Abstract
Embryonic stem cells (ESCs) have both the ability to self-renew and to differentiate into various cell lineages. Retinoic acid (RA), a metabolite of Vitamin A, has a critical function in initiating lineage differentiation of ESCs through binding to the retinoic acid receptors (RARs). Additionally, the Wnt signaling pathway plays a role in pluripotency and differentiation, depending on the activation status of the canonical and noncanonical pathways. The activation of the canonical Wnt signaling pathway, which requires the nuclear accumulation of β-catenin and its interaction with Tcf1/Lef at Wnt response elements, is involved in ESC stemness maintenance. The noncanonical Wnt signaling pathway, through actions of Tcf3, can antagonize the canonical pathway. We show that RA activates the noncanonical Wnt signaling pathway, while concomitantly inhibiting the canonical pathway. RA increases the expression of ligands and receptors of the noncanonical Wnt pathway (Wnt 5a, 7a, Fzd2 and Fzd6), downstream signaling, and Tcf3 expression. RA reduces the phosphorylated β-catenin level by 4-fold, though total β-catenin levels don't change. We show that RA signaling increases the dissociation of Tcf1 and the association of Tcf3 at promoters of genes that regulate stemness (e.g. NR5A2,Lrh-1) or differentiation (eg. Cyr61, Zic5). Knockdown of Tcf3 increases Lrh-1 transcript levels in mESCs and prevents the RA-associated, ∼4-fold increase in Zic5, indicating that RA requires Tcf3 to effect changes in Zic5 levels. We demonstrate a novel role for RA in altering the activation of these two Wnt signaling pathways and show that Tcf3 mediates some actions of RA during differentiation.
Keywords: Retinoic acid, Canonical Wnt signaling, Noncanonical Wnt signaling, embryonic stem cell differentiation, Transcription factor 7 (Tcf1), Transcription factor 7-like 1 (Tcf3)
Introduction
Embryonic stem cells (ESCs) are pluripotent and self-renewing cells that can differentiate into all three primary germ layers by using both extrinsic and intrinsic factors. Current research involving ESCs has demonstrated their importance in the mechanisms of lineage commitment, disease initiation, and cell therapy (reviewed in [1]). Retinoids, which consist of vitamin A and its derivatives, are signaling molecules that control aspects of embryonic development and cellular differentiation [2]. The biological effects of all-trans-retinoic acid (RA), the major bioactive retinoid, are mediated by its binding to retinoic acid receptors (RARs) and retinoid X receptors (RXRs) that then bind as heterodimers at specific DNA sites, known as retinoic acid response elements (RAREs) [3]. In the nucleus, RA binds to heterodimers of either RARα, β, or ϒ and RXRα, β, or ϒ, which initiates the modification of chromatin structure, changes the status of various epigenetic marks, and alters the regulation of various signaling pathways [3]. Also, RA can mediate its effects through secondary targets, which are activated by RA in an indirect manner. Both primary and secondary response genes can regulate various physiological processes, such as reproduction, embryonic development, epithelial differentiation, tissue repair, and immune function [4]. Additionally, RA has been used extensively for the treatment of various malignancies, such as neuroblastoma, acute promyelocytic leukemia, oral cavity cancer, non-small cell lung cancer, and metastatic breast cancer [5].
In addition to the role that RA and other retinoids have in ESC differentiation, Wnt signaling has been shown to act in concert with retinoid signaling [6]. Wnt ligands and their associated receptors act to regulate several cellular processes, such as embryonic cell differentiation, modification of cellular polarity properties, and determination of cellular fate [7]. However, the role that Wnt signaling has in regulating ESC pluripotency and differentiation is somewhat controversial. Several studies have demonstrated that activation of the Wnt signaling pathway can lead to differentiation [8], whereas other studies show that its activation maintains the pluripotent state [9,10]. In ESCs, Wnt ligands transduce their signaling effects through their corresponding Frizzled (Fzd) receptors via either the canonical (β-catenin dependent) or the noncanonical (β-catenin independent) pathway [11]. The canonical Wnt signaling pathway is activated through its ligands, which include Wnt1, 2, 3a, 8a, 8b, 10a, and 10b and through its receptors Fzd1, 5, 7 or 9 (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/). In the canonical pathway, the absence of a canonical Wnt ligand causes the sequestration of β-catenin by its degradation complex, which consists of adenomatous polyposis coli (APC), axin, disheveled (Dvl), casein kinase 1α (CK1α) and glycogen synthase kinase 3β (GSK3β) [7]. When β-catenin is associated with its degradation complex, β-catenin is initially phosphorylated by CK1α on Ser45, followed by phosphorylation on Ser33, Ser37, and Thr41 by GSK-3β [7]. The phosphorylated β-catenin is then polyubiquitinated by the β-transducin repeat-containing protein (β-TrCP1) complex for proteasomal degradation [7]. In the active state of the canonical pathway, the degradation of β-catenin is suppressed by the binding of a canonical Wnt ligand to a canonical Fzd receptor and a Low-density lipoprotein (LDL) coreceptor. The release of nonphosphorylated β-catenin from the degradation complex promotes the relocalization of the cytoplasmic pool of β-catenin into the nucleus, where it forms a complex with a specific T-cell factor/lymphoid enhancer factor (Tcf/Lef) for transcriptional activation [7]. Canonical Wnt signaling takes place when β-catenin and specific Tcf/Lef induce the transcription of several target genes, such as nuclear receptor subfamily 5, group A (NR5A2; Lrh-1), fibroblast growth factor (FGF20), dickkopf1 (DKK1), Wnt-1 inducible signaling pathway 1 (WISP1), and cyclin D1 (CCND1), reviewed in [12].
More recently, there have been reports demonstrating that the β-catenin-independent or the noncanonical Wnt signaling pathway is involved in many cellular processes, including cellular differentiation [13]. The ligands of the noncanonical Wnt signaling pathway include Wnt4, 5a, 5b, 6, 7a, 7b and 11 and the receptors of this pathway consist of Fzd2, 3, 4 and 6 (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/). The two major noncanonical Wnt signaling pathways are the Wnt/jun N-terminal kinase (JNK) pathway and the Wnt/calcium (Ca2+) pathway, reviewed in [14]. The activation of the Wnt/calcium (Ca2+) pathway ultimately leads to the binding of nuclear factor of activated T cells (NFAT) to specific DNA binding sites [15]. In addition, the induction of CaMKII can initiate the activation of nemo-like kinase (NLK), which has been shown to antagonize the downstream canonical Wnt signaling activities by phosphorylating Tcf1 [15]. Several studies have implicated the interconnectivity of the canonical and noncanonical Wnt signaling pathways in stem cell differentiation [16].
One mechanism by which the interconnectivity of the canonical and noncanonical Wnt signaling pathways may be achieved is that the noncanonical Wnt signaling pathway may function as a negative regulator of the canonical pathway, especially through the Tcf/Lef transcription factors [13]. Although there are several canonical and noncanonical Wnt ligands and Fzd receptors, target gene activation or repression is modulated through only four transcription factors: T-cell factor 1 (Tcf1; Transcription factor 7, Tcf7, NM_201634), Tcf3 (Transcription factor 7-like 1, Tcf7l1, NM_031283), Tcf4 (Transcription factor 7-like 2, Tcf7l2, NM_001198525) and Lymphoid enhancer-binding factor (Lef-1, Transcription factor 7-like 3, Tcf7l3, NM_016269), reviewed in [17]. Several reports suggest that Tcf1, Tcf4, and Lef1 function as transcriptional activators and that Tcf3 functions as a transcriptional repressor [18-22]. Ishitani and colleagues [23] found that upon activation, the noncanonical pathway could repress the canonical pathway through increased phosphorylation of Lef1 on Thr-155/Ser166 residues and of Tcf-4 on Thr-178/Thr-189 residues by NLK. Phosphorylation of Lef1 and Tcf4 prevents the binding of these transcription factors to their Wnt responsive elements (WREs) [23].
