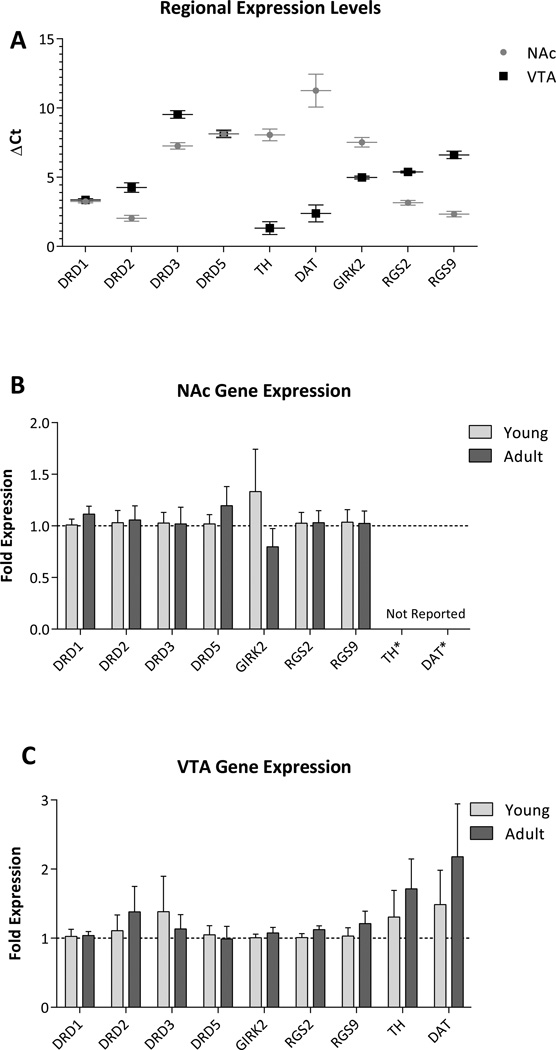
To examine dopamine-related molecular mechanisms that may underlie the different behavioral responses between pre-adolescent and adult rats, quantitative RT-PCR was performed using probes for the dopamine D1, D2, D3, D4, D5 receptors, as well as the dopamine transporter (DAT), tyrosine hydroxylase and dopamine receptor signaling molecules RGS2, RGS9 and GIRK2. We have previously reported that adult, MAM-treated rats display significant increases in D3 receptor expression throughout the NAc (Perez and Lodge, 2012). Here we demonstrate, that pre-adolescent rats do not display significant differences in the expression of a number of dopaminergic markers throughout either the NAc or VTA (3-way ANOVA; F1,180(Region) = 0.256, F1,180(Age) = 2.032, F8,180(Gene) = 1.738, F1,180(Region × Age) = 0.580, F=8,180(Region × Gene) = 0.395, F8,180(Age × Gene) = 1.054, F8,180(Region × Age × Gene) = 0.518, all p > 0.05; Fig 4B/C). It should be noted that dopamine D4 receptor expression was below the limits of detection, consistent with our previous observations (Perez and Lodge, 2012). Furthermore, NAc expression of TH (Adult: NAc: 8.05 ± 0.43 c.f. VTA: 1.32 ± 0.46 ΔCt) and DAT (Adult: NAc: 11.26 ± 1.19 c.f. VTA: 2.38 ± 0.61 ΔCt) were dramatically lower than that observed in the VTA, consistent with their restricted expression in dopamine neurons, and are not reported in Figure 4.
Figure 4.
Pre-adolescent rats do not display significant changes in the expression of dopaminergic markers in the NAc or VTA. Quantitative RT-PCR was performed on RNA extracted from the NAc and VTA of untreated pre-adolescent and adult rats. Expression data from adult rats (A) report the number of PCR cycles required for mRNA detection (compared to GAPDH) and confirm that mRNA expression of TH and DAT are largely restricted to the VTA and are therefore omitted from (B). Pre-adolescent rats display a similar expression of dopaminergic markers throughout both the NAc (B) and VTA (C) when compared to adult rats.
