Abstract
The importance of tick defensins is evidenced by their expression in a wide variety of tick tissues and prevalence across many tick genera. To date, the functional and biological significance of defensin-2 as a rickettsiastatic or rickettsiacidal antimicrobial peptide has not been addressed. In a previous study, defensin-2 transcription was shown to increase in Dermacentor variabilis ticks challenged with Rickettsia montanensis. In the present study, the hypothesis that defensin-2 is functional as a rickettsiastatic and/or rickettsiacidal antimicrobial peptide is tested. We show that defensin-2 plays a role in reducing burden after acquisition of Rickettsia montanensis through capillary feeding. Moreover, defensin-2 is shown to associate with R. montanensis in vitro and in vivo, causing cytoplasmic leakiness.
Keywords: tick, defensin, innate immunity, rickettsia.
Introduction
Ticks encounter and harbour many foreign microbes during their lifetime. The tick innate immune system has probably contributed significantly to their success as hosts and vectors. The tick immune system is characterized as having both cell-mediated and humoral defence components (Kopáček et al., 2010). Cell-mediated defence involves phagocytosis and encapsulation of foreign microbes that infiltrate the hemocoel (Eggenberger et al., 1990; Ceraul et al., 2002) The humoral arm of the defence response is driven by soluble antimicrobial peptides (AMPs) that are presumably secreted from the haemocytes and fat body into the hemocoel or from the midgut epithelium. AMPs can attack invading microbes through opsonization, agglutination, and/or lysing (Kopáček et al., 2010).
The tick midgut is the first point of contact for rickettsiae imbibed with a bloodmeal from an infected mammal. As the American dog tick, Dermacentor variabilis, imbibes a rickettsia-infected blood meal, genes encoding a number of AMPs and bacteriostatic factors are activated for transcription and translation, including defensin and D. variabilis kunitz-type serine protease inhibitor (Ceraul et al., 2007, 2011, 2008). Defensins are a ubiquitous class of AMPs that are active against Gram-positive and Gram-negative bacteria, fungi, virus and protozoans (Dugan et al., 2008; Smith & Nemerow, 2008; Kopáček et al., 2010; Tanaka et al., 2012). Tick defensins are 4–6.5 kDa cationic and anionic peptides with a secondary structure comprising α-helical and β-sheet structures that form a tertiary structure by way of three disulphide bonds (Kopáček et al., 2010; Wang & Zhu, 2011). Tick defensins also posses a γ-core within the C-terminus (Wang & Zhu, 2011). The γ-core is a feature common to defensins regardless of species and is responsible for their antimicrobial activities (Yount & Yeaman, 2004; Wang & Zhu, 2011). The cationic charge, amphipathicity and γ-core have been shown to mediate attraction, attachment and membrane insertion that facilitate the toroidal pore, barrel-stave or carpet mechanisms of pore-forming action by AMPs such as defensin (Yount & Yeaman, 2004; Brogden, 2005; Wang & Zhu, 2011).
Ticks are second only to dipterans in their importance as vectors of pathogens in humans and are the number one vector of pathogens of veterinary importance (Parola et al., 2005). Some of the most detrimental human tick-borne pathogens are members of the spotted fever group rickettsiae (SFG). SFG rickettsiae are obligate intracellular bacteria that replicate in the cytoplasm and nucleus of host cells and are transmitted by ticks. Although experimental studies suggest that there are some lethal effects associated with infection in D. variabilis by the aetiological agent of Rocky Mountain spotted fever, Rickettsia rickettsia, it is successfully acquired, maintained and transmitted both horizontally and vertically in nature (Niebylski et al., 1999). These observations suggest that even if ticks suffer long-term morbidity as a result of infection, they can control rickettsial infections long enough to transmit the pathogens, thereby perpetuating the zoonotic cycle.
Previous studies show that challenge of D. variabilis with SFG R. montanensis results in activation of defensin-1 and -2 and lysozyme (Ceraul et al., 2007). We hypothesize that defensin-2 is functionally active against R. montanensis. Experiments demonstrate that D. variabilis defensin-2 associates with and causes cytoplasmic leakage of R. montanensis. The present results suggest that defensin-2 function is one factor that limits R. montanensis infection of the tick.
Results
Defensin limits R. montanensis infection in vitro and in vivo
Purified R. montanensis (1 × 107), incubated with recombinant defensin, thioredoxin or phosphate-buffered saline (PBS), were placed on L929 cells, allowed to infect, and collected 24 h later. Rickettsial burden was assessed with quantitative reverse transcriptase-PCR (qRT-PCR). There was no significant difference between the two control treatments. The average burden in the recombinant defensin-2-treated R. montanensis decreased by 86% when compared with the PBS-treated rickettsia (P < 0.017) (Fig. 1A). To lend biological significance to our in vitro results, a defensin-2 neutralization experiment was performed. D. variabilis ticks were capillary-fed anti-defensin-2 IgG at increasing concentrations or preimmune IgG at 2 mg/ml, incubated for 1 h, and fed 2.4 × 105 total R. montanensis. Whole tissues were harvested 24 h post infection for RNA isolation. Rickettsial burden rose in direct proportion to the increasing concentrations of anti-defensin-2 IgG. Rickettsial burden in ticks fed 1.0 mg/ml and 2.0 mg/ml anti-defensin-2 IgG increased significantly more than that observed in the ticks treated with 2 mg/ml preimmune IgG (P = 0.004 and P = 0.043, respectively) (Fig. 1B). Collectively, the in vivo and in vitro burden assays demonstrate that the control of R. montanensis infection seen is specific to the defensin-2 response. Interestingly, the in vivo and in vitro burden assays, respectively, demonstrate that the growth-limiting effects of defensin-2 for R. montanensis are specific.