Tcf3 is a functional component of the Oct4/Sox2/Nanog circuitry for stem cell self-renewal [24]. Additionally, Chip-seq analyses demonstrated that many Tcf3-bound genes (at least 900 targets) were also bound by Oct4, Sox2 and Nanog [25]. In regard to its transcriptional repressive properties, Tcf3 binding to the promoter of Nanog reduced the transcription the stem cell marker, Nanog [19]. However, the loss of Tcf3 by RNA interference (RNAi) blocks the differentiation of ESCs [26]. These contrasting and somewhat contradictory results suggest that further assessment is needed concerning the roles of Tcf1 and Tcf3 in the activation and repression of specific target genes in ESCs before and during differentiation.
We explored the effects of RA on the canonical and noncanonical Wnt signaling pathways. We found that RA increased the expression of several canonical and noncanonical Wnt ligands and Fzd receptors, culminating in the repression of the downstream canonical Wnt signaling pathway. Additionally, RA treatment increased the expression of Tcf3, which plays a role in reducing downstream signaling of the canonical Wnt pathway in mESCs. Our data will contribute to the understanding of ESC biology, Wnt signaling, and retinoid pharmacology by describing a mechanism that delineates the opposing activities of Tcf1 and Tcf3 in the self- renewal and differentiation of wild type murine ESCs (WT mESCs).
Materials and Methods
Cell culture and chemicals
Wild-type murine ESCs (WT mESCs) and Cyp26a1 knockout (Cyp26a1−/−) mESCs were cultured in media containing 1 × 103 units/ml recombinant murine leukemia inhibitory factor (LIF; Cat. #LIF2010, Millipore, Billerica, MA) on gelatin-coated culture plates, as described in [27]. Cyp26a1−/− mESCs were generated by the disruption of both alleles of Cyp26a1 through homologous recombination, as previously described in [28]. HPLC grade all-trans-retinoic acid (RA; Cat. #R2625, Sigma, Saint Louis, MO) was dissolved in 100% ethanol. To induce differentiation, RA was used at a final concentration of 1 μM by dilution in the culture media and all experiments involving RA were performed under dim light conditions.
RNA isolation, reverse transcription, and QRT-PCR
mESCs (1 × 104) were seeded in 60 mm gelatin-coated plates. The following day, cells were treated with fresh culture media containing RA or vehicle. After 48 hours, mESCs were harvested in TRIzol (Cat. #15596-026, Life Technologies, Norwalk, CT) and total RNA extraction was performed, as described by the manufacturer. QRT-PCR was performed after reverse transcription of isolated total RNA. Primers pairs used for PCR analysis can be found in Supplementary Table 1.
Chromatin immunoprecipition (ChIP) assays
Approximately 1 × 106 WT mESCs and were treated with RA or vehicle. Post 48 hrs, treated cells were subjected to the one-step ChIP protocol, which employs formaldehyde cross-linking, as described in [27]. The following antibodies (2 μg) were used for these assays: rabbit polyclonal anti-Tcf3 (D15G11) antibody (Cat. #2883, Cell Signaling Technologies, Danvers, MA), rabbit polyclonal anti-β-catenin (Cat. #ab6302, Abcam, Cambridge, MA), rabbit polyclonal anti-HA antibody (Cat. #ab9110, Abcam), or secondary rabbit IgG (Cat. #sc-2027, Santa Cruz Biotechnology, Inc., Dallas, TX). The primer pairs used in these assays can be found in Supplementary Table 2.
Immunoprecipitation (IP) and Western blot analyses
WT mESCs were seeded and treated with RA as described above. For the IPs, cell lystates (500 μg) were incubated with either 1 μg rabbit polyclonal anti-β-catenin or secondary rabbit IgG. For Western blotting, the following antibodies were used: mouse monoclonal anti β-actin (1:10,000; Cat. #MAB1501, EMD Millipore, Billerica, MA), rabbit polyclonal anti-β-catenin (1:4000; Cat. #ab6302, Abcam), rabbit polyclonal anti-Tcf3 (described above), and rabbit polyclonal anti-phospho (Ser33/37/Thr41)-β-catenin (1:1000; Cat #9561, Cell Signaling Technology).
Luciferase assays
WT mESCs were transiently cotransfected with 3 μg of TOPFlash [29], FOPflash [29], NFAT luciferase [30] (Cat. #10959, Addgene, Cambridge, MA), or Cyp26a1 luciferase (Cyp26a1-luc) construct with 500 ng pRL Renilla luciferase vector control by using Lipofectamine LTX reagent (Cat.# 15338-100, LifeTechnologies), as described by the manufacturer's protocol. Post-transfection (24 hours), WT mESCs were treated with either RA or vehicle for 24 hours. Luciferase activity was determined as described by the manufacturer's protocol in the Dual-Luciferase Reporter Assay System (Cat. #E1910, Promega, Madison, WI).
Generation of stable Luciferase and Tcf1 cell lines
To generate stable luciferase cell lines, WT mESCs were co-transfected pLKO.1 and either TOPFlash, FOPflash, NFAT-luc [30] or Cyp26a1-luc. Also, the mESC-Tcf1HA cell line was generated by transfecting a HA-tagged Tcf1 cDNA driven by the CMV promoter [31]. Finally, shRNA constructs, cloned into the pLKO.1 puro vector [32], targeting Tcf3 were transduced into mESCs by lentiviral infection.
Statistical analysis
All experiments were conducted in at least three independent biological assays and results, where applicable, are presented as mean±SEM. All statistical analyses were performed using Prism 4.0a (GraphPad, Inc.).
Additional materials and methods
RNA isolation and reverse transcription, the ChIP assays, the IP/Western blotting analyses, and the generation of stable cell lines are further detailed in the Supplementary Materials and Methods.
Results
Retinoic acid (RA) increases the expression of canonical and noncanonical Wnt ligands and Frizzled (Fzd) receptors
To determine if RA treatment could initiate the activation of upstream Wnt signaling events, we assessed the transcript levels of various Wnt ligands and Fzd receptors of the both the canonical and noncanonical Wnt signaling pathways after treating WT mESCs with RA. Interestingly, we found increased expression of both canonical (Figure 1 A) and noncanonical (Figure 1 B) Wnt ligands and Fzd receptors. There was at least a 3-fold increase (as compared to that of untreated mESCs) in the transcript levels of the canonical Wnt ligands (Wnt 2, Wnt 3a and Wnt 8a) as well as the canonical Fzd receptor (Fzd 1) in cells treated with RA for 48 hours (Figure 1 A). After 48 hrs of RA administration we also detected significant fold increases in the transcript levels of the noncanonical Wnt ligands and receptors - Wnt 5a (∼4.5 fold increase), Wnt 7a (∼6.0 fold increase), Fzd 2 (∼3.2 fold increase), and Fzd 6 (∼6.0 fold increase) (Figure 1 B).
Figure 1. Treatment of WT mESCs with RA results in higher transcript levels of canonical and noncanonical Wnt ligands and receptors.
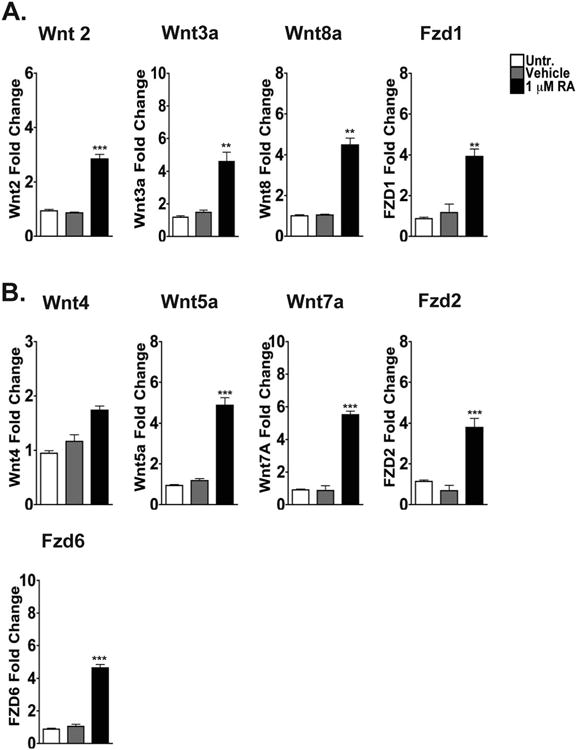
Quantitative Real-Time (QRT) PCR analysis was used to determine the transcript levels of canonical (A) and noncanonical (B) Wnt ligands and Frizzled receptors in WT mESCs cultured as untreated (white bars), with vehicle (grey bars), or with 1 μM RA (black bars) for 48 hours. Bars represent the mean of three independent experiments ± SEM where **, p<0.01 and ***, p<0.001. Note that the y-axes are not the same for all of the graphs.