Discussion
An augmented dopamine system function is a consistent observation in schizophrenia patients (Laruelle and Abi-Dargham, 1999; Abi-Dargham, 2004). Given that there is no observable pathology within the dopamine system, it is widely believed that it is the regulation of the dopamine neurons that may be altered in this disease. We have previously demonstrated, in the MAM model of schizophrenia, that the aberrant dopamine system function, typically associated with positive symptoms of schizophrenia, is directly attributable to a pathologically increased drive from the vHipp (Lodge and Grace, 2007). Interestingly, an augmented hippocampal function is a consistent observation in imaging studies of schizophrenia patients (Heckers et al., 1998; Malaspina et al., 1999; Medoff et al., 2001; Lahti et al., 2006; Schobel et al., 2009). Given that the positive symptoms of the disease typically appear following adolescence (Hafner et al., 1992), it is important to determine whether alterations in these key neuronal systems occurs prior to, or at the time of, the transition to psychosis. Here we demonstrate that the hippocampal regulation of dopamine neuron activity is similar between pre-adolescent and adult rats, and that the augmented dopamine neuron population activity is actually present prior to puberty in MAM-treated rats.
Distinct afferent regions have been reported to differentially modulate dopamine neuron activity states in adult rats (Floresco et al., 2003; Lodge and Grace, 2006; Lodge and Grace, 2006; Grace et al., 2007; Lodge, 2011). Specifically, NMDA-activation of the vHipp increases dopamine neuron population activity, i.e. the number of dopamine neurons firing spontaneously, whereas activation of the PPTg induces a transition from single spike firing to a bursting pattern (Floresco et al., 2003; Lodge and Grace, 2006). We demonstrate here that NMDA activation of the vHipp and PPTg produces a similar effect in preadolescent rats demonstrating that the afferent regulation of dopamine neuron activity (at least from the vHipp and PPTg) appears to be qualitatively similar in pre-adolescent and adult rats. Moreover, consistent with data obtained in adult rats (Lodge and Grace, 2007), pre-adolescent rats treated prenatally with MAM also display an increase in the number of spontaneously active dopamine neurons throughout the VTA. It should be noted that PPTg activation appeared to reduce dopamine neuron population activity in MAM-treated rats. While the mechanisms contributing to this effect have not been examined; it has been previously demonstrated that MAM-treated rats are more sensitive to excitatory drive leading to depolarization blockade of dopamine neurons (Valenti et al., 2011; Perez and Lodge, 2012). Hence, it is possible that PPTg activation may lead to depolarization block in MAM-treated rats; however, this requires further examination.
MAM-treated rats display an aberrant drive from the vHipp resulting in an augmented dopamine system function (Lodge and Grace, 2007). While the exact cause of the increased vHipp activity has not been conclusively demonstrated, it has been suggested that it may be attributable to deficits in fast-spiking interneurons, particularly those expressing parvalbumin. Specifically, a deficit in parvalbumin-containing interneuron function is a consistent observation in a diverse variety of animal models, as well as, in schizophrenia in humans (Lewis et al., 2005; Penschuck et al., 2006; Abdul-Monim et al., 2007; Behrens et al., 2007; Lodge et al., 2009). Indeed, we propose that a loss of parvalbumin interneuron function, particularly in the vHipp, may be the cause of the dopamine dysregulation and associated psychosis in schizophrenia (for review see: (Lodge and Grace, 2011)). We have previously demonstrated that adult MAM-treated rats display a region specific reduction in the number of parvalbumin positive interneurons (Lodge et al., 2009). Here we expand those previous observations and report that deficits in parvalbumin expression occur prior to puberty in MAM-treated rats. Specifically, hippocampal parvalbumin expression appears following birth, at around PN5, and increases dramatically, reaching adult levels by ~PN25, consistent with previous findings in untreated rats (Solbach and Celio, 1991). Although MAM-treated rats display similar parvalbumin expression up to PN15, they display a markedly attenuated increase in parvalbumin expression across puberty.