Figure 1.
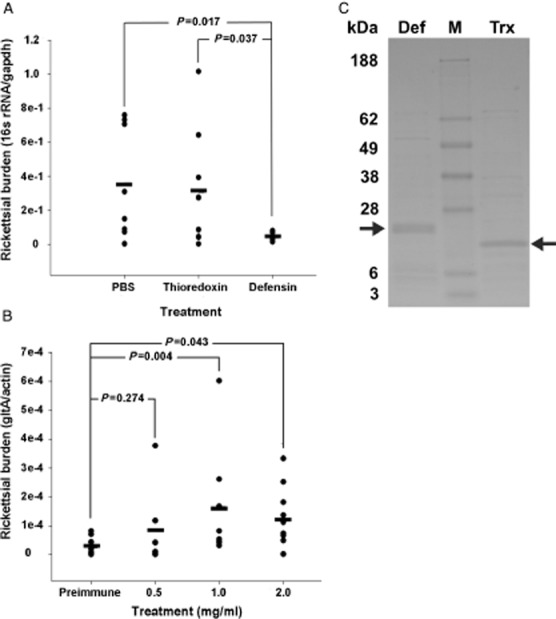
Defensin-2 limits Rickettsia montanensis infection in vitro and in vivo. (A) R. montanensis were incubated with recombinant defensin-2, phosphate-buffered saline (PBS) or recombinant thioredoxin, and then used to infect an L929 monolayer for 24 h. Rickettsial burden in the infected L929 cells was determined using quantitative reverse transcriptase-PCR (qRT-PCR). The bar represents the average for each treatment. A total of four separate experiments were performed in duplicate (two wells per treatment). The number of samples analysed per treatment are as follows: untreated, n = 8; thioredoxin, n = 8; defensin-2, n = 7. (B) Part-fed Dermacentor variabilis ticks were capillary-fed anti-defensin-2 IgG at increasing concentrations followed by an oral R. montanensis challenge. Whole tissue was dissected and rickettsial burden determined using qRT-PCR. Each individual tick is represented by a closed circle. The mean for each treatment is represented by a bar. Values represent the transcript abundance measured in the total number of ticks collected from at least two separate experiments. P values were determined using the t-test. Biological replicates are as follows: preimmune, n = 10; 0.5 mg/ml, n = 8; 1 mg/ml, n = 8; 2 mg/ml, 10. (C) Representative Imperial blue stained lithium dodecyl sulfate (LDS) polyacrylamide gel electrophoresis of recombinant defensin-2 (Def) and thioredoxin (Trx) purification. Defensin-2 migrated to ∼28 kDa due to the thioredoxin and histidine fusion tags. M, molecular weight marker. Arrows indicate purified recombinant defensin-2 and thioredoxin.
Incubation of R. montanensis with defensin causes cytoplasmic leakage
The most recognized function of defensins is pore formation. To address this, an in vitro lysis assay was performed and the data correlated to the live count data from the same experiments. An Imperial Blue-stained polyacrylamide gel indicated that the recombinant defensin-2 and thioredoxin proteins were relatively pure (Fig. 1C). Purified R. montanensis were incubated with defensin-2 or thioredoxin with agitation and the mixtures were separated through high-speed centrifugation into pellet and supernatant fractions. In addition, the lysis assay was performed using a synthetic defensin-2 peptide in increasing concentrations. The number of rickettsia/mm3 were counted using a small aliquot from each rickettsia sample incubated with the synthetic peptide. The same samples were used to blot for cytoplasmic leakage to correlate lysis with a decrease in live rickettsia and increasing concentrations of synthetic peptide. We theorized that perforations in the cytoplasmic membrane of R. montanensis as a result of defensin-2 treatment would cause the leakage of a cytoplasmic protein elongation factor-thermo stable (EF-Ts) into the supernatant.
Rickettsia montanensis EF-Ts was first expressed in Escherichia coli to determine the level of cross-reactivity of our antiserum generated against Rickettsia typhi cytoplasmic EF-Ts (Fig. 2A). A higher weight molecular host protein is known to cross-react with our EF-Ts antiserum. The higher molecular weight band was shown previously to be of host origin (Kaur et al., 2012). To support these previous observations, a lysate sample from uninfected L929 cells was subjected to Western blotting to demonstrate that EF-Ts antiserum cross-reacts with a host protein that migrates around 40 kDa (Fig. 2B). Thioredoxin-treated R. montanensis showed a strong EF-Ts band for the pellet, but not for the supernatant, while recombinant defensin-2-treated R. montanensis showed an EF-Ts band in both the pellet and supernatant lanes (Fig. 2C). These results are consistent between the two independent experiments performed. Similar results were documented for synthetic peptide-treated R. montanensis (Fig. 2D). The samples shown in Fig. 2D were subjected to longer electrophoretic runs to attain better separation between the host protein and rickettsial EF-Ts, which accounts for EF-Ts migration to ∼35 kDa.
Figure 2.