RA reduces the phosphorylation of β-catenin in a time-dependent manner
Changes in the phosphorylation status of β-catenin have been implicated in stem cell self-renewal and differentiation [9]. WT mESCs treated with RA for 48 hours showed approximately a 50% decrease in the amount of phosphorylated β-catenin compared to that of vehicle-treated WT mESCs (Figure 2 Aiii and 2 Bii). Also, we observed a significant reduction in phosphorylated β-catenin compared to that of vehicle-treated WT mESCs at 24 hr after RA treatment (Figure 2 Aiii and 2 Bii), indicating that the reduced phosphorylation of β-catenin is likely to be a secondary RA response. In contrast, we did not see significant changes in the levels of nonphosphorylated β-catenin (total β-catenin) protein in RA-treated cells (Figure 2 Ai and 2Bi). We conclude that RA reduces (as a secondary response) the phosphorylation of β-catenin, which would lead to the association of β-catenin with transcription factors involved in differentiation, such as Tcf3.
Figure 2. RA treatment reduces the level of phosphorylated β-catenin in WT mESCs.
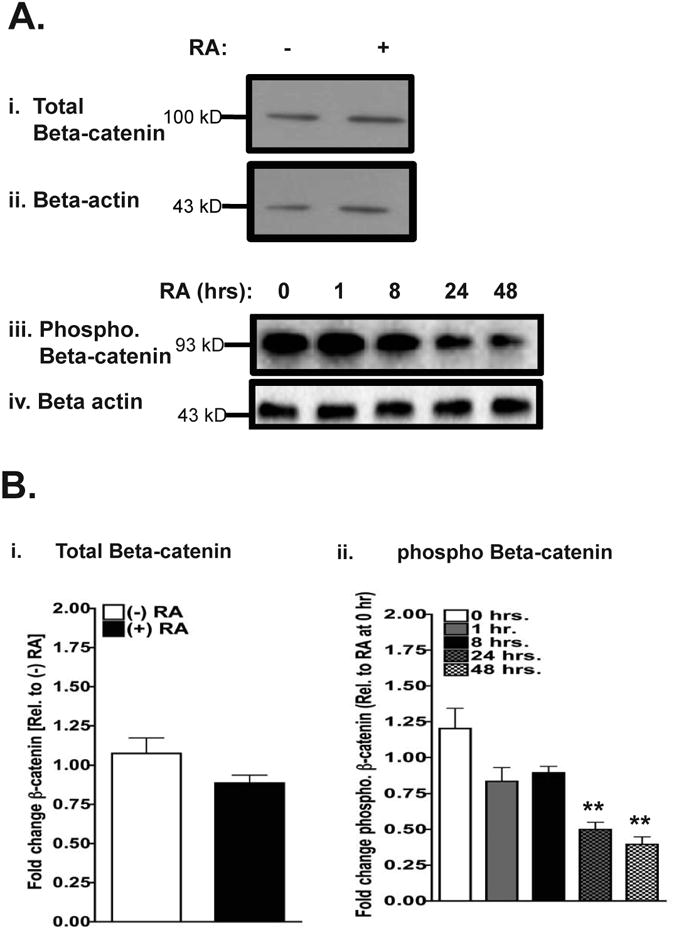
(A) Western blotting of WT mESCs 48 after vehicle or RA addition to determine the levels of total β-catenin (i), and the time course of changes in phosphorylated β-catenin (iii). All β-catenin and phosphorylated β-catenin blots are accompanied with β-actin Western blots, which served as loading controls (ii, and iv). The blots of Ai and ii are representative of three (n=3) separate experiments and the blots of Aiii and iv are representative of five (n=5) separate experiments.
(B) Quantitative analyses of total β-catenin levels after RA addition (i); time-course of changes in the phosphorylated β-catenin level (ii), based on Western blots are shown in (A). Bars represent the mean of three (Bi) or five (Bii) independent, biological repeats ± SEM, where **, p<0.01.
Since there was a significant decrease in the phosphorylated β-catenin levels at 24 hours post-RA addition, we determined possible changes in the transcript levels of the members of the β-catenin degradation complex. At 48 hours post RA addition, we detected no significant changes in the transcript levels of Axin 1, Disheveled 1, and Caesin kinase 1α (Supplementary Fig. 1).
The activities of the canonical and noncanonical Wnt signaling pathways are modified by RA
To determine the potential crosstalk between the canonical and noncanonical Wnt signaling pathways induced by RA, we performed luciferase assays that measure the activities of the canonical (TOPFlash-luciferase) and noncanonical (NFAT-luciferase) Wnt signaling pathways in WT mESCs cultured in the presence of RA or vehicle. The 8× TOPFlash construct has been used extensively to characterize the activation of canonical Wnt signaling downstream events [16,29]. We employed the NFAT-luciferase construct, which contains NFAT binding sites, to determine the activity of the noncanonical Wnt signaling pathway [33]. In vehicle-treated WT mESCs, there was a higher amount of TOPFlash-luciferase activity (∼35 units) compared to that of NFAT-luciferase activity (∼20 units; Figure 3 A). After 24 hours of RA treatment, we detected an 18% decrease in TOPFlash-luciferase activity, compared to the TOPFlash activity in RA (-) cells. Also, we detected a significant increase (65% increase) in NFAT-luciferase activity after RA addition (Figure 3 A).
Figure 3. RA increases downstream noncanonical Wnt signaling activity while decreasing downstream canonical Wnt signaling.
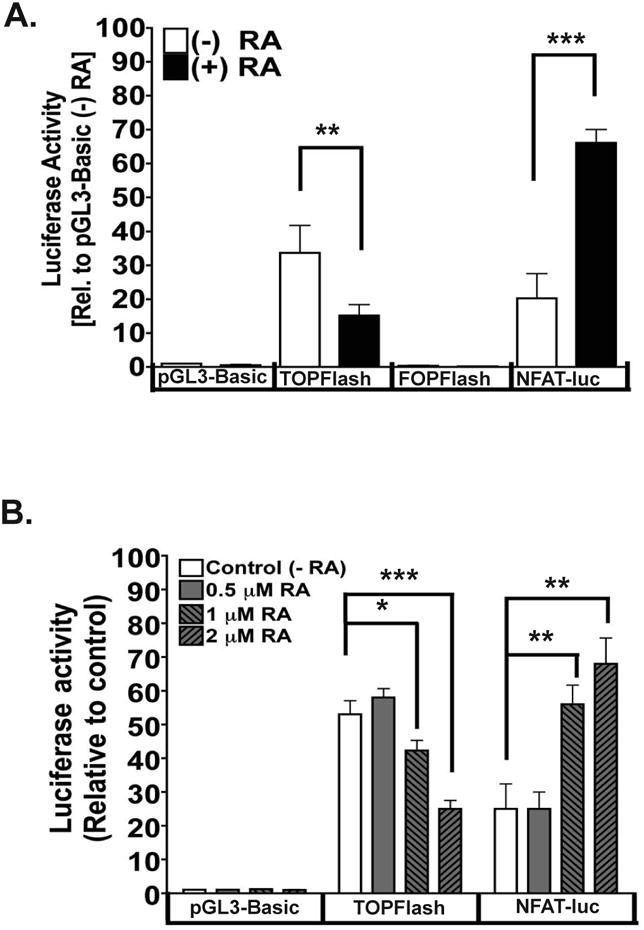
(A) Luciferase assays of WT mESCs transiently transfected with pGL3-Basic, TOPFlash, FOPFlash, or NFAT-luciferase and treated +/− RA for 24 hrs. Data represent three independent experiments in which the relative fold luciferase activity is compared to that of WT mESCs transfected with pGL3-Basic and cultured in the absence of RA.