It has been previously demonstrated that increases in dopamine neuron population activity result in an augmented locomotor response to low-dose amphetamine administration (Lodge and Grace, 2007; Lodge and Grace, 2008). Thus, adult MAM-treated rats display a hyper-responsivity to psychostimulant drugs (Moore et al., 2006; Lodge and Grace, 2007). Interestingly, this augmented response is not observed in pre-adolescent MAM-treated rats (Moore et al., 2006), at least in response to low-doses of amphetamine. Here we confirm that the increased response to low-dose amphetamine is not present in pre-adolescent rats; however, an augmented response was observed following a higher dose of amphetamine. This is consistent with previous ontogenic investigations of psychostimulant induced locomotion (Lanier and Isaacson, 1977; Bolanos et al., 1998; Frantz et al., 2007; Zakharova et al., 2009) demonstrating an attenuated response to systemic amphetamine in pre-adolescent rats. Similarly, our data demonstrate only modest increases in locomotion to vHipp stimulation in preadolescent saline and MAM-treated rats. Given that afferent regulation of dopamine neuron activity is similar in pre-adolescent and adult rats, it is likely that the different behavioral responses are attributable to developmental changes in the post-synaptic response to dopaminergic input. To examine this we performed quantitative RT-PCR to determine the expression of the dopamine receptors, as well as the dopamine transporter, tyrosine hydroxylase and signaling molecules associated with the response to dopamine (GIRK2, RGS2 & RGS 9) from pre-puberty to adulthood. We did not observe any significant alteration in the expression of the mRNA encoding the aforementioned markers of dopamine system function. Thus, the mechanisms underlying the differences in dopamine responsivity require further elucidation.
Previous studies by O’Donnell and colleagues have demonstrated dramatic differences in the response to dopamine ligands in pre-adolescent rats when compared to adult rats (Tseng et al., 2007; Tseng and O'Donnell, 2007; Benoit-Marand and O'Donnell, 2008; Huppe-Gourgues and O'Donnell, 2012; Huppe-Gourgues and O'Donnell, 2012). Specifically, the excitatory effect of the D2-like agonist, quinpirole, on prefrontal cortical interneurons was reportedly absent in pre-adolescent rats (Tseng and O'Donnell, 2007). Similarly, non-fast spiking interneurons of the cortex were observed to acquire an excitatory response to D1 receptor activation only after puberty (Tseng and O'Donnell, 2007). More recent work examining dopamine-glutamate interactions in the NAc have demonstrated that D2 receptor modulation of AMPA-mediated responses are different in pre-pubertal animals (Huppe-Gourgues and O'Donnell, 2012). Opposite effects of D1 receptor activation have also been observed in medium spiny neurons from pre-adolescent rats compared to adult(Huppe-Gourgues and O'Donnell, 2012). Taken together, it appears that the response of the prefrontal cortex and NAc to changes in dopaminergic transmission is dramatically different in pre-adolescent rats and likely underlies the apparent disparity between the electrophysiological and behavioral responses reported here. The exact mechanisms underlying this response are not currently known; however, our RT-PCR data suggest that it is likely independent of gross changes in mRNA expression of dopamine receptors; indeed, dopamine D1 and D2 binding, in the NAc, appears relatively constant from PN35 through adulthood (Teicher et al., 1995).
Here we suggest that pathological changes underlying schizophrenia may be present prior to the transition to psychosis and that the normal maturation of how the brain responds to dopamine may underlie the transition. Thus, we posit that changes in hippocampal and dopaminergic activity should be present in the prodrome and may be used as a predictor of illness. Indeed, recent work by Howes and colleagues have demonstrated elevations in dopamine synthesis capacity (measured by [18F]-DOPA PET imaging) in ultra-high-risk patients (Howes et al., 2009) and, furthermore, that dopamine synthesis capacity was significantly greater in patients that transitioned to psychosis (Howes et al., 2011), suggesting that augmented dopamine transmission may precede the onset of the psychotic illness. Given our hypothesis that aberrant dopamine system function is secondary to increased activity within hippocampal subfields, we posit that augmented hippocampal function should also be present prior to the onset of psychosis. Indeed, imaging studies examining regional cerebral blood volume in the hippocampus have demonstrated increases in baseline hippocampal activity in patients that transitioned to psychosis (Schobel et al., 2009). Thus, an increasing literature suggests that alterations in key neuronal systems are present prior to psychosis.
Here we demonstrate, in a rodent model of schizophrenia, that deficits in hippocampal parvalbumin expression and dopamine system function are present in pre-adolescent rats. Furthermore, the afferent regulation of dopamine system responsivity, at least from the vHipp and PPTg, appear to be functionally similar between pre-adolescent and adult rats. Interestingly, the behavioral response to hippocampal activation or psychostimulant drug administration was significantly attenuated in juvenile rats suggesting that the way the brain responds to dopamine changes across development. Taken together, these data suggest that deficits in the regulation of dopamine system function may be present prior to the emergence of behavioral deficits. Such information is relevant for the potential diagnoses of schizophrenia prior to the manifestation of psychosis and also raises the possibility of early therapeutic interventions. Indeed, a recent study has reported enduring benefits of adolescent pharmacotherapy in a rodent model of schizophrenia (Du and Grace, 2013).