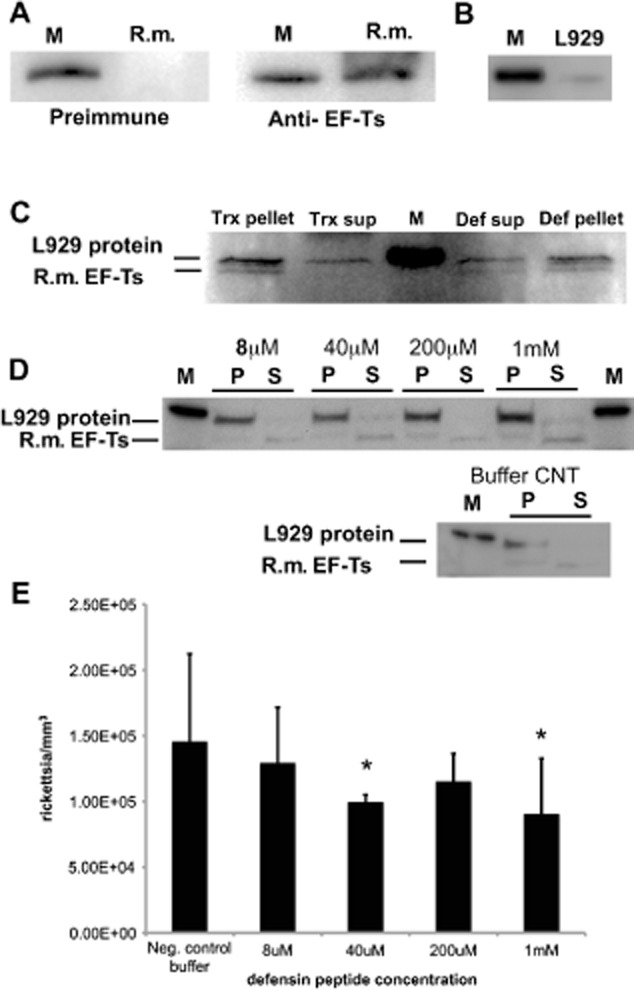
Defensin-2 causes cytoplasmic leakage in Rickettsia montanensis. R. montanensis were incubated with recombinant defensin-2 or thioredoxin (Trx) followed by centrifugation to separate the pellet and supernatant fractions. Alternatively, R. montanensis were treated with synthetic defensin-2 or diluent buffer (buffer control) followed by centrifugation to separate the pellet and supernatant fractions. Each fraction was subjected to lithium dodecyl sulfate (LDS) polyacrylamide gel electrophoresis, blotted to a polyvinylidene difluoride membrane, and probed with anti- elongation factor-thermo stable (EF-Ts) serum. (A) Lysate from Escherichia coli expressing recombinant EF-Ts from R. montanensis was detected by EF-Ts antiserum but not rabbit preimmune serum. Recombinant R. montanensis EF-Ts migrated to 40 kDa due to the histidine tag. (B) Nonspecific band detected by EF-Ts antiserum in uninfected L929 lysate. (C) R. montanensis EF-Ts was detected in the pellet from Trx-treated, as well as in the supernatant and pellet, from defensin-2-treated R. montanensis. EF-Ts was not observed in supernatant from Trx-treated R. montanensis. The nonspecific L929 and R. montanensis EF-Ts (35–40 kDa) proteins are indicated. (D) Pellet and supernatant fractions from R. montanensis treated with increasing concentrations of synthetic defensin-2 peptide or diluent buffer (Buffer CNT). R. montanensis EF-Ts ran closer to 35 kDa because of an increased running time on the polyacrylamide gels to ensure adequate separation from the nonspecific L929 protein that cross-reacts with EF-Ts antiserum. (E) Decrease in the number of live rickettsia/mm3 with increasing concentrations of synthetic defensin-2 peptide. Live counts were performed on the same samples used to generate the blots demonstrating lysis to correlate lysis with the decrease in live counts. A t-test was performed to test for statistical differences between the means for treatment with increasing concentrations of synthetic defensin-2 and the negative control buffer. P values for each synthetic defensin-2 concentration were 8 μM: P = 0.21; 40 μM: P = 0.027; 200 μM: P = 0.09; 1 mM: P = 0.018. Asterisks indicate significant differences from negative control buffer. M, molecular weight marker migrating to 40 kDa; R.m., R. montanensis; Def, defensin; sup, supernatant; CNT, control.
The number of live rickettsia in the samples used to demonstrate lysis post-treatment with synthetic defensin was estimated using a Live/Dead BacLight Viability kit (Life Technologies, Grand Island, NY, USA). The BacLight assay allows us to estimate the number of live rickettsia and correlate this number to the appearance of EF-Ts in the supernatant fractions from the lysis assay (cytoplasmic leakage). The numbers of live R. montanensis decrease with increasing concentrations of synthetic defensin-2 peptide tested (Fig. 2E). We noted that defensin-2, made either as a recombinant or synthetic peptide, showed the same trend. This indicates that our results are not caused by contaminants in the recombinant defensin-2 preparation or by an artifact of the synthetic peptide manufacturing process. Collectively, these results demonstrate that defensin-2 treatment causes leakage of cytoplasmic proteins from R. montanensis, which ultimately leads to bacterial death.