(B) Luciferase assays of WT mESC lines stably expressing pGL3-Basic, TOPFlash or NFAT-luciferase constructs treated with various concentrations [control (-RA), 0.5 μM, 1 μM or 2 μM] of RA for 48 hrs. Data represent three independent experiments in which the relative fold luciferase activity is compared to that of WT mESCs expressing pGL3-Basic and cultured in the absence of RA (vehicle control). For both (A) and (B) *, **, *** are p< 0.5, p<0.01 and p<0.001, respectively.
Additionally, the effects of RA on downstream Wnt signaling were dose-dependent (Figure 3 B). WT mESCs stably expressing comparable levels of exogenous TOPFlash luciferase, NFAT luciferase or Cyp26a1 luciferase constructs were used for these luciferase assays (Supplementary Fig. 2 A). The Cyp26a1 luciferase construct, which contains two RAREs in the Cyp26a1 promoter, functioned as a positive indicator of induction by RA (Supplementary Fig. 2 B). Here, we found that ESCs treated with RA had increased noncanonical Wnt signaling at the expense of downstream canonical Wnt signaling, suggesting that differentiation of ESCs may in part be dependent on the noncanonical Wnt signaling pathway.
The levels of transcripts of downstream canonical and noncanonical Wnt signaling target genes are changed by RA signaling
Many studies have demonstrated that Tcf1 functions to maintain the activation of the canonical Wnt signaling pathway, while Tcf3 can suppress this pathway [18,19,24,26,34,35]. To delineate further the role of RA on the transcript levels of Wnt signaling downstream targets, we also used Cytochrome P450 hydroxylase A1-null (Cyp26a1−/−) mESCs. Cyp26a1 is responsible for metabolizing RA into its polar metabolites, 4-oxo- and 4-hydroxy-RA [28]. Thus, Cyp26a1-deficient mESCs have higher intracellular concentrations of RA compared to those of their wild-type counterparts [28,36]. We observed a ∼0.5 fold and a ∼0.75 decrease in Tcf1 transcript levels in WT and Cyp26a1−/− mESCs, respectively (Figure 4 Ai). In contrast, we detected at least a 3.5 fold increase in Tcf3 transcript levels in WT and Cyp26a1−/− mESCs after RA administration. Also, the differences in the Tcf3 transcript levels between WT and Cyp26a1−/− mESC were statistically significant (Figure 4 Aii). In line with these data, we found a significant decrease in Liver receptor homolog-1 (Lrh-1; also known as Nuclear receptor subfamily 5, group A, member 2, NR5A2) transcript in both WT and Cyp26a1−/− mESCs; Lrh-1 is a direct target of Tcf1 [24] (Figure 4 Aiii) and Lrh-1 has been implicated in maintaining the pluripotent/stem cell state of mESCs [37]. Also, there were significant increases in the transcripts of Cysteine-rich, angiogenic inducer, 61 (Cyr61; ∼3.8 for WT mESCs and ∼7.7 for Cyp26a1−/− mESCs) and Zinc finger protein family member 5 (Zic5; ∼3.5 for WT mESCs and ∼5.0 Cyp26a1−/− mESCs) after 48 hours of RA treatment (Figure 4 Biv and v). Therefore, RA decreases the transcript levels of the stem cell marker, Lrh-1, and increases the transcript levels of the differentiation markers, Cyr61 and Zic5. These data suggest that RA reduces the downstream signaling events of Tcf1 and enhances those of Tcf3.
Figure 4. The downstream Wnt signaling target genes can be modified by RA.
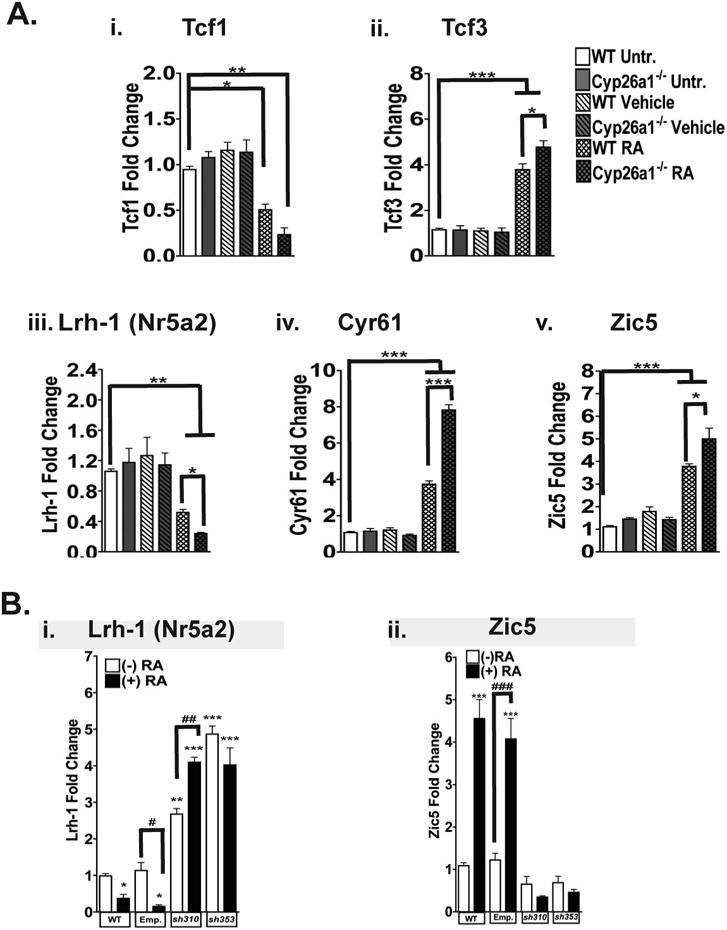
(A) QRT-PCR analysis of the levels of Tcf1 (i), Tcf3 (ii), Lrh-1 (iii), Cyr61 (iv), and Zic5 (v) transcripts in WT mESCs (white bars) and in Cyp26a1−/− (grey bars) mESCs cultured in untreated conditions (nonhatched bars), with vehicle (diagonally-hatched pattern) or with 1 μM RA (cross-hatched pattern) for 48 hours.
(B) QRT-PCR analysis of Lrh-1 (i) and Zic5 (ii) in mESCs expressing shRNA constructs targeted against Tcf3. WT mESCs and mESCs expressing the pKLO.1 vector without an shRNA construct (Emp.) and two shRNA constructs targeting Tcf3 (310 and 353) were treated with vehicle (white bars) and RA (black bars) as described in (A).
Bars, for both A and B, represent the mean of three independent experiments ± SEM where *, p<0.05 and **, p<0.01, and ***, p<0.001. For B, # represents statistical significance between vehicle- and RA-treated mESC lines where ##, p<0.01 and ###, p<0.001. Note that the y-axes are not the same for all of the graphs.
To determine if the expression of downstream Wnt targets could be altered by the combination of RA administration and changes in Tcf3 expression, we generated two mESC lines in which Tcf3 was knocked down by two different shRNA constructs (Supplementary Fig. 3 A and C). Additionally, we found that the shRNA constructs targeting Tcf3 were, in fact, Tcf3-specific because there were no significant changes in Tcf1 expression (Supplementary Fig. 3 B). In mESCs in which Tcf3 expression was ectopically reduced by shRNA (sh310 and sh353), the transcript level of Lrh-1 was increased in both vehicle- and RA-treated mESCs (Figure 4 Bi). Interestingly, in sh310 mESCs we observed a significant increase in the expression of Lrh-1 after RA administration compared to vehicle-treated sh310 mESCs (Figure 4 Bi). In sh310 and sh353 mESCs, we found levels of Zic5 expression comparable to that of WT mESCs (Figure 4 Bii). Also, we did not detect increased Zic5 expression in sh310 and sh353 mESCs after RA administration. For mESCs transduced with the empty pLKO.1 vector (Emp.), the expression of both Lrh-1 and Zic5 was similar to that of the parental mESCs after administration of both vehicle and RA (Figure 4 B).