Acknowledgements
This work was supported by the NIH (R01: MH090067) and a NARSAD award from the Maltz Family Foundation.
Footnotes
Dr. Lodge reports receiving consulting fees from Dey Pharmaceuticals while Dr Chen and Ms. Perez do not have any disclosures or conflicts of interest.
References
- Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. Journal of Psychopharmacology. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Science. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, van de Giessen E, Slifstein M, Kegeles LS, Laruelle M. Baseline and Amphetamine-Stimulated Dopamine Activity Are Related in Drug-Naive Schizophrenic Subjects. Biol Psychiatry. 2009;65:1091–1093. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Amitai N, Kuczenski R, Behrens MM, Markou A. Repeated phencyclidine administration alters glutamate release and decreases GABA markers in the prefrontal cortex of rats. Neuropharmacology. 2012;62:1422–1431. doi: 10.1016/j.neuropharm.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Bell DS. COMPARISON OF AMPHETAMINE PSYCHOSIS AND SCHIZOPHRENIA. Br J Psychiatry. 1965;111:701–707. doi: 10.1192/bjp.111.477.701. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, O'Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. European Journal of Neuroscience. 2008;27:1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: A behavioral and neurochemical analysis. Developmental Brain Research. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Valmaggia L, Stahl D, Byrne M, McGuire P. Disorganization/cognitive and negative symptom dimensions in the at-risk mental state predict subsequent transition to psychosis. Schizophrenia Bulletin. 2012;38:351–359. doi: 10.1093/schbul/sbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Franois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. International Journal of Neuropsychopharmacology. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli PDGSR, et al. Cognitive functioning in prodromal psychosis: A meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hafner H, Riecher-Rossler A, Hambrecht M, Maurer K, Meissner S, Schmidtke A, Fatkenheuer B, Loffler W, Van Der Heiden W. IRAOS: An instrument for the assessment of onset and early course of schizophrenia. Schizophr Res. 1992;6:209–223. doi: 10.1016/0920-9964(92)90004-o. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P. Dopamine synthesis capacity before onset of psychosis: A prospective [ 18F]-DOPA PET imaging study. American Journal of Psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Huppe-Gourgues F, O'Donnell P. D 1-NMDA receptor interactions in the rat nucleus accumbens change during adolescence. Synapse. 2012;66:584–591. doi: 10.1002/syn.21544. [DOI] [PubMed] [Google Scholar]
- Huppe-Gourgues F, O'Donnell P. Periadolescent changes of D 2-AMPA interactions in the rat nucleus accumbens. Synapse. 2012;66:1–8. doi: 10.1002/syn.20976. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Reynolds GP. Effect of subchronic phencyclidine administration on sucrose preference and hippocampal parvalbumin immunoreactivity in the rat. Neuroscience Letters. 2010;471:144–147. doi: 10.1016/j.neulet.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial seperation as an external sign of pubertal development in the male rat. Biology of Reproduction. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res Brain Res Rev. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Reviews Neuroscience. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- Lodge DJ. The medial prefrontal and orbitofrontal cortices differentially regulate dopamine system function. Neuropsychopharmacology. 2011;36:1227–1236. doi: 10.1038/npp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends in Pharmacological Sciences. 2011;32:507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46:89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pK(i) values. Journal of Pharmacology and Experimental Therapeutics. 1989;251:238–246. [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press Australia; 1986. [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. European Journal of Neuroscience. 2006;23:279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Perez SM, Lodge DJ. Aberrant dopamine D2-like receptor function in a rodent model of schizophrenia. J Pharmacol Exp Ther. 2012 doi: 10.1124/jpet.112.193201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solbach S, Celio MR. Ontogeny of the calcium binding protein parvalbumin in the rat nervous system. Anatomy and Embryology. 1991;184:103–124. doi: 10.1007/BF00942742. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: Role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–251. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter Jr JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O'Donnell P. Post-Pubertal Disruption of Medial Prefrontal Cortical Dopamine-Glutamate Interactions in a Developmental Animal Model of Schizophrenia. Biol Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebral Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Cifelli P, Gill KM, Grace AA. Antipsychotic Drugs Rapidly Induce Dopamine Neuron Depolarization Block in a Developmental Rat Model of Schizophrenia. The Journal of Neuroscience. 2011;31:12330–12338. doi: 10.1523/JNEUROSCI.2808-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]