Defensin-2 associates with R. montanensis in vitro and in vivo
Rickettsia montanensis (1 × 107) were incubated with recombinant defensin-2 for 30 min and then affixed to nickel grids. Rickettsiae were incubated with rabbit defensin-2 antiserum followed by incubation with 10 nm gold particle-conjugated anti-rabbit IgG. Grids were visualized with transmission electron microscopy (TEM) to determine if defensin-2 associates with the rickettsia (Fig. 3). The immunogold staining observed in the defensin-2 treated, preimmune probed (Fig. 3A) control is random and unorganized. Surface adhesion of the gold particles suggests that defensin-2 associates with the outer membrane of R. montanensis in vitro (Fig. 3B).
Figure 3.
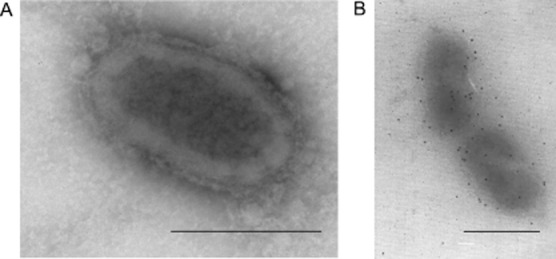
Defensin-2 associates with Rickettsia montanensis in vitro. Purified R. montanensis were incubated with recombinant defensin-2 and processed for negative staining electron microscopy. Rickettsia specimens incubated with preimmune serum (A) or defensin antiserum (B) Bars equal 5 μm.
In vivo staining for defensin-2 and R. montanensis was performed to determine if staining colocalizes to the same area in an attempt to lend biological significance to our in vitro findings. Infected D. variabilis midguts were sectioned on a cryo-microtome and incubated with rabbit defensin-2 and mouse R. montanensis antisera followed by incubations with Alexa Fluor 488 (defensin) anti-rabbit and Alexa Fluor 594 (rickettsia) anti-mouse sera. Sections were treated with SYTOX Blue nucleic acid stain and visualized on a Zeiss LSM 510 confocal microscope. In order for defensin-2 to be functional it needs to associate with the rickettsia. Defensin-2 staining appears to be localized with R. montanensis signal (Fig. 4A). We observe diffuse staining in sections from R. montanensis-infected tick midguts probed with normal rabbit and mouse control sera (Fig. 4 B). R. montanensis staining in control sera probed sections is absent (Fig. 4B).
Figure 4.
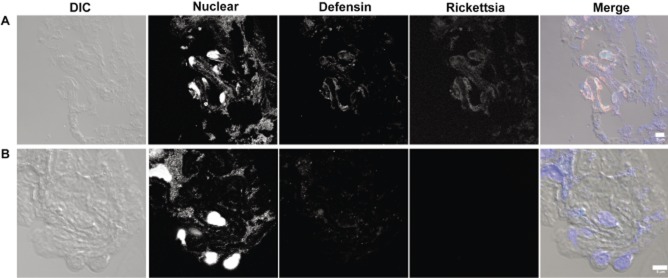
Defensin-2 associates with Rickettsia montanensis in vivo. (A) R. montanensis-infected tick midgut incubated with immune sera. (B) R. montanensis-infected tick midgut incubated with control sera. R. montanensis-infected Dermacentor variabilis midguts 48 h post-infection were probed with rabbit defensin and mouse R. montanensis antisera or rabbit and mouse preimmune serum. Rabbit and mouse antibodies were detected with anti-rabbit Alexa-488 (defensin) or anti-mouse Alexa-594 (rickettsia), respectively. SYTOX Blue was used to visualize the nucleus. DIC, differential interference contrast. Bars represent 5 μm.
Discussion
Tick defensins belong to a class known as ancient invertebrate-type defensins that are found in insects, arachnids, bivalvia and fungi (Wang & Zhu, 2011). Defensins from several genera possess antimicrobial activity for Gram-positive and Gram-negative bacteria, protozoa and fungi (Tsuji et al., 2007; Lu et al., 2009; Chrudimska et al., 2011; Wang & Zhu, 2011). In the present study, we showed that defensin-2 associates with R. montanensis, causing cytoplasmic leakage. We speculated that these antimicrobial properties of defensin-2 were responsible, in part, for the antimicrobial effect observed in vitro and in vivo.
Although cationic charge is one property that mediates the association between the bacterium and defensin (Ganz, 2003; Brogden, 2005), defensin-2, a weak anionic peptide (pI = 5.14; http://web.expasy.org/cgi-bin/compute_pi/pi_tool) associates with rickettsia. The present study does not explore the mechanism underlying defensin-2 association with rickettsia. Our findings present the possibility that defensin-2 interacts with ligands alternative to lipopolysaccharide (LPS) or that the LPS structure of R. montanensis is different from reported Gram-negative bacteria with respect to charge and facilitates the interaction with a weak anionic protein; however, charge does not appear to be the sole factor dictating the functional capacity of AMPs. Both cationic and anionic derivatives of dermcidin-derived peptides from eccrine sweat glands in humans possess broad range antimicrobial activity (Steffen et al., 2006). Interestingly, Steffen, et al. (2006) demonstrate that the dermcidin derivative, DCL-1 (pI = 5.07) requires 2 h before E. coli viability decreases 100% compared with the positive control peptide LL-37, which attains 100% killing in minutes. In designing experiments for the present study, we considered the effects that extended extracellular periods of time have on the viability of an obligate intracellular bacterium; however, it is feasible that anionic peptides function at slower rates and that greater loss in rickettsial viability will be attained when exposed to defensin-2 for a time period exceeding 30 min.