RA influences the binding properties of the transcription factors, Tcf1 and Tcf3, at their target promoter regions
Chromatin immunoprecipition (ChIP) assays were used to determine how RA alters the levels of Tcf1 and Tcf3 at target promoter regions (Figure 5). At the Lrh-1 promoter, a target of Tcf1 [24], we observed a statistically significant decrease in Tcf1 occupancy in WT mESCs treated with RA (∼0.45 bound DNA/input DNA versus ∼0.25 bound DNA/input; Figure 5 A, left panel). In contrast, we observed an increase in Tcf3 binding to the promoter of Lrh-1 in WT mESCs treated with RA (∼0.2 bound DNA/input DNA versus ∼0.5 bound DNA/input DNA; Figure 5 A, right panel). The data from the ChIP analysis confirm that Tcf3 functions as a transcriptional repressor of Lrh-1.
Figure 5. WT mESCs cultured in the presence of RA show reduced Tcf1 binding and increased Tcf3 binding at target promoter regions.
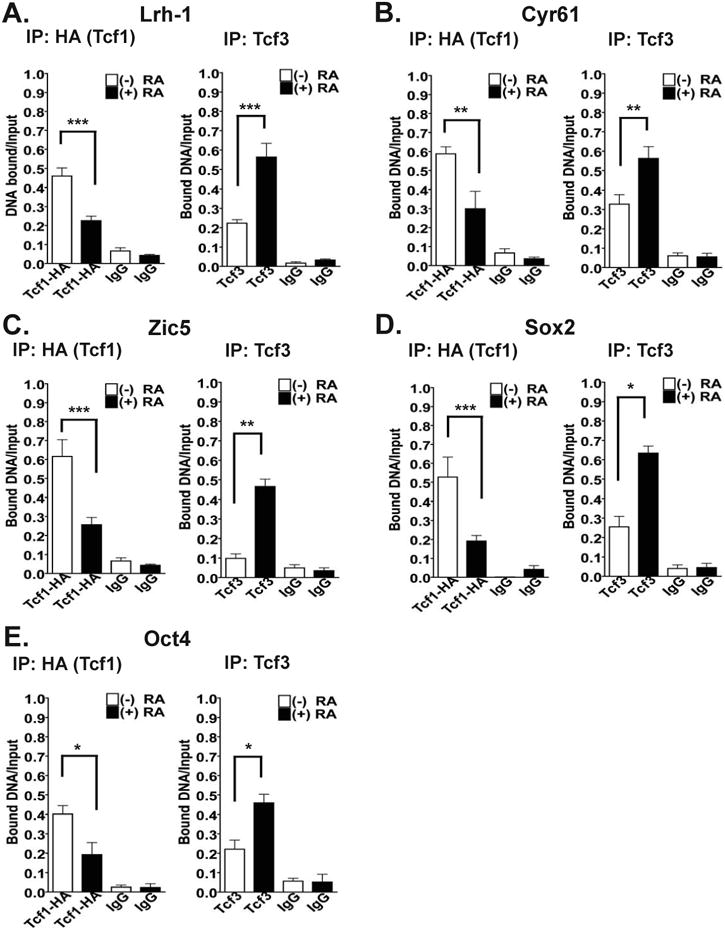
Quantitative ChIP assays for WT mESCs cultured in the absence (white bars) or in the presence (black bars) of RA for 48 hours. Soluble protein/chromatin complexes were immunoprecipated with either an anti-HA antibody, an anti-Tcf3 antibody, or IgG (negative control), and QRT-PCR was performed to amplify promoter regions of Lrh-1 (A), Cyr61 (B), Zic5 (C), Sox2 (D), and Oct4 (E). Each bar represents the ratio of the PCR signal intensities of the antibody- or IgG-bound DNA to that of the DNA input. The data in the bar graphs represent the mean of three independent experiments ± SEM, where *, p<0.05, **, p<0.01 and ***, p<0.001.
At the promoter regions of Cyr61 and Zic5, targets of Tcf3, we detected higher amounts of bound Tcf3 in the (+) RA condition (Figure 5 B and 5C). The ratios of bound DNA to input DNA for the Tcf3 ChIPs were as follows: Cyr61 (∼0.3 bound DNA/input DNA in the absence and ∼0.55 bound DNA/input DNA in the presence of RA) and Zic5 (∼0.1 bound DNA/input DNA in the absence and ∼0.45 bound DNA/input DNA in the presence of RA) (Figure 5B and 5C, right panels). Conversely, the ChIP analyses showed that the level of Tcf1 binding at the promoters of Cyr61 and Zic5 was statistically significantly reduced by ∼50% for Cyr61 and by ∼66.7% for Zic5 by RA treatment (Figure 5 B and C, left panels).
Since it is well established that RA plays a critical role in reducing the pluripotency of mESCs [38,39] and Tcf3 is involved in the Oct4, Sox2 and c-myc stem cell network [24], ChIP analysis was performed to determine if there is a correlation between the reduced expression of these stem cell target genes by RA and promoter occupancy by Tcf3. We found that Sox2 and Oct4 transcripts were reduced in WT mESCs treated with RA (Supplementary Fig. 4). We detected a higher amount of Tcf3 binding at these target gene promoters (Figure 5 D and 5E, right panels). Also, the increased binding of Tcf3 at these promoters was in contrast to reduced Tcf1 binding at the Sox2 and Oct4 promoters (Figure 5 D and 5E, left panels). These data are consistent with the observation that the transcript levels of Sox2 and Oct4 are statistically significantly reduced in WT mESCs that are cultured in the presence of RA for 48 hours (Supplementary Fig. 4). Thus, RA has a role in the stem versus differentiated states of ESCs by modifying the association or dissociation of the transcription factors, Tcf1 and Tcf3, at specific promoter regions (Figure 5).
β-catenin interactions with transcription factors remain constant in (-) RA- and (+) RA-treatment groups
In addition to the association of Tcf1 and Tcf3 at target promoters, we wanted to determine if RA could modify the binding of β-catenin to the target promoters mentioned in Figure 5. Based on ChIP assays, we found no significant changes in the association of β-catenin at the promoters of Lrh-1, Oct4, Cyr61, and Zic5 (Figure 6 A-D). As determined by immunoprecipitation/Western blotting assays, we found that β-catenin interacts with Tcf3 in both the absence and presence of RA (Figure 6 E). Although RA modifies the binding of Tcf1 and Tcf3 transcription factors to target promoters, β-catenin still interacts with these transcription factors in both the presence and absence of RA.
Figure 6. β-catenin remains bound to target promoter regions in both the absence and presence of RA.
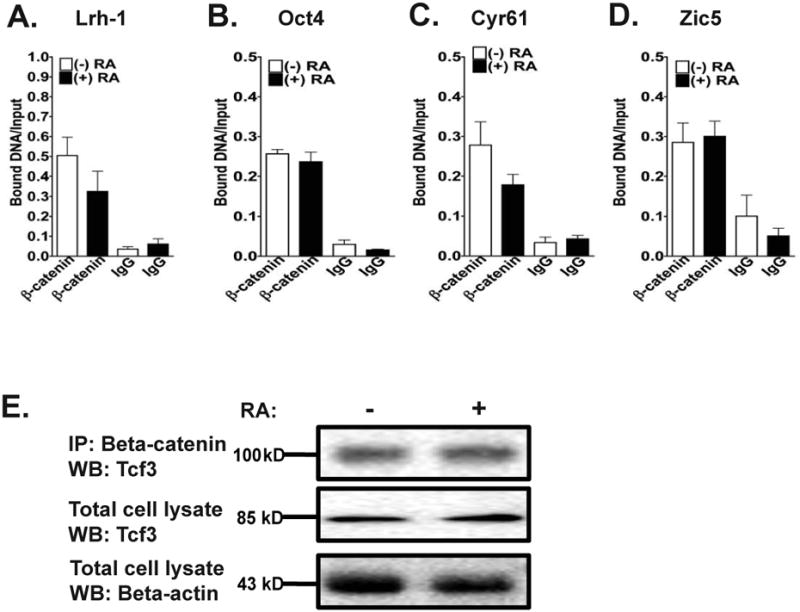
(A-D) Quantitative ChIP assays for WT mESCs cultured in the absence (white bars) or in the presence (black bars) of RA for 48 hours. Protein/chromatin complexes were immunoprecipitated with either an anti-β-catenin antibody or IgG and QRT-PCR was performed to amplify promoter regions of Lrh-1 (A), Oct4 (B), Cyr61 (C) and Zic5 (D). Note that the y-axes are different for the bar graphs.