After the initial attraction and attachment, AMPs are theorized to assume their folded secondary structure (Matsuzaki, 1999; Marcellini et al., 2009; Rahman et al., 2009; Manzo et al., 2012). A transmembrane pore is thought to form through three mechanisms and appears to be peptide concentration-dependent. All mechanisms are initiated with a parallel arrangement of the AMP at the surface of the phospholipid membrane. The barrel-stave model dictates that high peptide:lipid ratios encourage insertion of AMP into the membrane (Brogden, 2005). It is thought that the carpet mechanism works through peptide-mediated formation of micelles and that the torodial pore model is a function of peptide-induced membrane bending followed by pore formation (Brogden, 2005). Clues about the mechanism driving pore formation by defensin-2 (anionic) may come from data for the anionic peptide DCL-1 (net charge of-2). Recent data suggest that DCL-1 folds into an α-helical structure in the presence of bacterial membranes, self-associates in a time-dependent manner, and may form pores through the toroidal pore mechanism (Paulmann et al., 2012). This series of events is also documented for a defensin-like peptide, longicin, from the hard tick, Haemaphysalis longicornis. Similar to DCL-1, longicin remains unstructured in aqueous solution but assumes a secondary structure in membrane mimetic conditions (Rahman et al., 2009).
Our in vitro and in vivo antimicrobial assays lend support to the biological significance of defensin-2 as a rickettsiastatic or rickettsiacidal protein. We do not address defensin-2 function beyond its ability to limit rickettsial infection; however, the findings have implications for the role of defensin-2 in maintaining homeostatic commensal microflora populations. In Drosophila, antimicrobial bacteriostatic/-cidal peptides play critical roles in maintaining homeostasis with microflora as well as in fighting microbes that may harm the individual (Ryu et al., 2010). Endosymbionts and commensal microflora may be maintained at low levels in specific tissues to avoid detrimental physiological effects to the host (Login et al., 2011; Paredes et al., 2011).
It is unknown if activation of the midgut (local response) translates to a systemic response in the hemocoel or how this may impact colonization of hemocoelic tissues, such as the salivary glands and ovaries. In Drosophila, peptidoglycan recognition protein-LB, is responsible for controlling midgut immune activation and participates in activation of the systemic response after an oral challenge with the Gram-negative bacterium Erwinia carotovora carotovar-15 (Paredes et al., 2011). Considering this, midgut infection with rickettsia may prime hemocoelic immune effectors for an impending rickettsial invasion. In doing so, AMPs such as defensin-2 will limit transmigration of rickettsiae to the hemocoel, ovaries and salivary glands, resulting in less infection, which could have implications for transovarial or horizontal maintenance.
While the present study does not address the physiochemical mechanism of pore formation for defensin-2, we have correlated loss of rickettsial viability with defensin-2 induced membrane damage that results in the leakage of cytoplasmic contents. Future experiments will address the biochemical properties of defensin-2 as it relates to its antimicrobial function.
Experimental procedures
Rickettsia and host cells
Mouse fibroblast cells (L929, ATCC® CCL-1) were cultured in DMEM supplemented with 10% FBS at 5% CO2 at 34 °C. Host cells were inoculated with R. montanensis at 80% confluency. Infected host cells were grown for 5–6 days. R. montanensis were purified from host cells and enumerated as described (Ceraul et al., 2008).
Synthetic defensin-2 peptide
Six milligrams of synthetic defensin-2 (GenBank accession number: AAO18363.1) peptide was synthesized by Peptide 2.0, Inc. (Chantilly, VA, USA) at >96% purity. The signal sequence was identified using SignalP (http://www.cbs.dtu.dk/services/SignalP/) and excluded from synthesis. The final peptide sequence is NH2-TGERSEERSEEARASGCKADACKSYCKSLGSGGGYCDQGTWCVCN-COOH. The peptide was resuspended to 5 mM in acetonitrile:water (1:3) with 1% dimethylformamide (DMF) and 1% dimethyl sulphoxide (DMSO). Rabbit anti-defensin-2 polyclonal serum was generated against a synthetic peptide with the sequence NH2-EEARASGCKADACK-OH by Biosynthesis, Inc. (Lewisville, TX, USA). Biosynthesis, Inc. also provided the rabbit preimmune serum used in these experiments.
Expression and purification of recombinant defensin-2
The open reading frame for tick defensin-2 (AAO18363) was amplified as previously described (Ceraul et al., 2007) using primers AZ3519 (5′-CTTTGCATCTGCCTTGTCTTTCTC-3′) and AZ3518 (5′-AATTCCTGTAGCAGGTGCAGG-3′) and Hi-Fidelity Polymerase (Life Technologies, Grand Island, NY, USA), ligated to pET32a+ (Novagen, Darmstadt, Germany) and transformed into either Origami or RosettaGami (both Novagen) E. coli strains. pET32+a was also transformed for expression of thioredoxin as a control protein. Thioredoxin was expressed and purified simultaneously with defensin-2. All procedures were performed according to the respective manufacturers' protocols. Expression of recombinant defensin-2 was induced using either isopropyl β-D-1-thiogalactopyranoside (IPTG) or auto-induction medium composed of ZY broth supplemented with NPS (20 mM PO4, 1.25 mM SO4, 2.5 mM NH4, 20 mM Na, and 2.5 mM K), 5052 (0.5 g glycerol, 0.5 g glucose, and 0.2 g lactose) and 100 μg/ml ampicillin. Overnight cultures of transformed E. coli were diluted 50-fold in Luria–Bertani (LB) broth supplemented with 100 μg/ml ampicillin, grown to an optical density (OD)600nm of 0.5 and either supplemented with 1 mM IPTG or diluted fivefold into auto-induction medium. E. coli induced with IPTG was grown at 37 °C for 3–4 h, centrifuged at top speed for 10 min at 4 °C and the pellet stored at −80 °C until lysed for purification. E. coli diluted in auto-induction medium was grown at 28–30 °C overnight and the pellets collected and stored as described above. E. coli pellets were resuspended in 10 ml of NPI-10 (50 mM NaH2PO4●H2O, 300 mM NaCl, 10 mM imidazole) and lysed by pressure using a French press (ThermoFisher Scientific, Waltham, MA, USA) or LV1 low volume microfluidizer (Microfluidics, Newton, MA, USA). Recombinant defensin-2 was purified using Ni-NTA agarose magnetic beads or Superflow columns according to the manufacturer's protocol (Qiagen, Valencia, CA, USA). Each sample was electrophoresed on a 4–20% Bis-Tris gel (Life Technologies) and stained with Imperial Blue (ThermoFisher Scientific) to assess purity of each sample.
Antibacterial assay
Separate aliquots of R. montanensis (1 × 107), designated for the lysis and antibacterial assays, respectively, were incubated with 5 μg of recombinant defensin-2 or thioredoxin in a 30 μl volume of PBS for 30 min at 30 °C with shaking. R. montanensis was pelleted by centrifugation at top speed for 5 min. Thioredoxin and recombinant defensin-2-treated R. montanensis were washed with PBS (1X) and placed onto L929 host cells and allowed to incubate at 37 °C/5% CO2 for 24 h. Infected cells were washed with PBS three times and processed for RNA isolation. Rickettsial burden was determined using qRT-PCR by normalizing rickettsial 16 s rRNA to host cell gapdh transcript (Ceraul et al., 2008). 16 s rRNA was amplified using AZ4915 (5′-GTTCGGAATTACTGGGCGTA-3′) and AZ4916 (5′-AATTAAACCGCATGCTCCAC-3′). gapdh was amplified using AZ3674 (5′-TCAACGACCCCTTCATTGAC-3′) and AZ3675 (5′-ATGCAGGGATGATGTTCTGG-3′).
Lysis assay
Separate aliquots of R. montanensis (1 × 107) were prepared as described for the antibacterial assay above. Alternatively, R. montanensis was treated with 8 μM-1 mM synthetic defensin-2 or 0.9% NaCl buffer supplemented with the same volume of synthetic defensin diluent buffer (acetonitrile (1:3 ACN:water):1% DMF:1% DMSO) as a negative control buffer. The concentrations (8 μM–1 mM) were obtained using serial dilution. The negative control buffer was diluted equivalently to the highest peptide concentration of 1 mM. R. montanensis was incubated with defensin or the negative control buffer for 30 min at room temperature. R. montanensis were pelleted by centrifugation at 16 000 × g for 5 min. The R. montanensis pellet was separated from the supernatant and both were resuspended in lithium dodecyl sulfate (LDS) (1X) sample buffer with reducing agent (1X). Samples were heated to 70 °C for 10 min and separated on a 4–20% Bis-Tris gel (Life Technologies) and transferred to a polyvinylidene difluoride (PVDF) membrane using an iBlot semi-dry transfer apparatus (Life Technologies). Membranes were probed with preimmune or immune sera directed to R. typhi cytolasmic protein EF-Ts generated by Primm Biotechnologies (Cambridge, MA, USA). To determine if the R. typhi anti-EF-Ts serum recognized EF-Ts from R. montanensis, the open reading frame for EF-Ts from R. montanensis was cloned into pET101D (Life Technologies) and expressed from BL-21 Star E. coli (Life Technologies) using IPTG induction. Pellets from 2–4-h cultures were resuspended in LDS buffer, heated to 70 °C for 10 min and separated on 4–20% Bis-Tris gels and transferred to PVDF membranes. Blots were probed with EF-Ts anti-serum to confirm serum cross-reactivity.
Live counts
A 10-μl aliquot was removed from rickettsia incubated with 8 μM-1 mM synthetic defensin-2. Rickettsia were centrifuged at 16 000 × g for 5 min at 4 °C, the supernatant removed and the pellet resuspended in 100 μl of 0.9% NaCl. A 200-fold dilution was performed in 0.9% NaCl and the sample was counted on an iN CYTO C-Chip Improved Neubauer haemocytometer (Chungcheongnam-do, Korea) using a Nikon Eclipse E600 (Melville, NY, USA). Each experiment was performed in duplicate and a total of four experiments were completed. Values are reported as mean ± (sd) values. Statistical analysis was performed as described below.