(E) Immunoprecipitation (IP) assays were performed to determine the interaction of β-catenin and Tcf3. Protein lysates (500 μg) harvested from WT mESCs cultured in the absence or in the presence of RA for 48 hours were immunoprecipitated with an anti-β-catenin antibody and Western blotting was performed using an anti-Tcf3 antibody (top panel). Also, Western blots were performed to determine the expression of Tcf3 (middle panel) and β-actin (bottom panel) in 20 μg of the total cell lysate from both treatment groups. These IPs/Western blots are representative of three independent experiments.
Discussion
RA can induce changes in activation of both the canonical and the noncanonical Wnt signaling pathway in ESCs
Despite the large body of information regarding the role of Wnt signaling in stem cell and cancer biology, there are still many unanswered questions concerning the mechanistic events involved in Wnt signaling and ESC differentiation. The focus of this study was to augment knowledge in this field by investigating the role of retinoic acid (RA) has in the canonical and noncanonical Wnt signaling pathways in ESCs. Here, we found that RA increased the expression of the upstream targets of both the canonical (Wnt ligands, Frizzled receptors, and the reduction of phosphorylated β-catenin) and noncanonical (Wnt ligands and Frizzled receptors) Wnt signaling pathways (Figures 1 and 2).
Although we observed increased expression of upstream targets of both the canonical and noncanonical Wnt upstream signaling events, RA treatment of WT mESCs reduced downstream canonical Wnt signaling events as determined by reduced TOPFlash luciferase activity (Figure 3) and reduced target gene expression after RA treatment (Figure. 4). Conversely, WT mESCs treated with RA exhibited increased NFAT-luciferase activity, a reporter for noncanonical Wnt signaling activation (Figure 3), increased Tcf3 gene expression, and increased Tcf3 target gene transcript levels (Figure 4). Here, we observed that RA could activate one arm of the Wnt signaling pathway (Figure 3) while suppressing the second arm of this pathway. It has been demonstrated that the noncanonical Wnt signaling pathway, at the expense of the canonical Wnt signaling pathway, has the ability to promote murine endothelial cell differentiation [40], and the differentiation of human mesenchymal stem cells into various lineages [41].
RA modifies the expression and promoter binding of the transcription factors, Tcf1 and Tcf3
There is a wealth of information regarding the roles that Tcf1 and Tcf3 play in embryonic stem cell pluripotency/self-renewal and differentiation. The majority of these studies suggest that Tcf3 acts as a transcriptional repressor in murine embryonic stem cells [18,19,26]. Tcf3 has been described as a component of the core regulatory circuitry of ESCs, which also includes Oct4, Nanog, and Sox2 [18,24]. In addition, the transcriptional repressive activities of Tcf3 have been observed during gastrulation in the mouse [21], hair follicle stem cell differentiation in the mouse [21], and neural pattering in zebrafish [42]. Here, we found that RA reduced the expression of the stem cell markers Sox2 and Oct4 (Supplementary Fig. 4), as well as the transcription factor, Lrh-1 (NR5A2) (Figure 4 Aiii). Reduced transcript levels of these genes, after RA treatment, could result in part from increased Tcf3 expression (Fig. 4Aii). Additionally, the effects of RA on the transcript levels of Tcf1, Tcf3, Lrh-1, Cyr61, and Zic5 were enhanced in the Cyp26a1-deficient mESCs compared to WT mESCs (Figure 4 A). The increased expression of Tcf3 (Figure 4 Aii) upon RA addition would increase transcriptional repression by occupying promoter regions of these genes (Lrh-1, Sox2 and Oct4) that are involved in maintaining the self-renewal properties in mESCs (Figure 5). Interestingly, we found that after RA treatment there were: 1) an increase in transcript levels of Zic5 and Cyr61 (Figure 4 iv and v), proteins involved in mediating stem cell differentiation, and 2) an increase in binding of Tcf3 to these promoters (Figure 5).
Here, we examined if Tcf3 possibly could function as a gatekeeper regulating the expression of genes involved in stemness and differentiation. In mESCs transduced with shRNA targeting Tcf3 (Supplementary Fig. 3), we found higher transcript levels for Lrh-1 and lower transcript levels for Zic5, compared to those of WT mESCs and Emp. mESCs (Figure 4 Bi and ii). Also, there was no increase in the transcript level of Zic5 after RA administration. This observation stands in contrast to Zic5 transcripts in WT mESCs after RA administration (Figure 4 Bii). These results suggest that in the absence of Tcf3 there is increased transcription of Lrh-1 (a gene related to stemness) and reduced transcription of Zic5 (a gene related to differentiation). One explanation is that in mESCs with diminished Tcf3 levels there is no competitor for promoter binding of Tcf1 to induce or repress transcription. Similar to these data, in mESCs with ablated Tcf3 Yi and colleagues observed an increase in various stem cell markers such as Nanog, Klf4, Esrrb and Tbx3 [19]. Conversely, they found that in TCF3−/− mESCs markers of differentiation, including Id2, Cyr61, and Krt8, were reduced [19]. This information suggests that proper regulation of both the noncanonical and canonical Wnt signaling paths is critical for maintaining stemness or promoting differentiation.
Interestingly, we found that the association of β-catenin at the promoters of Lrh-1, Oct4, Cyr61, and Zic5 remains relatively unchanged in both (-) RA and (+) RA treatment groups (Fig. 6). This observation of relatively constant β-catenin/target promoter association in (-) RA- versus (+) RA-treatment groups may be explained by the fact that Tcf1, Tcf3, Tcf4, and Lef1 all have the same β-catenin binding domain at their N-termini [43]. Based on these data, β-catenin can associate with either Tcf1 or Tcf3 at target promoters and may not have a direct role in the binding of these transcription factors.
In a model proposed by Cole and colleagues [44], which was based on ChIP-on-chip microarrays, Tcf3 can exist in either an activating or a repressive complex in standard conditions. Thus, Tcf3 can have dual functions: 1) it can repress β-catenin target genes by recruiting specific co-repressor factors; 2) it can function as an activator by recruiting different sets of cofactors [45]. The repressive role (Lrh-1, Fig. 5) and activator role (Zic5 and Cyr61, Figure 5) of Tcf3 may be explained by the fact that many isoforms of the Tcf family are generated by alternative splicing and dual promoter usage [43]. For instance, Tcf3 isoforms that contain LVPQ and SXXSS motifs in their C-terminal context-dependent regulatory domain have been identified as repressors [46]. Also, Wnt target genes are regulated through the binding of Tcf/LEF factors to Wnt responsive elements (WREs) at the consensus sequence CCTTTGWW (W=A/T) via the high mobility group (HMG) box of Tcf/LEF factors [47,48]. Even though the HMG box is highly conserved among the Tcf family members, each member or isoform can have different affinities for specific WREs [49]. A possible hypothesis as to how Tcf3 may function both as a repressor and as an activator is that RA increases the expression of Tcf3 and may also increase the expression of specific isoforms that repress specific WREs, i.e. Lrh-1 (Figs. 4 and 5), and activate other WREs, i.e. Zic5 and Cyr61 (Figures 4 and 5). Further studies will have to be performed to determine if RA can change the expression patterns of the various isoforms of Tcf1 and Tcf3.
The noncanonical Wnt signaling pathway may cooperate with RA-induced ESC differentiation by antagonizing the canonical Wnt signaling pathway
A possible mechanism connecting retinoid signaling to the antagonistic effects of the noncanonical Wnt signaling pathway to that of the canonical pathway in mESCs is that the increased activation of the noncanonical Wnt pathway suppresses downstream events of the canonical pathway. Nemo-like kinase (NLK) and nuclear factor of activated T cells (NFAT) are downstream effectors in the noncanonical Wnt pathway and NLK can phosphorylate Tcf/Lef, prompting Tcf/Lef dissociation from target promoters and allowing increased Tcf3 binding [11]. Potentially, RA contributes to increased Tcf3 binding via activation of the noncanonical Wnt signaling pathway because we demonstrate that RA increases the expression of noncanonical Wnt ligands and Frizzled receptors (Figure 1). A recent study by Hoffman and colleagues found that Tcf3 functions as a transcriptional repressor to prepare mESCs for lineage differentiation [35]. Based on previous work [50], which employed ChIP-on-chip microarrays to determine potential RAREs in promoters in ESCs, we suggest that RA modifies the transcript levels of Tcf1 and Tcf3 as a secondary response because no RAREs were found in the promoters of Tcf1 or Tcf3.