Defensin-2-R. montanensis association assay negative staining electron microscopy of defensin-2 on rickettsia
Purified R. montanensis (1 × 107) were washed twice with PBS, resuspended in PBS and 5–10 μg of recombinant defensin-2 or thioredoxin, and incubated at 30 °C for 30 min with shaking. After incubation, R. montanensis were washed with PBS and fixed with 4F1G fixative (4% formaldehyde, 1% gluteraldehyde in 0.5 M NaH2 PO4 Buffer-pH 7.2). After 1-h fixation on ice, cells were washed and resuspended in HEPES buffer. Following resuspension, 10 μl of R. montanensis were spotted on nickel grids and allowed to air dry before an additional 10 μl were spotted onto the grids. A total of 1 × 107 rickettsia were spotted onto each grid. Grids were blocked with 5% bovine serum albumin + 0.1% Cold Water Fish Skin gelatin for 15 min at room temperature and incubated with the rabbit anti-defensin-2 primary antibody at room temperature (1:20) overnight. Rabbit preimmune serum was used to probe select samples previously incubated with defensin-2 to demonstrate specificity of primary antibody binding. To demonstrate the specificity of defensin-2 association with R. montanensis, rabbit anti-defensin-2 antibody was used to probe rickettsia that were incubated with thioredoxin control protein. Grids were washed three times with HEPES, and then incubated with the 10 nm gold particle-labelled anti-rabbit secondary antibody. Grids were fixed with 1% paraformaldehyde, washed with distilled water, and incubated with 1% ammonium molybdate before viewing. Samples were viewed on a JEOL 5700 Transmission Electron Microscope (Jeol USA, Inc. Peabody, MA, USA).
In vivo neutralization of defensin-2
IgG was purified from rabbit anti-defensin-2 or preimmune serum using MelonG IgG purification kit (ThermoFisher Scientific) and the concentration estimated using bicinchoninic acid protein assay (ThermoFisher Scientific) or Fluoroprofile protein quantitation kit (Sigma, St. Louis, MO, USA). Five microliters of IgG was provided to 4-day-part-fed D. variabilis in capillaries at concentrations of 0.5, 1 or 2 mg/ml. Preimmune IgG was provided at a 2-mg/ml concentration. To monitor evaporation of fluid from the capillaries, we placed a capillary containing 5 μl of solution (evaporation control) on a Petri dish. The capillary was not attached to the mouthparts of a tick. The fluid in capillaries attached to the ticks' mouthparts emptied before the fluid in the ‘evaporation control’ capillary. One hour after the IgG was imbibed, ticks were capillary-fed 8 μl of R. montanensis (30 000 bacteria/μl) suspended whole sheep's blood diluted 125-fold with 0.9% NaCl (2.4 × 105 total). The ticks were incubated overnight at 25 °C and 90–100% humidity. Whole tissues were collected for total RNA extraction (Qiagen, Valencia, CA, USA). After extraction, burden was estimated using qRT-PCR. Rickettsial gltA transcript was normalized with host actin transcript (Ceraul et al., 2011). We were unable to use the 16 s rRNA primers to estimate burden in vivo because of nonspecific amplification of symbiotic bacteria transcript.
In vivo immunofluorescence assay of infected tick midgut
The 4-day-part-fed D. variabilis ticks were capillary-fed R. montanensis and incubated for 48 h as described (Ceraul et al., 2007). Following incubation, midgut tissues from time-matched uninfected and R. montanensis-infected ticks was dissected and flash frozen in Tissue-Tek, optimum cutting temperature medium (Miles Inc., Elkhart, IN). Samples were stored at-80 °C until used for cryosectioning. Five-micron sections were cryosectioned and air-dried onto glass slides. After fixation, samples were blocked with PBS supplemented with 10% fetal bovine serum and washed twice with PBS. Samples were then incubated with rabbit anti-defensin-2 serum (1:400) and mouse anti-R. montanensis serum (1:100) for 1 h. A separate slide was incubated with normal rabbit and normal mouse control sera as a control. Following incubation with the primary antibodies, sections were washed three times with PBS for 5 min each. A 1:500 dilution of anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 594 secondary antibodies were applied for 30 min. Sections were washed three more times and counterstained with 1 μM SYTOX Blue nucleic acid stain in 10 mM Tris-HCl, 1 mM EDTA, pH 7.5 for 10 min at room temperature. Sections were mounted using Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA) and a coverslip. Slides were visualized and images captured at 400 X total magnification on a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Thornwood, NY, USA) and analysed using lsmix software package (Zeiss LSM Image Examiner Version 3.2.0.70).
Statistical analysis
Each experiment was repeated at least twice. Individual ticks were designated as biological replicates. Each treatment was represented by at least seven individual biological replicates collected from all experiments performed. An interquatrile range outlier test was performed to exclude outlier data points from statistical analysis. Data from each treatment group were subjected to the Shapiro–Wilk test and log transformed if lacking normality. An F-test was performed to assess equality of variances between groups. A one-tailed t-test was performed to compare each treatment with a control. All statistical analyses were performed using Microsoft Excel.
Acknowledgments
The authors would like to thank Dr Dan Sonenshine, Dr Kevin Macaluso, and Oklahoma State University's tick-rearing facility for the ticks used in this project. Work for this manuscript was supported by funds from the National Institutes of Health/National Institute of Allergy and Infectious Diseases 1K22AI08040-01A1 to SMC and R01AI043006 and R01AI017828 to Abdu F. Azad. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Ceraul SM, Sonenshine DE, Hynes WL. Resistance of the tick Dermacentor variabilis (Acari: Ixodidae) following challenge with the bacterium Escherichia coli (Enterobacteriales: Enterobacteriaceae) J Med Entomol. 2002;39:376–383. doi: 10.1603/0022-2585-39.2.376. [DOI] [PubMed] [Google Scholar]
- Ceraul SM, Dreher-Lesnick SM, Gillespie JJ, Rahman MS, Azad AF. New tick defensin isoform and antimicrobial gene expression in response to Rickettsia montanensis challenge. Infect Immun. 2007;75:1973–1983. doi: 10.1128/IAI.01815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraul SM, Dreher-Lesnick SM, Mulenga A, Rahman MS, Azad AF. Functional characterization and novel rickettsiostatic effects of a Kunitz-type serine protease inhibitor from the tick Dermacentor variabilis. Infect Immun. 2008;76:5429–5435. doi: 10.1128/IAI.00866-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceraul SM, Chung A, Sears KT, Popov VL, Beier-Sexton M, Rahman MS, et al. A Kunitz protease inhibitor from Dermacentor variabilis, a vector for spotted fever group rickettsiae, limits Rickettsia montanensis invasion. Infect Immun. 2011;79:321–329. doi: 10.1128/IAI.00362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrudimska T, Slaninova J, Rudenko N, Ruzek D, Grubhoffer L. Functional characterization of two defensin isoforms of the hard tick Ixodes ricinus. Parasit Vectors. 2011;4:63. doi: 10.1186/1756-3305-4-63. doi: 10.1186/1756-3305-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan AS, Maginnis MS, Jordan JA, Gasparovic ML, Manley K, Page R, et al. Human alpha-defensins inhibit BK virus infection by aggregating virions and blocking binding to host cells. J Biol Chem. 2008;283:31125–31132. doi: 10.1074/jbc.M805902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenberger LR, Lamoreaux WJ, Coons LB. Hemocytic encapsulation of implants in the tick Dermacentor variabilis. Exp Applied Acarol. 1990;9:279–287. doi: 10.1007/BF01193434. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Kaur SJ, Rahman MS, Ammerman NC, Beier-Sexton M, Ceraul SM, Gillespie JJ, et al. TolC-dependent secretion of an ankyrin repeat-containing protein of Rickettsia typhi. J Bacteriol. 2012;194:4920–4932. doi: 10.1128/JB.00793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopáček P, Hajdušek O, Burešová V, Daffre S. Tick innate immunity. In: Söderhäll K, editor. Invertebrate Immunity. New York, NY: Springer; 2010. pp. 137–162. [PubMed] [Google Scholar]
- Login FH, Balmand S, Vallier A, Vincent-Monegat C, Vigneron A, Weiss-Gayet M, et al. Antimicrobial peptides keep insect endosymbionts under control. Science. 2011;334:362–365. doi: 10.1126/science.1209728. [DOI] [PubMed] [Google Scholar]
- Lu X, Che Q, Lv Y, Wang M, Lu Z, Feng F, et al. A novel defensin-like peptide from salivary glands of the hard tick, Haemaphysalis longicornis. Protein Sci. 2009;19:392–397. doi: 10.1002/pro.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo G, Sanna R, Casu M, Mignogna G, Mangoni ML, Rinaldi AC, et al. Toward an improved structural model of the frog-skin antimicrobial peptide esculentin-1b(1-18) Biopolymers. 2012;97:873–881. doi: 10.1002/bip.22086. [DOI] [PubMed] [Google Scholar]
- Marcellini L, Borro M, Gentile G, Rinaldi AC, Stella L, Aimola P, et al. Esculentin-1b(1-18) – a membrane-active antimicrobial peptide that synergizes with antibiotics and modifies the expression level of a limited number of proteins in Escherichia coli. FEBS J. 2009;276:5647–5664. doi: 10.1111/j.1742-4658.2009.07257.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl Environ Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes JC, Welchman DP, Poidevin M, Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity. 2011;35:770–779. doi: 10.1016/j.immuni.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann M, Arnold T, Linke D, Ozdirekcan S, Kopp A, Gutsmann T, et al. Structure-activity analysis of the dermcidin-derived peptide DCD-1L, an anionic antimicrobial peptide present in human sweat. J Biol Chem. 2012;287:8434–8443. doi: 10.1074/jbc.M111.332270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Tsuji N, Boldbaatar D, Battur B, Liao M, Umemiya-Shirafuji R, et al. Structural characterization and cytolytic activity of a potent antimicrobial motif in longicin, a defensin-like peptide in the tick Haemaphysalis longicornis. J Vet Med Sci. 2009;72:149–156. doi: 10.1292/jvms.09-0167. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Ha EM, Lee WJ. Innate immunity and gut-microbe mutualism in Drosophila. Dev Comp Immunol. 2010;34:369–376. doi: 10.1016/j.dci.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Smith JG, Nemerow GR. Mechanism of adenovirus neutralization by Human alpha-defensins. Cell Host Microbe. 2008;3:11–19. doi: 10.1016/j.chom.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, Sahl HG, et al. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Maeda H, Galay RL, Boldbattar D, Umemiya-Shirafuji R, Suzuki H, et al. Tick longicin implicated in the arthropod transmission of Toxoplasma gondii. J Vet Sci Technol. 2012;3 doi: 10.4172/2157-7579.1000112. [Google Scholar]
- Tsuji N, Battsetseg B, Boldbaatar D, Miyoshi T, Xuan X, Oliver JH, Jr, et al. Babesial vector tick defensin against Babesia sp. parasites. Infect Immun. 2007;75:3633–3640. doi: 10.1128/IAI.00256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu S. The defensin gene family expansion in the tick Ixodes scapularis. Dev Comp Immunol. 2011;35:1128–1134. doi: 10.1016/j.dci.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proc Natl Acad Sci USA. 2004;101:7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]