Here, we outline a novel role for RA in stem cell biology through its activation of the noncanonical Wnt signaling pathway, which has been demonstrated to antagonize the canonical Wnt signaling pathway. Here, we used a mechanistic approach to determine the role of RA in Wnt signaling; however, we are currently investigating the biological effects (e.g. modification of cellular fate) that RA and noncanonical Wnt signaling have in ESC differentiation. In the absence of RA, canonical Wnt signaling is activated, which allows the binding of Tcf1 to specific WREs and maintains the expression of markers of stemness (Figure 7 A). RA reduces Tcf1 transcript level and increases Tcf3 transcripts, which promotes the expression of Tcf3-specific targets. Through the activation of the noncanonical Wnt signaling pathway RA increases the binding of Tcf3 to WREs, where Tcf3 functions as a transcriptional repressor or activator (Figure 7 B). These results could help to answer questions regarding the roles of Wnt signaling in regulating the balance between stemness and the differentiation of ESCs. Coordinating and modifying the expression of self-renewal and differentiation target genes in the canonical and noncanonical Wnt signaling pathways by using pharmacological agents, such as RA, could be useful in driving differentiation for therapeutic uses of stem cells.
Figure 7. A schematic describing the actions that RA has on canonical and noncanonical Wnt signaling.
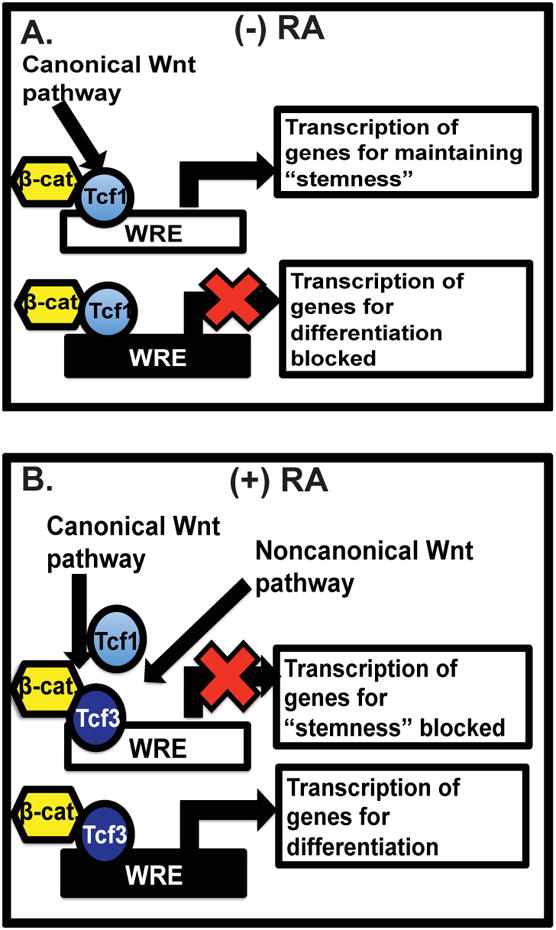
(A) In pluripotent conditions or in the absence of RA, the canonical Wnt signaling pathway activation culminates with Tcf1 and β-catenin binding to selective Wnt response elements (WREs; white boxes) and induction of the transcription of genes necessary for pluripotency/stemness.
(B) In presence of RA, the noncanonical Wnt signaling pathway is activated and its downstream effects cause a reduction in the expression of Tcf1 and in the binding of Tcf1 at specific Tcf1-specific WREs. This reduction in WRE promoter binding, along with increased expression of Tcf3, enhances the ability of Tcf3 to bind Tcf1-selective WREs, reducing the transcription of genes necessary for maintaining the pluripotent/stem cell state. RA allows the binding of Tcf3 to WREs that promote the transcription of specific genes that are expressed in a differentiated state (black box).
Supplementary Material
S1. WT mESCs cultured in RA do not show alterations in the β-catenin destruction complex.
QRT-PCR analysis determined that there were no significant changes in the levels of Axin 1 (A), Dishevelled 1 (B), and Caesin Kinase 1α (C) transcripts in WT mESCs cultured in the absence (white columns) or presence (black columns) of RA for 48 hours. Data are presented as the mean of three independent experiments.
S2. Confirmation of the expression of luciferase constructs in WT mESC stable cell lines.
(A) QRT-PCR analysis shows the expression of WT mESC lines stably expressing TOPFlash (AB1-TF), NFAT-luciferase (AB1-NF), FOPFlash (AB1-FF), and AB1-Cyp26a1 luciferase (AB1-Cypluc). AB1-TF1 line 1, AB1-NF line 2 and AB1-Cypluc line 1 were expanded and used to determine luciferase activities −/+ RA for subsequent experiments shown in Figure 3.
(B) Luciferase activities in WT mESCs transiently expressing the Cyp26a1-luciferase construct, in which the Cyp26a1 promoter drives luciferase activity, cultured in the absence (white columns) or in the presence of various concentrations (0.5 μM, 1 μM and 2 μM) of RA for 48 hours. Data in B are the means of three independent experiments ± SEM where ***, p<0.001. Note that the y-axes are not the same for both graphs.
S3. Generation of shRNA mESC stable lines to ablate the expression of Tcf3.
(A and B) QRT-PCR analyses of the expression of Tcf3 (A) and Tcf1 (B) in WT mESCs (grey bars) and in mESC clones expressing the empty pLKO.1 vector (Emp., horizontal pattern), a scrambled construct (Scr., checkered pattern), and two shRNA constructs targeting Tcf3 [310 (diagonally-hatched pattern) and 353 (cross-hatched pattern)]. Based on the QRT-PCR data, mESC- pLKO 2, Scr. 1, t310 cl4, t310 cl6, t353 cl1, and t353 cl2 were expanded for future experimentation. Bars for both A and B represent the mean of two experiments ± SEM.
(C) Western blotting confirming the knockdown of Tcf3 in shRNA mESC lines. The expression of Tcf3 (top panel) and β-actin (bottom panel) was determined in WT mESCs and mESCs expressing the parental shRNA vector (pLKO.1), a scrambled shRNA construct (Scr.), Tcf3 shRNA construct 1 (t310 clone 4 and clone 6), and Tcf3 shRNA construct 2 (t353 clone 1 and clone 2).
S4. RA promotes a reduction in stem cell state and increased differentiation of WT mESCs.
(A) QRT-PCR analysis demonstrates that RA reduces the expression of Oct4, a marker of pluripotency.
(B) QRT-PCR analysis confirms that RA induces the expression of Hoxa1, a marker of differentiation.
Data in A and B are the means of three independent experiments ± SEM where **, p<0.01 and ***, p<0.001. Note that the y-axes are not the same for both graphs.
Acknowledgments
We thank Drs. Kristian Laursen and Alison Urvalek for discussion regarding data from the ChIP analysis, and the members of the Gudas laboratory for scientific input.
Grant Support: The research was supported by NIH grants NCI R01 CA43796 and NIAAA R01 AA018332 to LJG. For a portion of this work, KOS was supported by NCI T32 CA062948.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors indicate no potential conflicts of interest.
Authors' Contributions: Kwame Osei-Sarfo: Conception and design, data analysis and interpretation, and manuscript writing
Lorraine J. Gudas: Conception and design, data analysis and interpretation, financial support, and manuscript writing
References
- 1.Alvarez CV, Garcia-Lavandeira M, Garcia-Rendueles ME, Diaz-Rodriguez E, Garcia-Rendueles AR, Perez-Romero S, Vila TV, Rodrigues JS, Lear PV, Bravo SB. Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J Mol Endocrinol. 2012;49:R89–111. doi: 10.1530/JME-12-0072. [DOI] [PubMed] [Google Scholar]
- 2.Gudas LJ. Retinoids and vertebrate development. The Journal of biological chemistry. 1994;269:15399–402. [PubMed] [Google Scholar]
- 3.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annual review of pharmacology and toxicology. 2006;46:451–80. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 4.Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Current opinion in cell biology. 1994;6:825–31. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 5.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annual review of pathology. 2011;6:345–64. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 6.Nusse R. Wnt signaling and stem cell control. Cell research. 2008;18:523–7. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- 7.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 8.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 9.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5668–73. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochemical and biophysical research communications. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 11.Katoh M. WNT signaling pathway and stem cell signaling network. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4042–5. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Dai X. A WNTer Revisit: New Faces of {beta}-Catenin and TCFs in Pluripotency. Science signaling. 2011;4:pe41. doi: 10.1126/scisignal.2002436. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends in genetics : TIG. 2000;16:279–83. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 14.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circulation research. 2010;106:1798–806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 15.Ishitani T, Kishida S, Hyodo-Miura J, Ueno N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J, Matsumoto K. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Molecular and cellular biology. 2003;23:131–9. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elizalde C, Campa VM, Caro M, Schlangen K, Aransay AM, Vivanco M, Kypta RM. Distinct roles for Wnt-4 and Wnt-11 during retinoic acid-induced neuronal differentiation. Stem cells. 2011;29:141–53. doi: 10.1002/stem.562. [DOI] [PubMed] [Google Scholar]
- 17.Wray J, Hartmann C. WNTing embryonic stem cells. Trends Cell Biol. 2012;22:159–68. doi: 10.1016/j.tcb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes & development. 2008;22:746–55. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi F, Pereira L, Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem cells. 2008;26:1951–60. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Molecular and cellular biology. 2006;26:7479–91. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–74. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- 22.Hikasa H, Sokol SY. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. The Journal of biological chemistry. 2011;286:12093–100. doi: 10.1074/jbc.M110.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Molecular and cellular biology. 2003;23:1379–89. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nature cell biology. 2011;13:762–70. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, Calabrese JM, Dennis LM, Volkert TL, Gupta S, Love J, Hannett N, Sharp PA, Bartel DP, Jaenisch R, Young RA. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–33. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam WL, Lim CY, Han J, Zhang J, Ang YS, Ng HH, Yang H, Lim B. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem cells. 2008;26:2019–31. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. The Journal of biological chemistry. 2010;285:14534–48. doi: 10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langton S, Gudas LJ. CYP26A1 knockout embryonic stem cells exhibit reduced differentiation and growth arrest in response to retinoic acid. Developmental biology. 2008;315:331–54. doi: 10.1016/j.ydbio.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 29.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Current biology : CB. 2003;13:680–5. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 30.Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. The Journal of biological chemistry. 2001;276:3524–30. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Lee JE, Cho YW, Xiao Y, Jin Q, Liu C, Ge K. UTX regulates mesoderm differentiation of embryonic stem cells independent of H3K27 demethylase activity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15324–9. doi: 10.1073/pnas.1204166109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Fromigue O, Hay E, Barbara A, Marie PJ. Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate. The Journal of biological chemistry. 2010;285:25251–8. doi: 10.1074/jbc.M110.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Developmental cell. 2010;19:521–32. doi: 10.1016/j.devcel.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman JA, Wu CI, Merrill BJ. Tcf7l1 prepares epiblast cells in the gastrulating mouse embryo for lineage specification. Development. 2013;140:1665–75. doi: 10.1242/dev.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricard MJ, Gudas LJ. Cytochrome p450 cyp26a1 alters spinal motor neuron subtype identity in differentiating embryonic stem cells. The Journal of biological chemistry. 2013;288:28801–13. doi: 10.1074/jbc.M113.474254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/beta-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem cells. 2010;28:1794–804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Seminars in cell & developmental biology. 2013 doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. Journal of cellular physiology. 2011;226:322–30. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X, Nakano K, Okada Y, Tanaka Y. Interleukin-1beta induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 2012;64:3355–63. doi: 10.1002/art.34555. [DOI] [PubMed] [Google Scholar]
- 42.Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–6. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao CD, Byers SW. Cell-context dependent TCF/LEF expression and function: alternative tales of repression, de-repression and activation potentials. Crit Rev Eukaryot Gene Expr. 2011;21:207–36. doi: 10.1615/critreveukargeneexpr.v21.i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cole MF, Young RA. Mapping key features of transcriptional regulatory circuitry in embryonic stem cells. Cold Spring Harbor symposia on quantitative biology. 2008;73:183–93. doi: 10.1101/sqb.2008.73.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. Journal of cell science. 2007;120:385–93. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- 46.Pukrop T, Gradl D, Henningfeld KA, Knochel W, Wedlich D, Kuhl M. Identification of two regulatory elements within the high mobility group box transcription factor XTCF-4. The Journal of biological chemistry. 2001;276:8968–78. doi: 10.1074/jbc.M007533200. [DOI] [PubMed] [Google Scholar]
- 47.Giese K, Cox J, Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992;69:185–95. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- 48.van de Wetering M, Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. The EMBO journal. 1992;11:3039–44. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hecht A, Stemmler MP. Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. The Journal of biological chemistry. 2003;278:3776–85. doi: 10.1074/jbc.M210081200. [DOI] [PubMed] [Google Scholar]
- 50.Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B, Davidson I. Cell-specific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Molecular and cellular biology. 2010;30:231–44. doi: 10.1128/MCB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. WT mESCs cultured in RA do not show alterations in the β-catenin destruction complex.
QRT-PCR analysis determined that there were no significant changes in the levels of Axin 1 (A), Dishevelled 1 (B), and Caesin Kinase 1α (C) transcripts in WT mESCs cultured in the absence (white columns) or presence (black columns) of RA for 48 hours. Data are presented as the mean of three independent experiments.
S2. Confirmation of the expression of luciferase constructs in WT mESC stable cell lines.
(A) QRT-PCR analysis shows the expression of WT mESC lines stably expressing TOPFlash (AB1-TF), NFAT-luciferase (AB1-NF), FOPFlash (AB1-FF), and AB1-Cyp26a1 luciferase (AB1-Cypluc). AB1-TF1 line 1, AB1-NF line 2 and AB1-Cypluc line 1 were expanded and used to determine luciferase activities −/+ RA for subsequent experiments shown in Figure 3.
(B) Luciferase activities in WT mESCs transiently expressing the Cyp26a1-luciferase construct, in which the Cyp26a1 promoter drives luciferase activity, cultured in the absence (white columns) or in the presence of various concentrations (0.5 μM, 1 μM and 2 μM) of RA for 48 hours. Data in B are the means of three independent experiments ± SEM where ***, p<0.001. Note that the y-axes are not the same for both graphs.
S3. Generation of shRNA mESC stable lines to ablate the expression of Tcf3.
(A and B) QRT-PCR analyses of the expression of Tcf3 (A) and Tcf1 (B) in WT mESCs (grey bars) and in mESC clones expressing the empty pLKO.1 vector (Emp., horizontal pattern), a scrambled construct (Scr., checkered pattern), and two shRNA constructs targeting Tcf3 [310 (diagonally-hatched pattern) and 353 (cross-hatched pattern)]. Based on the QRT-PCR data, mESC- pLKO 2, Scr. 1, t310 cl4, t310 cl6, t353 cl1, and t353 cl2 were expanded for future experimentation. Bars for both A and B represent the mean of two experiments ± SEM.
(C) Western blotting confirming the knockdown of Tcf3 in shRNA mESC lines. The expression of Tcf3 (top panel) and β-actin (bottom panel) was determined in WT mESCs and mESCs expressing the parental shRNA vector (pLKO.1), a scrambled shRNA construct (Scr.), Tcf3 shRNA construct 1 (t310 clone 4 and clone 6), and Tcf3 shRNA construct 2 (t353 clone 1 and clone 2).
S4. RA promotes a reduction in stem cell state and increased differentiation of WT mESCs.
(A) QRT-PCR analysis demonstrates that RA reduces the expression of Oct4, a marker of pluripotency.
(B) QRT-PCR analysis confirms that RA induces the expression of Hoxa1, a marker of differentiation.
Data in A and B are the means of three independent experiments ± SEM where **, p<0.01 and ***, p<0.001. Note that the y-axes are not the same for both graphs.


